Abstract
Atrial fibrillation (AF) is a complex disease with increasing prevalence in an aging population and longer survival with cardiovascular diseases. Whereas most clinical efforts have been aimed at predicting risk of AF sequelae such as stroke and heart failure, little is known on primary prevention. AF risk assessment is complicated by the existence of distinct subtypes of AF, such as lone AF or postoperative AF, in contrast to common AF in the elderly. Due to its often intermittent nature, diagnosing AF can be a challenge. Risk prediction becomes reasonable when specific interventions arise. Due to our limited understanding of AF pathophysiology and substantial lack of specific preventive strategies in the population, modification of the general cardiovascular risk profile has largely remained the only option. Initial attempts at combining established risk factors for AF such as age, sex, hypertension, body mass index, electrocardiographic characteristics, and cardiovascular disease in a risk‐prediction instrument have produced a robust algorithm. However, known risk factors only explain a fraction of the population‐attributable risk of AF, and the search for novel risk indicators is ongoing. More efficient monitoring for electrocardiographic precursors of AF and the field of genomics are evolving areas of AF risk factor research. A better understanding of the underlying substrate of AF will provide targets for prevention. In the future, clinical trials will be needed to establish risk categories, interventions, and their efficacy. Despite a relevant public‐health impact, knowledge on risk prediction and primary prevention of AF is still limited today.
There are no conflicts of interest to disclose.
Over the last decades, evidence about the anticipated epidemic of atrial fibrillation (AF) has been accumulated, supported by impressive data in Europe and the United States.1., 2., 3. Increasing incidence and longer survival with AF have led to realistic estimates of more than a doubling in AF prevalence, from 2.0% in 2008 to 4.3% in an adult northern European population. Projected numbers range from 25 to 30 million AF cases in Europe and to almost 16 million AF cases in the United States in 2050.2., 3. Major reasons for the rapid increase in AF are the aging of the population and better survival with cardiovascular (CV) diseases such as myocardial infarction (MI) and heart failure (HF).
Age is the most prominent risk factor for AF. At the age of 40, the lifetime risk of developing AF is 1 in 4.4 In contrast to coronary heart disease (CHD) risk, which decreases at older age,5 AF incidence remains high after accounting for competing risk of death. Modern treatment options and decreased case‐fatality of CV diseases such as CHD, HF, and valvular heart disease are likely to increase the number of patients at risk for AF.6 In patients with manifest AF, survival is markedly reduced, with comparable death rates in both sexes even after considering comorbid conditions.7., 8. Other significant and frequent complications of AF comprise thromboembolic events, with AF being the cause of stroke in roughly 1 out of 5 cases, and HF, which becomes manifest in approximately one‐half of AF patients.9., 10. Furthermore, AF is accompanied by reduced health‐associated quality of life and cognitive impairment.11 Symptoms and treatment of AF and its sequelae cause substantial direct and indirect costs.12 In contrast to the described scenarios that emphasize the significant public‐health impact of AF, disease prevention still is in its infancy. The reasons for the striking discrepancy are not fully explained. Several factors may account for the major mismatch.
Atrial fibrillation is a common, complex disease. Despite an increasingly detailed picture of trigger substrates of AF and structural and electromechanical remodeling, disease mechanisms including hypertrophy and fibrosis are little understood. Whereas the concept that AF is not a distinct disease entity but merely a consequence of HF can no longer be advocated, the exact pathophysiology of AF that would provide insight into risk markers for developing disease and targets for intervention remains largely unknown. The phenotype of AF is heterogeneous. Rhythm‐based classification schemes subdivide AF into paroxysmal (ie, self‐terminating), persistent (episodes that last longer than 7 days), and permanent AF.13
Frequently, but not inevitably, a progression of intermittent to permanent types of AF is observed during the clinical course.14 This classification system has been questioned because it only vaguely reflects symptoms, the extent and severity of the underlying disease, prognosis, or indications for therapeutic intervention.15 Counterintuitively, stroke rates in paroxysmal AF patients with risk factors are comparable to persistent or permanent AF,16 and mortality is also increased.17 An additional category is lone AF, observed in individuals without overt cardiopulmonary disease,18 although fibrotic changes in the atria can be detected histologically.19 Lone AF usually becomes manifest earlier in life and may have a stronger genetic predisposition than common AF.20
Diagnosis of AF can be challenging due to the sometimes intermittent nature of the disease. In addition, not all AF episodes are symptomatic.21 Recent advances in monitoring of arrhythmias through continuous pacemaker registration suggest that the majority of AF episodes, in particular nocturnal AF, remain asymptomatic.22 Disease misclassification in primary prevention study samples without continuous monitoring may thus underestimate prevalence measures and risk factor effects on AF and may render correct diagnosis of AF onset more complicated.
