Abstract
Background:
The etiology of chronic fatigue syndrome (CFS) is unknown. Orthostatic intolerance (OI) is common in CFS patients. Recently, small heart with low cardiac output has been postulated to be related to the genesis of both CFS and OI.
Hypothesis:
Small heart is associated with OI in patients with CFS.
Methods:
Study CFS patients were divided into groups of 26 (57%) CFSOI(+) and 20 (43%) CFSOI(−) according to the presence or absence of OI. In addition, 11 OI patients and 27 age‐ and sex‐matched control subjects were studied. Left ventricular (LV) dimensions and function were determined echocardiographically.
Results:
The mean values of cardiothoracic ratio, systemic systolic and diastolic pressures, LV end‐diastolic dimension, LV end‐systolic dimension, stroke volume index, cardiac index, and LV mass index were all significantly smaller in CFSOI(+) patients than in CFSOI(−) patients and healthy controls, and also in OI patients than in controls. A smaller LV end‐diastolic dimension (<40 mm) was significantly (P<0.05) more prevalently noted in CFSOI(+) (54%) and OI (45%) than in CFSOI(−) (5%) and controls (4%). A lower cardiac index (<2 L/min/mm2) was more prevalent in CFSOI(+) (65%) than in CFSOI(−) (5%, P<0.01), OI (27%), and controls (11%, P<0.01).
Conclusions:
A small size of LV with low cardiac output was noted in OI, and its degree was more pronounced in CFSOI(+). A small heart appears to be related to the genesis of OI and CFS via both cerebral and systemic hypoperfusion. CFSOI(+) seems to constitute a well‐defined and predominant subgroup of CFS. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Chronic fatigue syndrome (CFS) is an important health problem, characterized by persistent and relapsing severe, disabling fatigue that is not resolved by rest, causing a marked reduction of working activity.1, 2, 3 Despite the public health burden imposed by CFS, effective diagnostic, treatment, and prevention strategies are not available because the etiology, risk factors, and pathophysiology remain unknown.
Recently, patients with orthostatic intolerance (OI) have become a focus of clinical attention.4, 5, 6 These patients predictably develop symptoms of disabling fatigue, dizziness, diminished concentration, tremulousness, and nausea while standing. Simple activities such as eating, showering, or low‐intensity exercise may profoundly exacerbate these symptoms, which may significantly impair even the most basic activities of daily living. Many of the primary symptoms of OI are often seen in patients with disabling CFS.7, 8, 9 Both CFS and OI affect many young people, predominantly women.
We have recently reported that “small heart” with low cardiac output is prevalent in CFS patients.10, 11, 12 Furthermore, Fu et al13 have recently reported that patients with postural orthostatic tachycardia, which is often noted in patients with chronic OI, have small hearts coupled with reduced blood volume compared with healthy controls, as determined by magnetic resonance imaging and blood volume measurements.
In the present study, left ventricular (LV) size and function were evaluated echocardiographically in CFS patients with and without OI, and also in OI patients without CFS, to clarify the differences in cardiac size and function among these patients and healthy controls.
Methods
Study Population
Consecutive patients with chronic fatigue who visited our clinic, were diagnosed with CFS, and gave informed consent to participate were included in the present study. Chronic fatigue syndrome was diagnosed according to the revised case definition of Fukuda et al.2
The study population comprised 46 patients with CFS, 16 men and 30 women, with a mean age of 30 ± 8 years. These patients were divided into CFSOI(+) (26, 57%) and CFSOI(−) (20, 43%) groups, according to the presence or absence of OI. Orthostatic intolerance was defined as instability in maintaining normal consciousness without significant symptoms such as disabling fatigue, dizziness, diminished concentration, tremulousness, sweating, lightheadedness, visual disturbance, palpitations, and nausea while standing, or any perturbation that mimics the hemodynamic consequences of standing.8 In addition, we studied 11 patients with OI but without CFS (group OI) and 27 healthy control subjects (controls; Table 1). Postural orthostatic tachycardia syndrome (POTS) was diagnosed in 7 (27%) of the CFSOI(+) patients and in 3 (27%) of the OI patients with an increase of heart rate >30 beats/minute during a 10‐minute standing test.
