Abstract
Background:
In patients with acute type A aortic dissection (AAD), localization of the primary entry tear to be excluded is of major importance for intervention.
Hypothesis:
There are reliable indirect computed tomography (CT) findings to predict the entry site.
Methods:
In 83 patients with type A AAD whose primary entry tears were identified surgically between 2003 and 2009, we retrospectively examined the diagnostic CT scans regarding pericardial effusion, the largest short‐axial diameter of the aorta, widths of true and false lumens, and false lumen thrombosis at 6 levels of thoracic aorta from the aortic root to the descending aorta.
Results:
The primary entry sites identified intraoperatively were proximal ascending in 21 patients, middle ascending in 21, distal ascending in 21, arch in 17, and descending or unknown in 16. The multivariate logistic analysis revealed that pericardial effusion (odds ratio [OR]: 2.2, 95% confidence interval [CI]: 1.2–3.4, P < 0.001) and dilated ascending aorta (OR: 1.6, 95% CI: 1.1–2.4, P = 0.012) were the significant CT findings to predict the entry tear in the ascending aorta. It also revealed that the significant CT finding to predict the entry tear distal to the aortic arch was nonthrombosed false lumen in the descending aorta (OR: 1.2, 95% CI: 1.1–2.1, P = 0.048).
Conclusions:
We can predict the primary entry site by the preoperative CT findings in patients with type A AAD, considering pericardial effusion, aortic diameter, widths of true and false lumens, and false lumen thrombosis at different anatomic levels. Clin. Cardiol. 2012 DOI: 10.1002/clc.21991
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Acute aortic dissection (AAD) is a life‐threatening disease that requires immediate diagnosis and treatment. Acute type A AAD, involving the ascending aorta regardless of the site of intimal entry tear, should be repaired immediately to avoid fatal extension to the pericardium, the pleural space, the coronary arteries, and aortic root.1 Surgical intervention aims to exclude the entry tear, defined as a disruption of the flap continuity. Therefore, determining the localization of the entry tear is important in planning open aortic replacement or percutaneous endografting.
Computed tomography (CT), because of its speed and wide availability, is currently the most common diagnostic‐imaging method for aortic dissection, with reported accuracy ranging from 88% to 100%.2., 3., 4. However, visualization of the primary entry tear from true to false lumens was reported to be difficult, even using multidetector‐slice CT scanners, which have facilitated faster scanning times, improved thin‐section reconstructions, and decreased pulsation artifacts in aortic imaging.5
In this study, we investigated the reliable indirect CT image findings to predict the location of the primary entry tear, which should be verified and surgically excluded in patients with type A AAD.
Methods
Patients
Between January 2003 and December 2009, 83 consecutive patients (46 men and 37 women; mean age, 63 ± 13 y) with type A AAD underwent surgery on an emergency basis at Nagoya Daini Red Cross Hospital, a community‐based teaching hospital. The diagnosis was confirmed with enhanced CT scans, indicating the involvement into the ascending aorta. Table 1 summarizes the demographic and clinical characteristics of the patients. The institutional review board approved this retrospective observational study, and the approval included a waiver of informed consent.
Table 1.
Patients' Demographics and Clinical Characteristics
| Demographics | N (%) or Mean ± SD (range) |
|---|---|
| Mean age, y | 63 ± 13 (32–87) |
| Male sex | 46 (55%) |
| Hypertension | 60 (72%) |
| CVD | 7 (8%) |
| CAD | 6 (7%) |
| Hyperlipidemia | 13 (16%) |
| DM | 5 (6 %) |
| Smoking | 37 (44%) |
| Marfan syndrome | 2 (2%) |
| Time from onset to diagnosis (h) | 5.5 ± 10 (0·8–62) |
| Migrating pain | 15 (18%) |
| Non–chest pain/non–back pain | 19 (23%) |
| Shock | 5 (6%) |
| Neurological deficit | 24 (29%) |
| Malperfusion of extremities | 19 (23%) |
Abbreviations: CAD, coronary artery disease; CVD, cerebrovascular disease; DM, diabetes mellitus; SD, standard deviation.
Computed Tomography Scans and Analysis
We retrospectively reviewed preoperative CT images in the study patients. All CT scans were performed on an 8‐ or 16‐slice CT scanner with the anatomic coverage from the thoracic inlet through the pubic symphysis at 5‐mm intervals. Imaging was performed during the arterial‐enhancement phase using an automated bolus tracking technique after intravenous injection of 100 mL nonionic contrast material.
