Abstract
B‐type natriuretic peptide (BNP) and N‐terminal‐proBNP (NT‐proBNP) are increasingly recognized as prognostic markers in patients with acute coronary syndrome (ACS). The need for novel and more effective tools for risk assessment cannot be more emphasized than in older patients with ACS given their atypical presentation, multiple comorbidities, and higher risk for mortality and morbidity. Accurate interpretation of B‐type NP values in older patients with ACS, however, may be confounded by several aging‐related physiologic changes. Advanced age, reduction in body mass, and kidney function and anemia have been associated with higher BNP and NT‐proBNP concentrations, and may create challenges with interpreting NP levels in the elderly. This review highlights the need to better understand the physiology of BNP and NT‐proBNP in older individuals and their prognostic value in older patients with ACS. Clin. Cardiol. 2012 doi: 10.1002/clc.22035
Dr. de Lemos has received grant support from Roche Diagnostics and Abbott Diagnostics. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
The prognostic role of B‐type natriuretic peptide (BNP) and N‐terminal‐proBNP (NT‐proBNP) in acute coronary syndromes (ACS) remains the subject of vigorous investigation reflecting the growing interest in improving risk stratification strategies for affected patients. While a compelling body of evidence demonstrates that these biomarkers independently predict adverse outcomes, a few issues of uncertainty limit their routine use in clinical decision making. One is the question of whether existing evidence can be extrapolated to the older population. This is an important issue as approximately 82% of deaths from coronary artery disease (CAD) occur in individuals age 65 or older.1 Fundamental to examining the value of BNP and NT‐proBNP in older persons with CAD is identifying aging‐related factors that may influence their levels. This review summarizes current literature describing the value of these biomarkers in older patients with ACS. The relationship of BNP and NT‐proBNP with advanced age and its associated physiologic alterations is also reviewed.
Influence of Age on ACS Presentation and Outcomes
The aging population represents an increasing CAD burden and imposes challenges for age‐appropriate medical care. In the United States, the average age at first myocardial infarction (MI) is 64.5 years for men and 70.4 years for women.1 Moreover, it has been reported that elderly patients are more likely to develop ACS with atypical symptoms (ie, absence of chest pain), nondiagnostic electrocardiograms, and multiple comorbid conditions, making them a risk stratification and diagnostic challenge.2 Advancing age is an independent risk factor for short‐term and long‐term mortality post‐MI.2 The odds for in‐hospital death escalate by 70% for every 10‐year increase in age3 and the risk of electrical and mechanical complications progressively increases with advancing age. The rate of heart failure (HF), in particular, increases markedly with age and has been associated with aging‐related alterations in left ventricular (LV) wall thickness, LV ejection fraction, diastolic filling patterns, and vascular stiffness,4 known stimuli of the NP system. Taken together, these data accentuate a need for better risk stratification tools for a complex disease in an equally complex population.
Older age has also been associated with increased complication rates from medical and interventional therapies in ACS. Elderly patients often present with comorbid kidney disease that may magnify their risk for drug‐related adverse events.5 Rates of blood transfusions for bleeding complications rise with age in patients who are treated either noninvasively or invasively.6 Particularly among individuals treated with percutaneous coronary intervention, advanced age has been directly associated with contrast‐induced nephropathy, stroke/transient ischemic attack, and access‐site and other vascular complications.7., 8. As a result of these increased risks of complications from medical and invasive therapies in the elderly, it is particularly important that accurate risk assessment tools are used to identify the highest‐risk patients in whom the risk/benefit will be favorable for aggressive management for ACS.
Impact of Aging–Related Factors on NPs
BNP is a hormone derived from a precursor pro‐BNP that is released from the ventricles in response to increased ventricular wall stress, including that caused by ischemia. Pro‐BNP is then cleaved into a biologically active BNP and its more stable inactive form NT‐proBNP. While data indicate that NT‐proBNP is cleared exclusively through the kidney and has a half‐life of approximately 120 minutes, BNP has a markedly shorter half‐life of about 20 minutes because it is cleared not only via the kidneys but via binding to NP clearance receptors (NPR‐C) and catabolism by neutral endopeptidases.9
In addition, several aging‐related changes have also been shown to influence NP levels (Table 1). Wallen et al10 were among the first to document that BNP levels were significantly higher in healthy 80‐year‐olds compared with the 40‐year‐old reference group. These findings are concordant with a report of increasing BNP with age in an Olmsted community‐based study, an association that persisted after adjusting for age‐related changes in blood pressure, kidney function, atrial volume, and LV mass.11
Table 1.