Table 1.
Comparative Echocardiographic Data Among the Study Groups
| CFSOI(+) | CFSOI(−) | OI | Controls | |
|---|---|---|---|---|
| No. of patients | 26 | 20 | 11 | 27 |
| M/F | 7/19 | 9/11 | 2/9 | 10/17 |
| Age (y) | 28 ± 8 | 32 ± 8 | 31 ± 7 | 32 ± 7 |
| Cardiothoracic ratio (%) | 38 ± 5a,b | 42 ± 4 | 42 ± 3c | 44 ± 4 |
| Heart rate (bpm) | 69 ± 15 | 72 ± 13 | 68 ± 11 | 70 ± 12 |
| SBP (mm Hg) | 109 ± 11a,b | 115 ± 13 | 112 ± 14c | 121 ± 11 |
| DBP (mm Hg) | 66 ± 12a,b | 72 ± 12 | 68 ± 13c | 75 ± 12 |
| IVS (mm) | 8 ± 1 | 9 ± 1 | 8 ± 1 | 9 ± 1 |
| PW (mm) | 8 ± 1 | 9 ± 1 | 8 ± 1 | 9 ± 1 |
| LVEDD (mm) | 39 ± 5b,d | 45 ± 5 | 40 ± 5b,d | 45 ± 4 |
| LVEDD <40 (mm) | 14 (54%)a,b | 1 (5%) | 5 (45%)a,b | 1 (4%) |
| LVESD (mm) | 25 ± 5b,d | 28 ± 3 | 24 ± 5b,d | 27 ± 2 |
| LAD (mm) | 25 ± 5 | 27 ± 5 | 24 ± 4 | 27 ± 4 |
| AoD (mm) | 26 ± 4 | 26 ± 4 | 25 ± 3 | 27 ± 5 |
| RVD (mm) | 15 ± 4 | 17 ± 4 | 15 ± 2 | 15 ± 3 |
| Stroke volume (mL) | 45 ± 13b,d | 64 ± 17 | 49 ± 11a,b | 63 ± 13 |
| Stroke volume index (mL/m2) | 30 ± 7b,d | 38 ± 7 | 33 ± 7c | 39 ± 7 |
| Cardiac output (L/min) | 3.0 ± 0.7b,d | 4.5 ± 1.1 | 3.3 ± 1.0b,d | 4.4 ± 1.0 |
| Cardiac index (L/min/m2) | 2.0 ± 0.3b,d | 2.7 ± 0.6 | 2.2 ± 0.5a,c | 2.7 ± 0.6 |
| Cardiac index <2 (L/min/m2) | 17 (65%)b,d | 1 (5%) | 3 (27%) | 3 (11%) |
| Fractional shortening (%) | 37 ± 4 | 38 ± 3 | 40 ± 7 | 39 ± 3 |
| LVEF (%) | 67 ± 5 | 68 ± 4 | 71 ± 8 | 69 ± 4 |
| LV mass index (g/m2) | 62 ± 16b,d | 77 ± 14 | 64 ± 13a,c | 77 ± 16 |
Abbreviations: AoD, aortic root diameter; CFSOI(+), patients with chronic fatigue syndrome and orthostatic intolerance; CFSOI(−), patients with chronic fatigue syndrome but without orthostatic intolerance; DBP, diastolic blood pressure; F, female; IVS, interventricular septum thickness; LAD, left atrial dimension; LV, left ventricular; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; M, male; OI, patients with orthostatic intolerance but without chronic fatigue syndrome; PW, left ventricular posterior wall thickness; RVD, right ventricular dimension; SBP, systolic blood pressure.
P<0.05 vs CFSOI(−).
P<0.01 vs controls.
P<0.05 vs controls.
P<0.01 vs CFSOI (−).
All studied participants gave informed consent, and the study protocol was approved by the ethics committee of our institution.