For each patient, we first recorded the presence of pericardial effusion and enhancement of the kidneys. Next, the largest short‐axial diameter of the outer contour of the aorta perpendicular to the curvature, widths of true and false lumens, and presence of thrombosis in the false lumen were recorded at 6 levels of thoracic aorta: aortic root (level A), ascending aorta at bifurcation of the pulmonary artery (level B), mid portion of the arch (level C), descending aorta at bifurcation of the pulmonary artery (level D), descending aorta at the level of the aortic root (level E), and descending aorta at the lower cardiac border (level F), as illustrated in the Figure 1. From the widths of true and false lumens, we determined the predominance of the true or false lumen at each level of the aorta.
Figure 1.
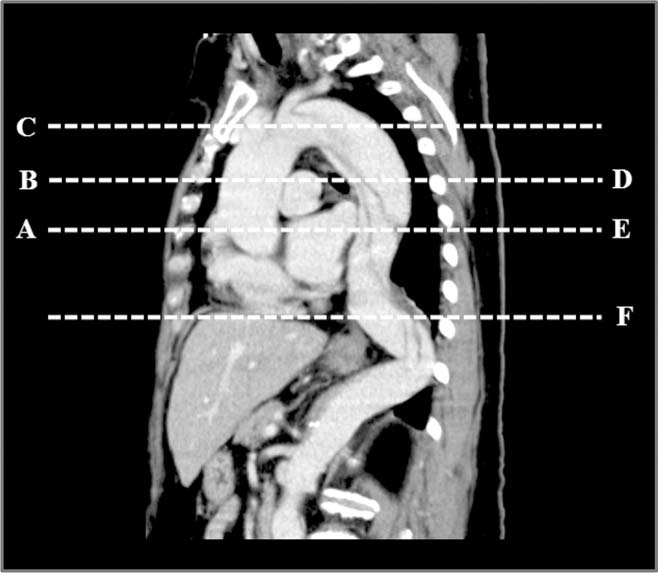
The diagnostic CT scans were examined at 6 levels of thoracic aorta in patients with type A AAD: aortic root (level A), ascending aorta at bifurcation of the pulmonary artery (level B), mid portion of the arch (level C), descending aorta at bifurcation of the pulmonary artery (level D), descending aorta at the level of the aortic root (level E), and descending aorta at the lower cardiac border (level F). Abbreviations: AAD, acute aortic dissection; CT, computed tomography.
Intraoperative Identification of the Intimal Tear
Our surgical strategy for type A AAD has been to eliminate the primary entry tear. When the primary entry tear was present in the arch, the arch was totally or partially replaced. If the arch appeared normal at the inspection, it was considered unnecessary to replace the aortic arch, and only the ascending aorta was replaced.
Patients routinely underwent median sternotomy. Cardiopulmonary bypass was established by cannulation of both the right axillary and femoral arteries for the arterial return, and by cannulation of the right atrium for venous drainage. After systemic cooling to 25°C at the bladder, the aorta was opened to identify the location of the primary entry tear during the deep hypothermic circulatory arrest and selective antegrade cerebral perfusion: proximal ascending aorta, mid ascending aorta, distal ascending aorta, arch, and descending aorta or unknown.
Our selective cerebral perfusion was perfusion through the right axillary artery with clamping of the innominate artery and perfusion through the cannulae inserted into the left common carotid artery and left subclavian artery in the Trendelenburg position. Blood was perfused into cerebral arteries at a rate of 10 mL/kg/minute, and cerebral perfusion pressure was adjusted to maintain a radial artery pressure of 40–70 mm Hg. When the eventual arch replacement or distal aorto‐prosthesis anastomosis was completed, cerebral perfusion was stopped and antegrade systemic perfusion was reinstituted though the prosthesis. The proximal anastomosis was performed during rewarming.
Statistical Analysis
Continuous variables are presented as mean ± SD and categorical variables are presented as frequencies and percentages. The CT findings were compared among the patients, according to the entry sites identified intraoperatively. The Fisher exact test was used for comparing categorical variables, and the Mann‐Whitney U test for comparison of continuous variables. Variables with statistical significant set at P < 0.1 were included in a multivariate logistic regression model to identify independent CT findings for prediction of the primary entry tear. All statistical analyses were performed with StatView 5.0 (Abacus Concepts Inc., Berkeley, CA) and a P value <0.05 was considered statistically significant.