Aging Factors Increasing BNP/NT‐proBNP Levels in the Elderly
| Aging Factors | Proposed Mechanism |
|---|---|
| Increased fibrosis and cardiac stiffness | Increased ventricular wall stress |
| Decreased vascular compliance | Increased ventricular wall stress |
| Diminished renal function | Impaired clearance; coexisting structural/coronary heart disease; hypervolemia |
| Decreased lean body mass | Decreased suppressive effect from lean body mass (mediated by androgens) |
| Decreased androgens | Reduction in direct suppressive effect of androgens on proBNP synthesis |
| Anemia | Hemodynamic and ischemic changes; neuroendocrine upregulation |
BNP, B‐type natriuretic peptide; NP, natriuretic peptide.
Apart from advanced age itself, other noncardiac factors may also influence BNP and NT‐proBNP levels, including kidney function, body mass composition, and anemia. The age‐related decline in glomerular filtration rate (GFR) and the higher rates of diabetes and hypertension in an aging population contribute to an increasing prevalence of chronic kidney disease (CKD) in older individuals.12 Although NT‐proBNP levels appear to be more strongly affected by worsening renal dysfunction than BNP,13 both NPs have been shown to predict all‐cause mortality irrespective of CKD stage.14 An inverse association between body mass index (BMI) and NP levels has been consistently documented.15., 16., 17. Data from the Dallas Heart Study demonstrated an inverse association between lean mass, but not fat mass, with BNP and NT‐proBNP levels (Figure 1),18 an effect that appears to be mediated through a suppressive effect of androgens on NP synthesis.19 This finding may be particularly important in the elderly, given the known reduction in lean mass/adipose tissue ratio and testosterone that occurs with aging18: lower lean mass and testosterone may contribute to age‐associated increases in NP levels. The prevalence of anemia is significantly higher in the older population and highest among those age 85 years and older.20 An inverse relationship between lower hemoglobin concentrations and higher BNP and NT‐proBNP levels has also been reported.21., 22., 23.
Figure 1.
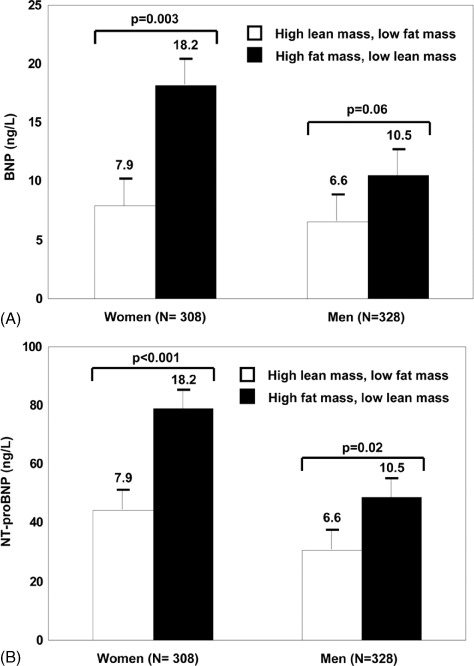
Difference in association of BNP and NT‐proBNP levels with fat and lean mass. Mean level and SE of BNP (A) and NT‐proBNP (B) are shown for 2 subgroups of study participants: (1) below‐median fat mass and above‐median lean mass (open bars); and (2) above‐median fat mass and below‐median lean mass (black bars). Reproduced from Das et al18 (Circulation. 2005;112:2163–2168) with permission of Wolters Kluwer Health via Copyright Clearance Center.
In aggregate, the above findings suggest that noncardiac factors play a relatively greater role in influencing BNP and NT‐proBNP levels in older vs younger individuals. However, although these interactions have not been prospectively evaluated in specific populations of elderly adults with ACS, several studies have shown that BNP and NT‐proBNP are independent predictors of all‐cause and cardiovascular mortality in healthy older adults, after accounting for BMI and renal function.24., 25., 26.
BNP and NT‐proBNP in Older Patients with ACS: Current Evidence
While B‐type NPs are not as useful as cardiac troponins for the diagnosis of MI, higher BNP levels predict higher risk for post‐MI death and HF independent of troponin status.27 In the setting of ST‐elevation MI (STEMI), high BNP concentrations on admission have been associated with an increased risk of 30‐day mortality; patients with BNP > 80 pg/mL had a 7‐fold higher mortality risk compared to those with BNP < 80 pg/ml.28 A study by Omland et al29 showed that NT‐proBNP is a strong and independent predictor of long‐term mortality in STEMI patients, independent of age and LV ejection fraction (LVEF).