Delayed, and in some cases difficult, diagnosis of AF may be one reason why more effort has been put into predicting and preventing sequelae of AF than into the prevention of the disease itself. Current AF guidelines devote much room and detail to rapidly emerging risk assessment and anticoagulation regimens to reduce stroke risk.13 Over decades, risk scores for stroke, one of the most threatening complications of AF, have been developed and validated.23., 24. Currently the CHADS2 and more refined CHA2DS2‐VASc scores are most widely used.25., 26. Oral anticoagulation can effectively reduce incidence of stroke,27 but at the risk of major bleeding such as intracranial hemorrhage. Risk scoring systems have been introduced to assess excess risk of bleeding in AF patients eligible for anticoagulation, the dilemma here being that stroke risk factors overlap with bleeding risk to a large degree.28 Patient characteristics and preferences balancing the risk of stroke and bleeding play an important role in the recommendations.29 The approval of effective new oral anticoagulants such as the direct thrombin inhibitor dabigatran with potentially lower event rates of major bleeding, will change anticoagulation regimens, and will require re‐evaluation of bleeding‐risk algorithms.30., 31.
For primary prevention, evidence is still largely missing. Initial attempts at compiling risk‐prediction algorithms for incident AF were developed in thoracic surgery patients.32 Postoperative AF is a frequent complication in cardiac and noncardiac surgery.33 It may represent a specific subtype of AF that is triggered by periprocedural hemodynamics and imbalance of the sympathetic and parasympathetic tone, resulting in cardiac stress. The exact structural and electrical pathomechanisms leading to postoperative AF are unknown. Direct surgical manipulation and trauma alone or on top of a preexisting substrate have been held responsible for postoperative AF. Enhanced local and systemic inflammation and oxidative stress can be measured. Systemic biomarkers of the acute phase response, inflammation, and oxidative stress are elevated in patients developing postoperative AF.34., 35. It has been suggested that there may be a genetic component to postoperative AF, as shown for the interleukin‐6 promoter gene that appears to modulate the inflammatory response to surgery and susceptibility to postoperative AF.36 Modern atrial tissue metabolomic and proteomic analyses support oxidative stress, acute phase, and apoptotic pathways to be involved. Down‐regulation of glycolysis and pyruvate metabolism and enhanced ketone‐body metabolism can be observed.37., 38. This rudimentary understanding of the pathophysiology of postoperative AF would explain why medication such as beta‐blockers, drugs affecting the renin‐angiotensin‐aldosterone system, statins, and corticoids have been attributed protective effects.39., 40., 41. The antiarrhythmic drugs amiodarone and sotalol may stabilize the proarrhythmic substrate for AF and reduce the incidence of postoperative AF, at the cost of serious side effects.42
The incidence of postoperative AF depends on patient characteristics and type of surgery. After heart surgery, incidence rates can rise to almost 50%. Postoperative AF is accompanied by higher morbidity including HF and stroke, and consecutively longer hospitalization, mortality, and increased costs.43 In addition, first diagnosis of AF after surgery predisposes to recurrent AF during later life. Most known risk parameters for postoperative AF are derived from cardiac or thoracic surgery experience. Indicators of risk of developing AF consistently comprise age, male sex, race, and type and extent of surgery.44 Prior AF episodes are also predictive of recurrent AF in the perioperative phase.45 Among novel risk factors recently suggested are obesity and electrocardiographic (ECG) characteristics, such as P‐wave duration on a 12‐lead ECG.32 Large‐scale validation of risk schemes is outstanding and their ability to guide preventive therapies needs to be assessed. The transportability of risk algorithms to nonthoracic surgery has not been demonstrated yet.
At the population level, postoperative AF accounts only for a small proportion of AF cases, and the pathophysiology of AF occurrence most likely differs for common AF observed in the community. Although the exact links with cardiac structural, contractile, and electrical remodeling processes remain to be elucidated, consistently reported risk indicators of AF are sex, advancing age, body mass index, hypertension, HF, MI, and valvular heart disease.46., 47. Although there is considerable overlap with other CV diseases, there are differences in magnitude and ranking of risk factors. Diabetes, though consistently related to incident AF,48 and smoking are less strongly associated with AF than with other CV diseases. Risk factors more specific to AF are ECG changes, such as PR interval as possible precursors of AF.
Most of these variables are easily available in clinical practice and have been combined in a first attempt at providing a risk‐prediction tool for incident AF in the community.49 The risk algorithm showed good model fit and calibration. Interestingly, echocardiographic information on cardiac structure and function did not seem to improve the risk function to a clinically relevant extent, except for subgroups with HF or valvular heart disease in which cardiological workup is indicated anyway. Additional measurement of the inflammatory biomarker C‐reactive protein or B‐type natriuretic peptide as an indicator of cardiac stress improved the discriminatory ability of the risk function marginally.50 But the benefit in risk information gained through measurement of the 2 biomarkers may not be justifiable for screening due to the related costs.