Results
Fifty‐five patients (66%) underwent only ascending aorta replacement; 7 (8%) had a hemiarch and 18 (22%) had ascending aorta + total arch replacement. Aortic root was replaced with the modified Bentall procedure in 3 patients (4%). Thirty‐one patients (37%) underwent concomitant procedures, including commissural resuspension of the aortic valve, coronary artery bypass grafting, and replacement of abdominal aorta. Cardiopulmonary bypass and cardiac ischemia times were 291 ± 85 minutes and 131 ± 48 minutes, respectively.
The sites of primary entry tears identified intraoperatively were the proximal ascending aorta in 21 patients, middle ascending aorta in 21, distal ascending aorta in 21, arch in 17, and descending or unknown in 16. In 8 patients, ≥2 sites of intimal tears were identified.
The hospital mortality rate was 15.7% (13 patients). The causes of hospital death were multiorgan failure (n = 3), stroke (n = 3), low cardiac output (n = 3), sepsis (n = 2), pulmonary cause (n = 1), and rupture of descending aorta (n = 1). The mortality rates were significantly higher in patients with primary entry tears at the arch and descending aorta than in those with entry tears at the ascending aorta (23.7% vs 6.3 %, P < 0.05). There was no hospital death in the patients with >2 entry sites. The morbidity rate was 65%, including respiratory failure, cerebral events, bleeding, infection, renal failure, and gastrointestinal complication.
There were no correlations between the localization of the primary entry tears and the patients' characteristics, including age, sex, hypertension, cerebrovascular disease, coronary artery disease, hyperlipidemia, diabetes mellitus, and smoking. There were also no correlations between the localization of the primary entry tears and the patients' symptoms, including pains and neurological deficits, except for pain migration. Pain migration was documented in 35% of the patients with the entry tear at the arch (level C) vs in 16% in other patients (P = 0.04). In contrast, only 5% of the patients with the entry tear at level B complained of pain migration.
Table 2 shows that pericardial effusion was observed on CT scans in the patients with entry tears at the proximal and middle ascending aorta, whereas it was not detected in those with entry tears distal to the distal ascending aorta. The frequency of poor renal enhancement on CT scans showed no differences by the entry site. With regard to the diameter of the aorta, as shown in Table 2 the diameters at level B were significantly larger in the patients with entry tears at the middle and distal ascending aorta than in those with the other entry sites. At levels other than B, the diameters did not differ by the entry site. Table 3 demonstrates that the false lumen was larger than the true lumen in most patients at levels A, B, and C. At levels D, E, and F, the true lumen was more predominant in the patients with entry tears at the middle ascending aorta than in those with the other entry sites. More patients with entry tears distal to the descending aorta had predominant true lumens at level A than those with other entry sites. Table 4 demonstrates that the false lumen was thrombosed in all patients with entry tears at the aortic arch. The false lumen at levels A and B was more frequently thrombosed in the patients with entry tears distal to the descending aorta than in those with other entry sites. In the multivariate logistic analysis performed with 5 significant variables—pericardial effusion, dilated ascending aorta (>48 mm), predominant true lumen in the ascending aorta, predominant true lumen in the descending aorta, and false lumen thrombosis in the descending aorta—the significant CT findings to predict the entry tear in the ascending aorta were pericardial effusion (odds ratio [OR]: 2.2, 95% confidence interval [CI]: 1.2–3.4, P < 0.001) and dilated ascending aorta (OR: 1.6, 95% CI: 1.1–2.4, P = 0.012). Furthermore, the significant CT finding to predict the entry tear distal to the aortic arch was nonthrombosed false lumen in the descending aorta (OR: 1.2, 95% CI: 1.1–2.1, P = 0.048).
Table 2.