However, data on the value of these biomarkers in older patients with ACS remain limited. To date, only 2 studies have investigated the prognostic value of NPs, specifically in the elderly population.30., 31. Drewniak et al30 studied 161 patients aged 79 ± 8 years who were admitted with either STEMI or non‐STEMI. NT‐proBNP levels were measured during hospital admission and repeated at 6 months. Results demonstrated that patients who died had admission NT‐proBNP levels ∼3‐fold higher than those who survived (12,237 vs 4606 pg/mL, P = 0.0001). NTpro‐BNP levels remained a strong and independent predictor of 6‐month mortality after multivariate analysis. Although this study was the first to document the prognostic role of NPs in an exclusive cohort of older patients with ACS, important limitations included the small patient population, use of NT‐proBNP only, absence of data on in‐hospital morbidity (eg, HF, cardiogenic shock), and lack of adjustment for other confounding factors such as creatinine clearance, history of acute MI, and cardiac troponins.
More recently, Lorgis et al31 explored the correlation between admission NT‐proBNP levels and 1‐year mortality among 3291 older patients admitted for acute MI and enrolled in the RICO survey (a French regional survey for MI). In their study, the mean age was 68 ± 14 years and the median NT‐proBNP level was 1053 pg/mL. Patients were divided into 4 groups according to increasing age: Group 1 (interquartile range: 43–51 years); Group 2 (interquartile range: 57–64 years); Group 3 (interquartile range: 70–75 years); and Group 4 (interquartile range: 79–85 years). NT‐proBNP median values increased 10‐fold from the youngest group (367 pg/mL) to the oldest age group (3774 pg/mL). Similarly, mortality was significantly higher in the oldest age group (24.2%) when compared the youngest group (3%). After multivariate analysis adjusting for traditional risk factors including troponin, creatinine clearance, and LVEF, NT‐proBNP levels remained a strong and independent predictor of mortality at 1 year across all age groups, including those age 79 to 85 years. Moreover, NT‐proBNP provided more robust prognostic value when used in combination with the Global Registry of Acute Coronary Events (GRACE) risk score (Figure 2).
Figure 2.
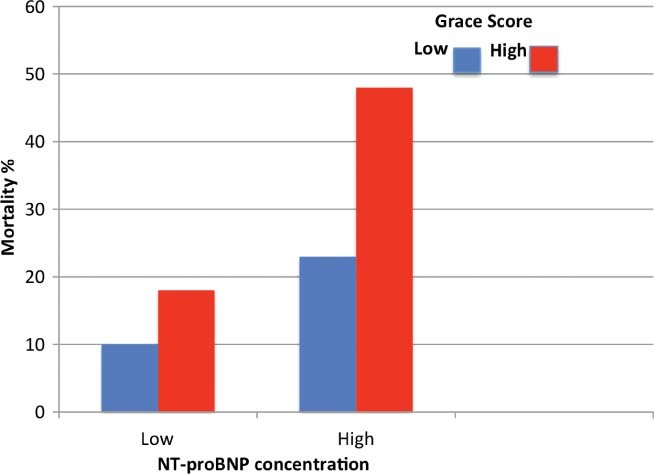
Mortality risk at 1 year based on N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) concentration and Global Registry of Acute Coronary Event (GRACE) risk score in the oldest patients. Reproduced from Lorgis et al31 (BMJ. 2009;6;338:b1605) with permission from BMJ Publishing Group Ltd. via Copyright Clearance Center.
Conclusion and Future Directions
The continuing search for a better approach to risk‐stratify and manage elderly patients with ACS invokes the increasingly recognized value of B‐type NPs in predicting death and other adverse events. Since older patients stand to derive greater absolute treatment benefits than younger patients given their higher risk of morbidity and mortality, the applicability of BNP and NT‐proBNP in this group, either alone or as part of a multimarker schema, seems intuitive. Recent data show a promising role of these biomarkers in prognostication of older patients in the setting of ACS. Despite higher concentrations of BNP and NT‐proBNP with increasing age, these peptides retain their prognostic value and strongly predict mortality in older patients with ACS. Moreover, these biomarkers seem to provide additional prognostic role above and beyond that provided by major predictors such as the GRACE risk score and other traditional biomarkers. However, because the causes of elevated BNP and NT‐proBNP may be more heterogeneous among elderly individuals, additional study is needed to clarify the role of B‐type NPs in guiding therapy (ie, noninvasive or invasive) of older ACS patients.