The initial risk score was validated in independent cohorts with similar performance characteristics.51 Despite well‐known differences in AF risk factor burden in African Americans, the magnitude of association was comparable to whites and the risk function performed equally well. However, the risk factors combined only accounted for a maximum of 64% of the population‐attributable risk across cohorts. Efforts are underway to further improve the risk function and assess its performance in different types of samples.
Multiple novel risk indicators have been suggested in context with AF over the last decade that may help to improve the initial risk score. Among them race,52 sleep apnea,53 and kidney disease54 have been studied most extensively. Some risk factors may apply in specific individuals, as has been reported for thyroid status.55 Increasing availability of rhythm control or monitoring devices, and the identification of ECG predictors of AF, may support detection of precursors of AF and early diagnosis of disease in populations at high CV risk.56
A rapidly evolving field of AF risk assessment is genetic epidemiology, which will provide insights into pathophysiology and risk stratification. The susceptibility to AF is heritable, and a positive family history in at least 1 parent almost doubles the risk of developing AF, in particular in younger individuals.57 In these initial studies that published results on the relevance of parental AF, ECG validation of AF was available in both generations. In practice, a family history of AF is more difficult to accurately obtain than for drastic events such as stroke or MI. More objective genetic markers will be necessary to achieve reliable risk estimates. Whereas familial AF is highly heritable,58 common types of AF are complex diseases with a less well‐established genetic background. From candidate and genome‐wide association studies we have gained insights into the genetic architecture and potential pathophysiological mechanisms of AF across different ethnicities.59., 60. The risk conferred by the identified genetic variants, however, is modest and will not significantly improve risk prediction of AF in the general population at the present stage. Large‐scale consortium efforts are ongoing with the aim to estimate the value of genetic testing for disease prediction. Deeper insights are needed to comprehensively elucidate the role of genomics in AF risk.
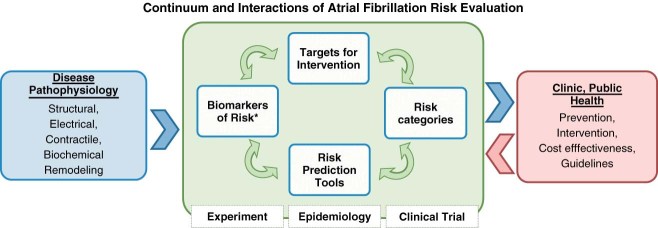
In the practice setting, the gain in information using additional risk factors always needs to be weighed against the increasing complexity of the risk‐prediction model and additional costs arising from the determination of the respective risk factor. Importantly, risk assessment in AF is supposed to help guide clinical decision‐making. For this reason, specific actions should arise from a certain risk score. Present risk categories are data‐driven and do not result in specific interventional recommendations, except for the intuitive optimization and treatment of modifiable risk factors with the hope to modify the substrate of AF. Clinical trials will be needed to establish risk categories, test interventions, and assess the cost‐benefit ratio of novel risk factors (Figure 1).
Figure 1.
Generation, evaluation, and refinement of AF risk‐prediction tools and primary prevention. * Biomarkers in the broad sense of objectively measurable and quantifiable characteristics correlated with normal biology and disease. Arrow means “informs.” Abbreviations: AF, atrial fibrillation.
In conclusion, we are only at the beginning of valid and reliable risk prediction in AF. Initial risk‐prediction algorithms for incident AF perform satisfactorily but need to show their transportability to different settings and ethnicities. Known risk factors explain only a moderate proportion of the population‐attributable risk and leave room for improvement. Convincing preventive strategies need to be developed based on the identified risk factors. In return, their performance will help to refine risk categories and choice of risk indicators. The success story for coronary artery disease with a significant decline in CHD deaths teaches us that primary prevention can be highly effective. Risk factor research at all levels, from understanding pathophysiological mechanisms and the epidemiology of AF to the identification of precursors of disease, will inform the development of accurate risk‐stratification instruments, optimize early diagnosis of AF, and, ultimately, optimize the implementation of preventive strategies to reduce the public health burden of AF.