Blood Pressure Measurement
Arm blood pressure was measured using a cuff digital pressure monitor (HEM‐907; Omron, Kyoto, Japan) in the sitting position.
Chest Roentgenography
Cardiothoracic ratio was calculated as the cardiac transverse diameter to the thoracic transverse diameter in the posteroanterior projection on a chest roentgenogram.
Echocardiography
All individuals underwent standard M‐mode and 2‐dimensional echocardiography. Left ventricular dimensions were measured according to the recommendations of the American Society of Echocardiography.14 Left ventricular volume was calculated by the Teichholz formula,15 and ejection fraction was calculated. Left ventricular mass was calculated according to the formula of Devereux and indexed to body surface area.14
Statistical Analysis
Values are presented as the mean ± SD. Comparisons of echocardiographic values or parameters between the study groups were performed with analysis of variance followed by the Student unpaired t test. Proportional data were analyzed by the χ 2 test, with Yates' correction. The value of significance was set at P<0.05.
Results
Results are summarized in the Table 1 and Figures 1 and 2. The mean cardiothoracic ratio was significantly smaller in the CFSOI(+) group than in the CFSOI(−) group and in controls. The ratio was also significantly smaller in the OI group than in controls. Both systolic and diastolic blood pressures were significantly lower in CFSOI(+) than in CFSOI(−) and in controls. Both pressures were also significantly lower in OI than in controls. The mean values of LV end‐diastolic dimension (LVEDD), LV end‐systolic dimension (LVESD), cardiac index, and LV mass index were all significantly smaller in CFSOI(+) and in OI than in CFSOI(−) and in controls. The mean stroke volume index was significantly smaller in CFSOI(+) than in CFSOI(−) and in controls, and also significantly smaller in OI than in controls. A smaller LVEDD (<40 mm) was significantly more prevalently noted in CFSOI(+) (54%) and OI (45%) than in CFSOI(−) (5%) and controls (4%). A lower cardiac index (<2 L/min/m2) was more prevalently noted in CFSOI than in the other 3 groups (Table 1). The individual data of LVEDD and cardiac index in each group are shown in Figures 1 and 2. The mean values of both LV fractional shortening and LV ejection fraction were comparable among the groups (Table 1).
Figure 1.
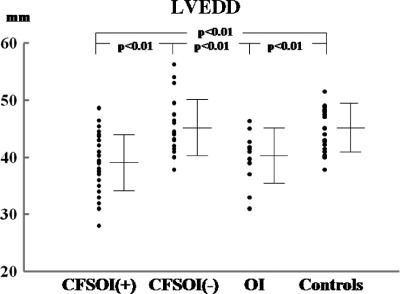
Comparison of LVEDD among the study groups. Data are presented as mean ± SD. Abbreviations: CFSOI(+), patients with chronic fatigue syndrome and orthostatic intolerance; CFSOI(−), patients with chronic fatigue syndrome but without orthostatic intolerance; LVEDD, left ventricular end‐diastolic dimension; OI, patients with orthostatic intolerance but without chronic fatigue syndrome.
Figure 2.
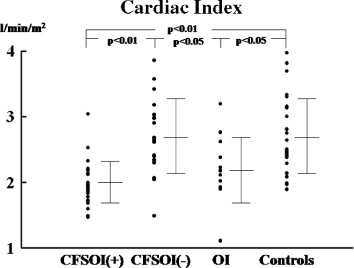
Comparison of cardiac index among the study groups. Data are presented as mean ± SD. Abbreviations: CFSOI(+), patients with chronic fatigue syndrome and orthostatic intolerance; CFSOI(−), patients with chronic fatigue syndrome but without orthostatic intolerance; OI, patients with orthostatic intolerance but without chronic fatigue syndrome.