CT Findings: Pericardial Effusion and the Largest Short‐Axial Diameter (mm) of the Outer Contour of the Aorta
| Entry Site Identified at Surgery | |||||
|---|---|---|---|---|---|
| Proximal Ascending (n = 21) | Middle Ascending (n = 21) | Distal Ascending (n = 21) | Arch (n = 17) | Descending/ Unknown (n = 16) | |
| Pericardial effusion | 5 (24%) | 13 (62%) | 0a | 0a | 0a |
| Level of aorta | |||||
| A | 41 ± 4.6 | 43 ± 5.9 | 47 ± 8.4 | 44 ± 6.5 | 41 ± 3.7 |
| B | 43 ± 6.1 | 48 ± 6.6a | 49 ± 10.2a | 44 ± 6.0 | 44 ± 6.1 |
| C | 36 ± 3.5 | 38 ± 6.2 | 41 ± 6.3 | 40 ± 8.6 | 37 ± 6.3 |
| D | 30 ± 4.2 | 29 ± 4.1 | 31 ± 4.1 | 31 ± 4.6 | 32 ± 4.7 |
| E | 29 ± 4.2 | 28 ± 3.4 | 31 ± 4.4 | 30 ± 3.6 | 29 ± 2.9 |
| F | 28 ± 4.4 | 26 ± 3.3 | 30 ± 3.7 | 29 ± 3.6 | 28 ± 4.7 |
Abbreviation: CT, computed tomography.
P < 0.05.
Table 3.
CT Findings: Predominance of False Lumen Where the False Lumen Was Wider Than the True Lumen
| Level of Aorta | Entry Site Identified at Surgery | ||||
|---|---|---|---|---|---|
| Proximal Ascending (n = 21) | Middle Ascending (n = 21) | Distal Ascending (n = 21) | Arch (n = 17) | Descending/ Unknown (n = 16) | |
| A | 18 (86%) | 13 (62%) | 19 (90%) | 17 (100%) | 6 (38%) |
| B | 18 (86%) | 13 (62%) | 19 (90%) | 17 (100%) | 10 (63%) |
| C | 18 (86%) | 10 (48%) | 19 (90%) | 17 (100%) | 12 (75%) |
| D | 16 (76%) | 8 (38%)a | 14 (67%) | 15 (88%) | 12 (75%) |
| E | 16 (76%) | 8 (38%)a | 14 (67%) | 13 (76%) | 10 (63%) |
| F | 14 (67%) | 8 (38%)a | 14 (67%) | 13 (76%) | 10 (63%) |
Abbreviation: CT, computed tomography.
P < 0.05.
Table 4.
CT Findings: Thrombosis in the False Lumen
| Level of Aorta | Entry Site Identified at Surgery | ||||
|---|---|---|---|---|---|
| Proximal Ascending (n = 21) | Middle Ascending (n = 21) | Distal Ascending (n = 21) | Arch (n = 17) | Descending/ Unknown (n = 16) | |
| A | 3 (14%) | 13 (62%) | 8 (38%) | 0 | 10 (63%) |
| B | 2 (10%) | 10 (48%) | 7 (33%) | 0 | 10 (63%) |
| C | 2 (10%) | 7 (33%) | 8 (38%) | 0 | 4 (25%) |
| D | 2 (10%) | 7 (33%) | 7 (33%) | 0 | 0 |
| E | 2 (10%) | 7 (33%) | 7 (33%) | 0 | 0 |
| F | 2 (10%) | 7 (33%) | 5 (24%) | 0 | 0 |
Abbreviation: CT, computed tomography.
Discussion
The principal result of this study was that we can predict the entry site by the diagnostic indirect CT findings in patients with type A AAD. Our results suggest that pericardial effusion and dilated ascending aorta may predict the primary entry tear in the ascending aorta and that nonthrombosed false lumen in the descending aorta may predict the primary entry tear distal to the aortic arch. Although not statistically significant, predominant true lumen in the descending aorta seemed to predict the primary entry tear in the ascending aorta. Also, predominant false lumen in the ascending aorta seemed to predict the entry tear in the arch and predominant true lumen in the ascending aorta seemed to predict the entry tear in the descending aorta.
The primary aim of surgical treatment for type A AAD is to eliminate the primary entry tear so as to restore true lumen flow and induce false lumen thrombosis. When the entry tear is present in the arch, the arch was totally or partially replaced. Otherwise, it is unnecessary to replace the aortic arch and only the ascending aorta was replaced. Therefore, preoperative prediction of the intimal tear by CT, based upon our results, may be helpful for surgical‐intervention planning.
