Abstract
Background:
Adequately recording diastolic heart sounds and systolic time intervals over longer periods is difficult. Thus, information on the circadian variation of these parameters in an ambulatory population is lacking. Moreover, age‐related changes in the prevalence of diastolic heart sounds and measurements of systolic time intervals in an asymptomatic population have not been studied in continuous recordings.
Hypothesis:
Diastolic heart sounds and systolic time intervals will have age and circadian variations that reflect known changes in cardiac function due to aging and circadian rhythms.
Methods:
We studied 128 asymptomatic subjects wearing an ambulatory monitor with acoustic cardiography. The recording spanned a mean duration of 14 hours, including sleep. Data were analyzed for the presence of third (S3) and fourth (S4) heart sounds and for systolic time intervals.
Results:
In these asymptomatic subjects, S3 was significantly more prevalent in those age <40 years than in those age >40 years, and significantly more pronounced during sleep in the younger group. Also, S4 was significantly more prevalent in those age >40 years and significantly more pronounced during sleep in those age >40 years. In contrast, time intervals reflecting systolic function showed less circadian variation and less worsening with age.
Conclusions:
The nocturnal increase of S4 in the elderly reflects diastolic impairment—likely a result of changes in diastolic filling patterns with increasing age. An S3 after the age of 40 is a relatively uncommon finding and therefore should be a specific sign of cardiac disease. Continuous monitoring of diastolic heart sounds and systolic time intervals is possible using acoustic cardiography. © 2011 Wiley Periodicals, Inc.
Patricia Arand, PhD, is an employee of Inovise Medical. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
There have been few studies involving continuous assessment of diastolic heart sounds and systolic time intervals over long periods of time. One previous study on 1329 asymptomatic adults over a wide age range reported the prevalence of third (S3) and fourth (S4) heart sounds using a 10‐second recording technique.1 However, this study did not address postural and circadian influences. With advances in technology, acoustic cardiography has emerged as a portable and reliable form of phonocardiography2 that can be applied to ambulatory subjects for up to 48 hours of continuous monitoring (Figure 1). Focused on systolic time intervals and diastolic heart sounds, acoustic cardiography allows reliable assessment of hemodynamics via dual‐function electrocardiographic (ECG)/sound sensors placed on the chest in addition to 2 ECG electrodes.3, 4, 5, 6 Parameters produced by this technique include those to evaluate diastolic function (the presence of S4, which has been shown to be associated with increased left ventricular [LV] stiffness)7 and to assess systolic function8 (the presence of S3, EMAT [electrical mechanical activation time; interval from onset of Q wave to first heart sound S1], and LVST [left ventricular systolic time; interval from S1 to second heart sound S2]).
Figure 1.
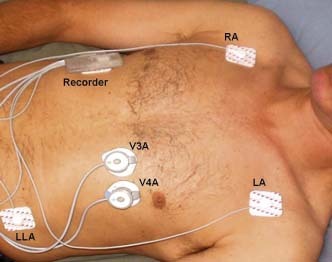
Ambulatory acoustic cardiography monitor. This figure shows the placement of the acoustic cardiography recorder unit (5 × 7.6 × 1.9 cm, weight 43 g) and the ECG/sound sensors. Regular ECG monitoring electrodes are placed on the right arm, left arm, and left leg locations for ECG monitoring. Combined ECG/sound sensors are placed in the precordial V3 and V4 positions. The recorder unit is placed on either the patient's chest or abdomen. Abbreviations: ECG, electrocardiography; LA, left arm; LLA, modified left leg position; RA, right arm.
The purpose of this study is to understand the circadian and age‐related changes in diastolic heart sounds and systolic time intervals in a group of ambulatory, asymptomatic (exclusion of symptomatic coronary artery disease and heart failure) subjects over a wide age range. This study is important to define the incidence of diastolic heart sounds and normal ranges for systolic time intervals as an age, postural, and diurnal/nocturnal reference for use in comparison with populations with cardiac disease, such as heart failure.
Methods
Acoustic cardiographic Holter data were collected from a convenience sample of asymptomatic subjects age >18 years. The study received institutional review board approval from Liberty Institutional Review Board, DeLand, FL.