Discussion
“Small heart,” the concept that the heart is too small compared with the body to function adequately, was previously proposed by Master in 1944 as so‐called neurocirculatory asthenia in young adults, and was associated with a small heart shadow on a chest roentgenogram.16, 17 The symptoms usually appear during youth, and such patients occasionally consult doctors because of easy fatigability, general malaise, weakness, nervousness, trembling, and sweating, as well as a variety of cardiovascular complaints, including chest pain, palpitation, dyspnea or shortness of breath, dizziness, and fainting. These symptoms are also common in patients with CFS and/or OI, although the disease concept of both CFS and OI was developed and postulated later.2, 4, 5, 6, 7, 8, 9 Although Master hypothesized that these symptoms were caused by diminished cardiac output due to a congenital or constitutionally small heart,16 the pathophysiological significance of small heart has not been established as a cardiovascular disease manifesting low cardiac output syndrome.
Recently, we reported that a small heart shadow on a chest roentgenogram was prevalent in CFS patients.10 Echocardiographic evaluation demonstrated that cardiac function was actually impaired, with low cardiac output due to a small LV in many CFS patients.10, 11, 12 Moreover, hemodynamic abnormalities including smaller LV chamber size and diminished cardiac stroke volume and cardiac output during the exacerbation phase significantly improved during the remission phase, suggesting a direct relationship between the severity of their symptoms and impaired cardiac function.11 Following our reports, Hurwitz et al reported that severe CFS patients have lower cardiac volume assessed by echocardiography and lower total blood volume, plasma volume, and red blood cell volume measured by dual tag blood volume assessments than controls, suggesting a comorbid hypovolemic condition.18 Interestingly, in 2003 Peckerman et al reported that, using impedance cardiography in the supine and standing positions, cardiac output is significantly lower in a severe CFS group than in controls and less‐ill patients.19 Many CFS patients have low cardiac output, and the resulting low circulatory flow state may make it difficult for patients to meet the demands of daily activity, and it may also lead to fatigue and other conditions. Indeed, CFS patients have a variety of possible cardiovascular complaints, including chest pain, palpitation, dyspnea or shortness of breath, cold feet, dizziness, and fainting, although all of these symptoms are not necessarily attributable to cardiovascular dysfunction.12
In the present study, CFS patients had a high prevalence of OI, and echocardiographic examination revealed that CFS patients with OI, and also OI patients, have smaller LV chambers and poorer cardiac performance with lower cardiac output than controls. Small heart coupled with reduced cardiac function seems to be related to the genesis of OI as a predisposing factor for cerebral hypoperfusion on standing. High prevalence of lower cardiac index <2 in the CFS patients with OI (65%) suggests that these patients have a more severe form of small heart, with impaired systemic circulation and cerebral hypoperfusion while in an upright position, than the patients with simple OI. Chronic fatigue syndrome with OI seems to constitute a well‐defined and predominant subgroup of CFS.
Orthostatic intolerance is characterized by the inability to remain upright without severe signs and symptoms such as hypotension, tachycardia, lightheadedness, pallor, fatigue, weakness, dizziness, diminished concentration, tremulousness, and nausea.20, 21, 22 The POTS, defined by symptoms of OI associated with an excessive increase in heart rate on orthostatic challenge, has been identified as a common form, but not the only form, of chronic OI.6, 22, 23, 24 Many symptoms of OI appear to be related to reduced cerebral blood flow. Symptoms are associated with inadequate systemic venous return to the right heart or thoracic hypovolemia, although precise mechanisms remain to be clarified.4, 5, 25 In addition, reduced cerebral perfusion induced by excessive lower‐body venous pooling with delayed orthostatic hypotension has been suggested to be involved in the orthostatic component of fatigue in CFS patients.26 Recently, by using a cardiac magnetic resonance imaging technique, Fu et al13 assessed precisely the heart size and mass in patients with POTS