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehta RH, Rathore SS, Radford MJ, et al. Acute myocardial infarction in the elderly: differences by age. J Am Coll Cardiol. 2001;38:736–741. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Goldberg RJ, Dabbous O, et al; for the Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163:2345–2353. [DOI] [PubMed] [Google Scholar]
- 4. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. [DOI] [PubMed] [Google Scholar]
- 5. Shanmugasundaram M, Alpert JS. Acute coronary syndrome in the elderly. Clin Cardiol. 2009;32:608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander KP, Newby LK, Cannon CP, et al; for the American Heart Association Council on Clinical Cardiology; Society of Geriatric Cardiology. Acute coronary care in the elderly, part I: Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. [DOI] [PubMed] [Google Scholar]
- 7. Thomas MP, Moscucci M, Smith DE, et al.; for the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Outcome of contemporary percutaneous coronary intervention in the elderly and the very elderly: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Clin Cardiol. 2011;34: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Appleby CE, Ivanov J, Mackie K, et al. In‐hospital outcomes of very elderly patients (85 years and older) undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv. 2011;77: 634–641. [DOI] [PubMed] [Google Scholar]
- 9. Sarzani R, Dessi‐Fulgheri P, Paci VM, et al. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996;19:581–585. [DOI] [PubMed] [Google Scholar]
- 10. Wallen T, Landahl S, Hedner T, et al. Brain natriuretic peptide in an elderly population. J Intern Med. 1997;242:307–311. [DOI] [PubMed] [Google Scholar]
- 11. Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. [DOI] [PubMed] [Google Scholar]
- 12. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 13. Vickery S, Price CP, John RI, et al. B‐type natriuretic peptide (BNP) and aminoterminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46:610–620. [DOI] [PubMed] [Google Scholar]
- 14. Austin WJ, Bhalla V, Hernandez‐Arce I, et al. Correlation and prognostic utility of Btype natriuretic peptide and its amino‐terminal fragment in patients with chronic kidney disease. Am J Clin Pathol. 2006;126:506–512. [DOI] [PubMed] [Google Scholar]
- 15. Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 16. Horwich TB, Hamilton MA, Fonarow GC. B‐type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. [DOI] [PubMed] [Google Scholar]
- 17. James SK, Lindahl B, Siegbahn A, et al. N‐terminal pro‐brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)‐IV substudy. Circulation. 2003;108:275–281. [DOI] [PubMed] [Google Scholar]
- 18. Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112: 2163–2168. [DOI] [PubMed] [Google Scholar]
- 19. Chang AY, Abdullah SM, Jain T, et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol. 2007. 2;49: 109–116. [DOI] [PubMed] [Google Scholar]
- 20. Steensma DP, Tefferi A. Anemia in the elderly: how should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007;82:958–966. [DOI] [PubMed] [Google Scholar]
- 21. Fukuta H, Ohte N, Mukai S, et al. Anemia is an independent predictor for elevated plasma levels of natriuretic peptides in patients undergoing cardiac catheterization for coronary artery disease. Circ J. 2008;72:212–217. [DOI] [PubMed] [Google Scholar]
- 22. Nybo M, Benn M, Mogelvang R, et al. Impact of hemoglobin on plasma pro‐B‐type natriuretic peptide concentrations in the general population. Clin Chem. 2007;53:1921–1927. [DOI] [PubMed] [Google Scholar]
- 23. Desai AS, Bibbins‐Domingo K, Shlipak MG, et al. Association between anemia and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP): findings from the Heart and Soul Study. Eur J Heart Fail. 2007;9:886–891. [DOI] [PubMed] [Google Scholar]
- 24. Wallen T, Landahl S, Hedner T, et al. Brain natriuretic peptide predicts mortality in the elderly. Heart. 1997;77:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N‐terminal pro‐B‐type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kistorp C, et al. N‐terminal pro‐brain natriuretic peptide, C‐reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 27. de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B‐type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. [DOI] [PubMed] [Google Scholar]
- 28. Mega JL, Morrow DA, de Lemos JA, et al. B‐type natriuretic peptide at presentation and prognosis in patients with ST‐segment elevation myocardial infarction: an ENTIRETIMI‐23 substudy. J Am Coll Cardiol. 2004;44:335–339. [DOI] [PubMed] [Google Scholar]
- 29. Omland T, Persson A, Ng L, et al. N‐terminal pro‐B‐type natriuretic peptide and longterm mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. [DOI] [PubMed] [Google Scholar]
- 30. Drewniak W, Snopek G, Zarukiewicz M, et al. Prognostic value of the N‐terminal pro‐B‐type natriuretic peptide in the elderly with acute myocardial infarction. Kardiol Pol. 2008;66:750–755. [PubMed] [Google Scholar]
- 31. Lorgis L, Zeller M, Dentan G, et al; RICO Survey Working Group. Prognostic value of N‐terminal probrain natriuretic peptide in elderly people with acute myocardial infarction: prospective observational study. BMJ. 2009;338:b1605. [DOI] [PMC free article] [PubMed] [Google Scholar]