A total of 132 subjects were recruited from a fitness center and local business facilities near Portland, OR. Four subjects were excluded, due to presence of sick sinus syndrome (1 subject) and insufficient length or quality of recording (3 subjects). Potential subjects were screened by study staff with a brief cardiac history to exclude persons with symptomatic coronary artery disease or heart failure. Informed consent was obtained from all subjects. There were no subjects undergoing oncological chemotherapy. History of previous or current smoking status was not recorded. Baseline demographics, medications, and medical history, especially history of hypertension and cardiovascular drug therapy, were recorded on all subjects.
Acoustic Cardiographic Holter Recordings
Standard ECG electrodes were placed in modified limb locations, and dual‐purpose Audicor sensors (Inovise Medical, Inc., Beaverton, OR) were placed in either the standard V4 position or both the V3 and V4 positions while the subject was in a supine position. Adequate data quality was confirmed by visual assessment by study personnel. Cardiac acoustic and ECG data were recorded simultaneously for a mean duration of 14 hours including sleep time (awake, 7.5 ± 3.2 h; sleep, 7.7 ± 1.3 h).
The data were analyzed for the presence of diastolic heart sounds (S3 and S4) and for systolic time intervals including EMAT, LVST, and left ventricular diastolic perfusion time (LDPT; interval from the S2 to the next Q wave onset). The strength of S3 and S4 is a combination of intensity and persistence, and is expressed on a scale from 0 to 10; if the strength is ≥5.0, S3 or S4 is considered to be present.
Statistical Analysis
Results are given as mean ± SD for continuous variables with Gaussian distribution. Categoric data are presented as exact numbers and proportions. P values <0.05 were considered statistically significant.
Results
Basic demographic and clinical characteristics are summarized in the Table 1. Note the increasing prevalence of hypertension with age. More than half (63%) of the population were not taking any medications. The remainder of the subjects were taking noncardiac medications (18%), antihypertensives (15.4%), statins (12.2%), insulin (2.4%), and β‐blockers (1.6%).
Table 1.
Demographics and Clinical Characteristics
| No. | % Male | Height, cm | Weight, kg | BMI, kg/m2 | Medical History | |
|---|---|---|---|---|---|---|
| All | 128 | 58% | 174 ± 11 (157–198) | 80.6 ± 18.7 (47.6–149.7) | 26.6 ± 5.2 (19.0–50.3) | 79% None |
| 17% HT | ||||||
| 2.3% Prior MI | ||||||
| 1.6% DM | ||||||
| Age < 30 y | 18 | 67% | 173 ± 11 (158–198) | 76.5 ± 15.9 (58.1–113.4) | 25.5 ± 3.0 (21.6–33.0) | None |
| Age 30–39 y | 24 | 71% | 179 ± 10 (157–198) | 83.3 ± 18.3 (55.3–145.1) | 26.0 ± 4.0 (20.9–37.5) | 4% Other than cardiac and DM |
| Age 40–49 y | 23 | 52% | 176 ± 10 (160–193) | 80.5 ± 19.5 (58.1–149.7) | 25.9 ± 5.2 (20.4–40.2) | 4% HT |
| 9% DM | ||||||
| Age 50–59 y | 26 | 58% | 172 ± 11 (157–193) | 79.8 ± 19.9 (50.3–124.7) | 26.8 ± 6.2 (19.0–50.3) | 31% HT |
| 4% Prior MI | ||||||
| Age 60–69 y | 23 | 48% | 171 ± 11 (160–188) | 80.8 ± 14.2 (54.4–133.8) | 27.8 ± 4.4 (19.7–47.6) | 30% HT |
| Age ≥70 y | 14 | 50% | 172 ± 9 (157–188) | 83.6 ± 25.3 (47.6–122.5) | 28.1 ± 7.6 (19.2–38.7) | 43% HT |
| 14% Prior MI |
Data are presented as mean ± SD, range (min–max). Abbreviations: BMI, body mass index; DM, diabetes mellitus; HT, hypertension; MI, myocardial infarction.
Figure 2 shows that heart rate decreased significantly during sleep in all age groups. There was also a decrease in awake heart rate as age increased but no significant age‐related difference in nocturnal heart rate, with only 2 subjects (1.6%) having taken β‐blocking agents.
Figure 2.