and found that cardiac size and mass and blood volume were much smaller in the patients than in healthy sedentary controls. The marked orthostatic tachycardia in these patients seemed to be a physiologic compensatory response to a smaller stroke volume, and exercise training improved this syndrome in most patients.13 In their assessment of both sympathetic baroreflex sensitivity and cardiovagal baroreflex sensitivity, the function of the autonomic nervous system was intact in the patients,13 although other researchers have postulated autonomic nervous dysfunction with exaggerated sympathetic nervous activation over compensatory levels while standing as a major mechanism behind the symptoms.4, 5, 20, 24, 27
Elucidation of the pathophysiology of CFS and OI may lead to better therapeutic strategies. Recently, xenon–computed tomography blood‐flow studies demonstrated that CFS patients have global cerebral hypoperfusion with reduced absolute cortical blood flow in broad areas, especially in bilateral middle cerebral artery territories, compared with healthy controls.28 Impaired cerebral oxygenation due to reduced cerebral hemodynamics in young CFS patients with OI during an active standing test was suggested from the findings of continuous measurement of cerebral oxygenated hemoglobin using near‐infrared spectroscopy.29 In the present study, low systolic and diastolic blood pressures were noted in OI patients with and without CFS compared with those in control subjects. Using 24‐hour ambulatory blood pressure monitoring, Newton et al30 have consolidated the evidence that lower blood pressure occurs in CFS patients, and lower nighttime blood pressure seems to be a significant problem that may lead to the enhanced diurnal variation. Reasonable potentiation of cerebrovascular flow without exaggerated activation or perturbation of the autonomic nervous system may be needed for effective treatment. However, administration of nonselective vasoconstrictive agents may cause a simple reduction of cerebral blood flow via elevation of cerebrovascular resistance, although intravenous infusion of phenylephrine, a sympathetic nerve α 1 stimulator, has been reported to improve OI, as a result of producing significant peripheral vasoconstriction and venoconstriction in some OI patients.31 Volume repletion by increasing sodium intake or by treatment with fludrocortisones may theoretically improve OI and also symptoms of CFS by replenishing intravascular volume.4, 7, 9, 22, 25 Military antishock trousers and elastic stockings may also be effective via potentiation of venous return, resulting in increased cardiac output.26 Although CFS patients are limited by the discomfort of an increased perception of exertion, there are some data to support the notion that an appropriately designed exercise program is beneficial.13, 32, 33, 34 Various triggers including loss of appetite, diarrhea, and sweating can cause dehydration accompanied by preload reduction, leading to further decreases in stroke volume and cardiac output, thereby impairing both systemic and cerebral circulation and exacerbating symptoms.
Study Limitations
Our study has some limitations. First, the causal relationship between the low cardiac output observed in the patients and a variety of symptoms remains to be clarified. That low cardiac output is attributable in part to the decreased demand by the possible loss of muscle volume due to immobility or reduction of physical effort in these patients cannot be ruled out. Second, the underlying disease of OI, such as postural orthostatic tachycardia, delayed orthostatic hypotension, and neurally mediated hypotension, was not identified in our study patients. The mechanism or cause of OI may not be similar among the patients, especially concerning the possible role of autonomic dysfunction. Another limitation is a lack of measurement of cerebral blood flow. Quantitative assessment of cerebral blood flow in recumbent and upright positions is required to clarify the genesis of OI and also a possible link between OI and low cardiac output in CFS.
Conclusion
In conclusion, a small size of LV with reduced cardiac function is common in patients with OI and its degree appears to be more pronounced in CFS patients with OI. Chronic fatigue syndrome with OI seems to constitute a well‐defined and predominant subgroup of CFS. Small heart with impaired cardiac performance due to decreased preload appears to be an important target for the treatment of CFS and OI.