References
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 3. Stefansdottir H, Aspelund T, Gudnason V, et al. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–1117. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones DM, Larson MG, Beiser A, et al. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. [DOI] [PubMed] [Google Scholar]
- 6. McManus DD, Gore J, Yarzebski J, et al. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 8. Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new‐onset atrial fibrillation. JAMA. 2011;305:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22: 983–988. [DOI] [PubMed] [Google Scholar]
- 10. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107: 2920–2925. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds MR, Lavelle T, Essebag V, et al. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new‐onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse Events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152: 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holstenson E, Ringborg A, Lindgren P, et al. Predictors of costs related to cardiovascular disease among patients with atrial fibrillation in five European countries. Europace. 2011;13: 23–30. [DOI] [PubMed] [Google Scholar]
- 13. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 14. De Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. [DOI] [PubMed] [Google Scholar]
- 15. Lubitz SA, Benjamin EJ, Ruskin JN, et al. Challenges in the classification of atrial fibrillation. Nat Rev Cardiol. 2010;7:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–975. [DOI] [PubMed] [Google Scholar]
- 17. Friberg L, Hammar N, Pettersson H, et al. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort‐Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28: 2346–2353. [DOI] [PubMed] [Google Scholar]
- 18. Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation: a population‐based study over three decades. N Engl J Med. 1987;317:669–674. [DOI] [PubMed] [Google Scholar]
- 19. Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 20. Ellinor PT, Yoerger DM, Ruskin JN, et al. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. [DOI] [PubMed] [Google Scholar]
- 21. Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89: 224–227. [DOI] [PubMed] [Google Scholar]
- 22. Boriani G, Botto GL, Padeletti L, et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2‐VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke. 2011;42:1768–1770. [DOI] [PubMed] [Google Scholar]
- 23. Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008;31:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new‐onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. [DOI] [PubMed] [Google Scholar]
- 25. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 26. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 27. Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline‐adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high‐risk patients with atrial fibrillation: the Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153:1006–1012. [DOI] [PubMed] [Google Scholar]
- 28. Olesen JB, Lip GY, Hansen PR, et al. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost. 2011;9:1460–1467. [DOI] [PubMed] [Google Scholar]
- 29. Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13:723–746. [DOI] [PubMed] [Google Scholar]
- 30. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 31. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:1144–1150. [DOI] [PubMed] [Google Scholar]
- 32. Amar D, Shi W, Hogue CW Jr, et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:1248–1253. [DOI] [PubMed] [Google Scholar]
- 33. Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127:779–786. [DOI] [PubMed] [Google Scholar]
- 34. Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111: 2881–2888. [DOI] [PubMed] [Google Scholar]
- 35. Ramlawi B, Otu H, Mieno S, et al. Oxidative stress and atrial fibrillation after cardiac surgery: a case‐control study. Ann Thorac Surg. 2007;84:1166–1172. [DOI] [PubMed] [Google Scholar]
- 36. Mandal K, Jahangiri M, Mukhin M, et al. Association of anti‐heat shock protein 65 antibodies with development of postoperative atrial fibrillation. Circulation. 2004;110:2588–2590. [DOI] [PubMed] [Google Scholar]
- 37. Mayr M, Yusuf S, Weir G, et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–594. [DOI] [PubMed] [Google Scholar]
- 38. Kourliouros A, Yin X, Didangelos A, et al. Substrate modifications precede the development of atrial fibrillation after cardiac surgery: a proteomic study. Ann Thorac Surg. 2011;92:104–110. [DOI] [PubMed] [Google Scholar]
- 39. Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. [DOI] [PubMed] [Google Scholar]
- 40. Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA‐3 (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery) study. Circulation. 2006;114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 41. Chen WT, Krishnan GM, Sood N, et al. Effect of statins on atrial fibrillation after cardiac surgery: a duration‐and dose‐responsemeta‐analysis. J Thorac Cardiovasc Surg. 2010;140:364–372. [DOI] [PubMed] [Google Scholar]
- 42. Crystal E, Garfinkle MS, Connolly SS, et al. Interventions for preventing post‐operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2004;CD003611. [DOI] [PubMed] [Google Scholar]
- 43. Rostagno C, La Meir M, Gelsomino S, et al. Atrial fibrillation after cardiac surgery: incidence, risk factors, and economic burden. J Cardiothorac Vasc Anesth. 2010;24:952–958. [DOI] [PubMed] [Google Scholar]
- 44. Onaitis M, D'Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90:368–374. [DOI] [PubMed] [Google Scholar]
- 45. Mathew JP, Parks R, Savino JS, et al; MultiCenter Study of Perioperative Ischemia Research Group . Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. JAMA. 1996;276:300–306. [PubMed] [Google Scholar]
- 46. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 47. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 48. Huxley RR, Filion KB, Konety S, et al. Meta‐analysis of cohort and case‐control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schnabel RB, Aspelund T, Li G, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marcus GM, Alonso A, Peralta CA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 54. Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123: 2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295: 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons >/= 60 years old (from the Framingham Heart Study). Am J Cardiol. 2011;107: 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fox CS, Parise H, D'Agostino RB Sr, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291: 2851–2855. [DOI] [PubMed] [Google Scholar]
- 58. Hodgson‐Zingman DM, Karst ML, Zingman LV, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benjamin EJ, Rice KM, Arking DE, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010; 42:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]