Although CT is regarded as the most reliable approach for immediate and accurate diagnosis of aortic dissection, transesophageal echocardiography (TEE) is also a useful method, especially for the localization of the tear.6 However, TTE often fails in the descending aorta. In addition, TEE is rarely used as the first imaging test in daily practice, possibly due to its lower availability compared with CT, lack of experienced physicians, and the fear of a rise in arterial blood pressure in critically ill patients.7
To our knowledge, there have been few studies evaluating the ability of CT scans to demonstrate intimal tears. Visualization of intimal tears has been reported in some studies using ultrafast electron‐beam CT and spiral CT.8., 9. Images obtained using multidetector techniques are far superior, due to faster acquisition time and thinner sections, to those acquired using single‐detector techniques. We occasionally found that tears were quite small, necessitating close scrutiny for detection by CT, such as a series of intercostal or lumbar origins sheared off by the dissection. Because of these limitations of CT scans for detection of intimal tears, we tried, in the present study, to identify the indirect CT findings to predict the location of the intimal tears.
Of course, we need further investigation to verify the specificity and sensitivity of the CT findings to predict the location of the entry sites, considering the following limitations of our study. First, we did not collect the CT findings of the abdominal aorta and iliac arteries. It is usual that a large number of tears are present in the region of the branch arteries, particularly the renal arteries.10 Second, the predominance of the true or false lumen and false lumen thrombosis, which were focused as predictive CT findings in the present study, may be variable according to the number and size of intimal tears. Phantom studies have shown that communications between the true and false lumens downstream to a proximal intimal tear may help prevent true lumen collapse, especially if the tear is small.11 Phantom studies also have demonstrated that an opening in the flap large enough to facilitate pressure equilibration between the true and false lumens allowed the true lumen to collapse.12 The third limitation is the relatively small sample sizes.
Conclusion
We can predict the primary entry tear by the preoperative CT findings in patients with type A AAD, considering pericardial effusion, aortic diameter, widths of true and false lumens, and false lumen thrombosis at different anatomic levels of the thoracic aorta.
References
- 1. Lansman SL, Saunders PC, Malekan R, et al. Acute aortic syndrome. J Thorac Cardiovasc Surg. 2010;140(6 suppl):S92–S97. [DOI] [PubMed] [Google Scholar]
- 2. Small JH, Dixon AK, Coulden RA, et al. Fast CT for aortic dissection. Br J Radiol. 1996;69:900–905. [DOI] [PubMed] [Google Scholar]
- 3. LePage MA, Quint LE, Sonnad SS, et al. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol 2001;177:207–211. [DOI] [PubMed] [Google Scholar]
- 4. Castañer E, Andreu M, Gallardo X, et al. CT in nontraumatic acute thoracic aortic disease: typical and atypical features and complications. Radiographics. 2003;23:S93–S110. [DOI] [PubMed] [Google Scholar]
- 5. Kapoor V, Ferris JV, Fuhrman CR. Intimomedial rupture: a new CT finding to distinguish true from false lumen in aortic dissection. AJR Am J Roentgenol. 2004;183:109–112. [DOI] [PubMed] [Google Scholar]
- 6. Evangelista A, Avegliano G, Aguilar R, et al. Impact of contrast‐enhanced echocardiography on the diagnostic algorithm of acute aortic dissection. Eur Heart J. 2010;31:472–479. [DOI] [PubMed] [Google Scholar]
- 7. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 8. Hamada S, Takamiya M, Kimura K, et al. Type A aortic dissection: evaluation with ultrafast CT. Radiology. 1992;183:155–158. [DOI] [PubMed] [Google Scholar]
- 9. Quint LE, Platt JF, Sonnad SS, et al. Aortic intimal tears: detection with spiral computed tomography. J Endovasc Ther. 2003;10:505–510. [DOI] [PubMed] [Google Scholar]
- 10. McMahon MA, Squirrell CA. Multidetector CT of aortic dissection: a pictorial review. Radiographics. 2010;30:445–460. [DOI] [PubMed] [Google Scholar]
- 11. Chung JW, Elkins C, Sakai T, et al. True‐lumen collapse in aortic dissection, part I: evaluation of causative factors in phantoms with pulsatile flow. Radiology. 2000;214:87–98. [DOI] [PubMed] [Google Scholar]
- 12. Williams DM, LePage MA, Lee DY. The dissected aorta, part I: early anatomic changes in an in vitro model. Radiology. 1997;203:23–31. [DOI] [PubMed] [Google Scholar]