References
- 1. Shafran SD. The chronic fatigue syndrome. Am J Med. 1991;90: 730–739. [PubMed] [Google Scholar]
- 2. Fukuda K, Straus SE, Hickie I, et al; International Chronic Fatigue Syndrome Study Group . The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. [DOI] [PubMed] [Google Scholar]
- 3. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–236. [DOI] [PubMed] [Google Scholar]
- 4. Rosen S, Cryer PE. Postural tachycardia syndrome: reversal of sympathetic hyperreponsiveness and clinical improvement during sodium loading. Am J Med. 1982;72:847–850. [DOI] [PubMed] [Google Scholar]
- 5. Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. [DOI] [PubMed] [Google Scholar]
- 6. Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J MedSci. 1999;317:75–77. [DOI] [PubMed] [Google Scholar]
- 7. Schondorf R, Freeman R. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci. 1999;317:117–123. [DOI] [PubMed] [Google Scholar]
- 8. Schondorf R, Benoit J, Wein T, et al. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75:192–201. [DOI] [PubMed] [Google Scholar]
- 9. Streeten DH, Thomas D, Bell DS. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2000;320:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Miwa K, Fujita M. “Small heart syndrome” in patients with chronic fatigue syndrome. Clin Cardiol. 2008;31:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miwa K, Fujita M. Cardiac function fluctuates during exacerbation and remission in young adults with chronic fatigue syndrome and “small heart.” J Cardiol. 2009;54:29–35. [DOI] [PubMed] [Google Scholar]
- 12. Miwa K, Fujita M. Cardiovascular dysfunction with low cardiac output due to small heart in patients with chronic fatigue syndrome. Intern Med. 2009;48:1849–1854. [DOI] [PubMed] [Google Scholar]
- 13. Fu Q, Vangundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010; 55:2858–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schiller NB, Shah PM, Crawford M, et al. Recommendations for the quantification of the left ventricle by two‐dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 15. Teichholz LE, Kreulen T, Herman MV, et al. Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. [DOI] [PubMed] [Google Scholar]
- 16. Master AM. Neurocirculatory asthenia due to small heart. Med Clin North Am. 1944;28:577–588. [Google Scholar]
- 17. Miwa K, Fujita M. Is small heart syndrome a “heart” disease or low output syndrome? Int J Cardiol. 2011;146:95–96. [DOI] [PubMed] [Google Scholar]
- 18. Hurwitz BE, Coryell VT, Parker M, et al. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond). 2010;118:125–135. [DOI] [PubMed] [Google Scholar]
- 19. Peckerman A, Lamanca JJ, Dahl KA, et al. Abnormal impedance cardiography predicts symptom severity in chronic fatigue syndrome. Am J Med Sci. 2003;326:55–60. [DOI] [PubMed] [Google Scholar]
- 20. Furlan R, Jacob G, Snell M, et al. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–2159. [DOI] [PubMed] [Google Scholar]
- 21. Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105:2274–2281. [DOI] [PubMed] [Google Scholar]
- 22. Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr. 2004;145:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rowe PC, Bou‐Holaigah I, Kan JS, et al. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet. 1995;345:623–624. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. [DOI] [PubMed] [Google Scholar]
- 25. Fouad FM, Tadena‐Thome L, Bravo EL, et al. Idiopathic hypovolemia. Ann Intern Med. 1986;104:298–303. [DOI] [PubMed] [Google Scholar]
- 26. Streeten DH. Role of impaired lower‐limb venous innervations in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2001;321:163–167. [DOI] [PubMed] [Google Scholar]
- 27. Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. 2000;48: 218–226. [DOI] [PubMed] [Google Scholar]
- 28. Yoshiuchi K, Farkas J, Natelson BH. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. 2006;26:83–86. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka H, Matsushima R, Tamai H, et al. Impaired postural cerebral hemodynamics in young patients with chronic fatigue with and without orthostatic intolerance. J Pediatr. 2002;140:412–417. [DOI] [PubMed] [Google Scholar]
- 30. Newton JL, Sheth A, Shin J, et al. Lower ambulatory blood pressure in chronic fatigue syndrome. Psychosom Med. 2009;71:361–365. [DOI] [PubMed] [Google Scholar]
- 31. Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute α‐1 adrenergic and β‐1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. 2002;106:2946–2954. [DOI] [PubMed] [Google Scholar]
- 32. Wilson A, Hickie I, Lloyd A, et al. The treatment of chronic fatigue syndrome: science and speculation. Am J Med. 1994;96:544–550. [DOI] [PubMed] [Google Scholar]
- 33. Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. Br Med J. 1997;314:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wearden AJ, Morriss RK, Mullis R, et al. Randomised, double‐blind, placebo‐controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome [published correction appears in Br J Psychiatry. 1998;173:89]. Br J Psychiatry. 1998;172: 485–490. [DOI] [PubMed] [Google Scholar]