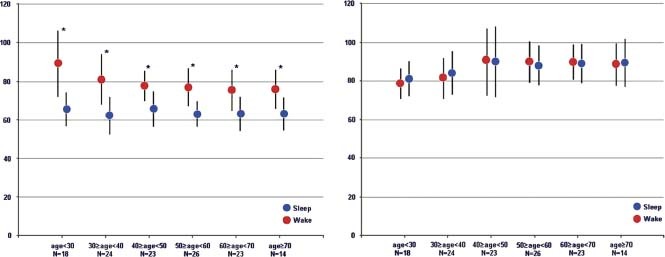
Heart rate (bpm) and electromechanical delay (ms). Left panel: Heart rate in each decade for awake and sleep. *P value <0.05 for awake vs sleep within 1 decade. Right panel: Electromechanical delay (EMAT = QS1, time interval from onset of Q wave to mitral valve component of S1). Abbreviations: EMAT, electrical mechanical activation time; S1, first heart sound.
We analyzed the S4 as a parameter of diastolic function and systolic time intervals (EMAT, LVST, and %LVST [LVST/R‐R interval]) as well as S3 as parameters of systolic function. Parameters that reflect systolic function showed less circadian variation—with the exception of the physiologic S3 age <40 years—and lower incidence in elderly age groups than the S4, which reflects diastolic function.
Parameters Reflecting Systolic Function
Electromechanical activation time had a slightly increasing trend from age <30 years (80 ± 8 ms) to the 30‐to‐39‐year decade (82 ± 11 ms), and then rose in all age decades >40 years to a rather constant value of 89 ± 12 ms (Figure 2). Electromechanical activation time had little circadian variation, with a nonstatistical change from awake to sleep across all age groups. There was a gradual trend for shortening of diurnal %LVST with increasing age until 40 years of age. After age 40, both awake and sleep %LVST were constant regardless of increasing age (Figure 3). Only in the groups aged <40 years was there was a significant shortening in %LVST from awake to sleep (40.3 ± 4.1% awake vs 36.3 ± 4.0% sleep). The ratio of EMAT to LVST (EMAT/LVST) was also constant across all age groups and showed no significant circadian variation (mean daytime range 0.27–0.28 vs mean nocturnal range 0.24–0.26).
Figure 3.
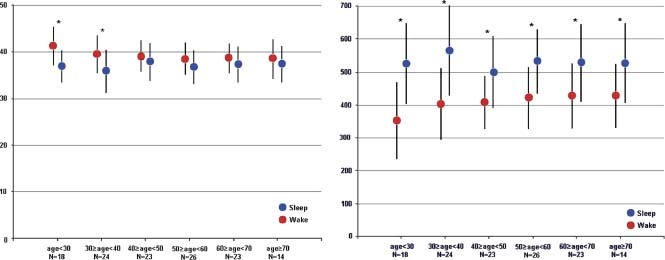
Percent LV systolic time, % of R‐R interval, and LV diastolic perfusion time (ms). Left panel: %LVST (LVST / R‐R interval) in each age decade for awake and sleep. * P value <0.05 for awake vs sleep within 1 decade. Right panel: LDPT (time from S2 to the next onset of Q wave) for awake and sleep. * P value <0.05 for awake vs sleep within 1 decade. Abbreviations: LDPT, left ventricular diastolic perfusion time; LV, left ventricular; LVST, left ventricular systolic time; %LVST, percent left ventricular systolic time; S2, second heart sound.
The S3 was significantly more prevalent in persons age <40 years compared with persons age >40 years (13% vs 3.6%) and significantly more pronounced during sleep in the younger group (19.6% sleep vs 6.8% awake) as compared with the older group (4.8% sleep vs 2.4% awake) (Figure 4).
Figure 4.
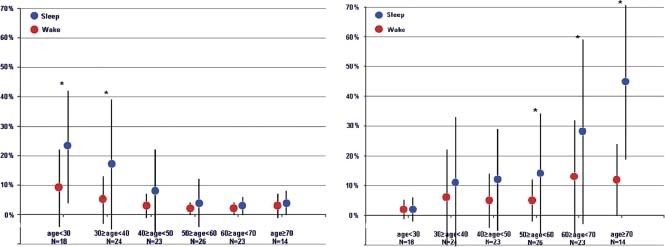
Presence of S3 (%) and S4 (%). Left panel: Percentage of S3 detection (S3 strength ≥5.0) in each age decade for awake and sleep. * P value <0.05 for awake vs sleep within 1 decade. Right panel: Percentage of S4 detection (S4 strength ≥5.0) in each age decade for awake and sleep. * P value <0.05 for awake vs sleep within 1 decade. Abbreviations: S3, third heart sound; S4, fourth heart sound.
Parameters Reflecting Diastolic Function
The S4 was significantly more prevalent in persons age >60 years compared with those age <40 years (23.5% vs 5.6%) and significantly more pronounced during sleep for all ages >50 years, but particularly in the ages >60 years (34.4% sleep vs 12.6% awake) (Figure 4). Left ventricular diastolic perfusion time was significantly longer during sleep as compared with awake in all age groups. There was no significant age‐related difference in sleep LDPT, whereas awake LDPT was significantly longer in persons aged >30 years than in the younger group (Figure 3).
Because there was a proportion of subjects with a history of hypertension, who were being currently treated, we analyzed the data by dividing the population into those with and without a history of hypertension. There were no significant differences in diastolic heart sounds or systolic time intervals between those 2 groups. We attribute the lack of significant differences to the fact that the subjects with history of hypertension were currently receiving and complying with medical treatment and had presently controlled blood pressures.
Discussion
The S3 occurs approximately 120–160 ms after the S2 and is caused by an abrupt limitation of LV inflow during early diastole that causes vibration of the cardiohemic system. Our findings confirm those of others in the relatively high prevalence of a physiological S3 in those age <40 years.9 The low prevalence of S3 in asymptomatic subjects age >40 years (<5% mean awake value) strengthens the findings of others that a detectable S3 in older subjects is specific for cardiac pathology10 and is associated with elevated LV filling pressures.11
In 1962, Rodin and Tabatznik12 studied the effect of posture on heart sounds and found that physiological S3 disappeared when standing up but persisted in patients with poorly controlled heart failure. Our findings would suggest the low prevalence of S3 in those age >40 years, both while upright and asleep. In those age <40 years, the prevalence of S3 decreases from 19.6% while sleeping to 6.8% while awake. The 3‐fold increase in an S3 during sleep is most likely due to the effects of posture on increased venous return with reduced afterload due to lower blood pressure and increased diuresis at night.
The significance of an S4 was debated in the 1970s and 1980s,13, 14, 15 when the prevalence was found to be as high as 73% in healthy persons. Some of the controversy was due to the difference between auscultated S4 vs those detected by phonocardiography. Later studies using acoustic cardiography have found lower prevalence of the S4 1 and its presence associated with increased LV end‐diastolic stiffness7 and impaired relaxation.16 The findings of this study suggest an age‐related increase in the prevalence of the S4 similar to age‐related changes in LV filling patterns determined using echocardiography by other researchers. In a randomly recruited population of 539 people, Kuznetsova et al17 measured the prevalence of LV diastolic dysfunction using echocardiography. They found a significant decline in the early (E) to late atrial (A) ratio (E/A ratio) with age due to a significant decrease in E velocity as well as an increase in A velocity. The S4 is produced by the abrupt deceleration of the A wave and a stiff LV. The finding of altered E and A velocities with aging is consistent with our findings of an increase in the prevalence of the S4 with age, particularly in those age >60 years.
Heart rate decreased significantly in all age groups during sleep, with only 2 subjects using β‐blocking agents. The decrease in awake heart rate with increasing age reflects, aside from a lower level of physical activity, the development of a subclinical sinoatrial node dysfunction with aging in ambulatory adults.18 Left ventricular diastolic perfusion time is determined largely by the heart rate and the timing of the S2.19 The LV diastolic perfusion time increased significantly in all age groups during sleep and was relatively constant in the older age groups. The decrease in heart rate at night may have benefits beyond the reduced oxygen consumption such that it provides maximal myocardial perfusion and preserves coronary reserve. Current research in nocturnal nondipping of heart rate has shown the risk of future cardiovascular events to be as much as 2.4× higher in those whose heart rate does not exhibit the typical nocturnal decline.20 The results of this study may prove useful when studying patients with cardiac disease, namely heart failure, for comparison of altered measures of heart rate and diastolic perfusion time at night.
In this asymptomatic population we found little age‐related change in parameters related to systolic function. Electromechanical activation time was constant at an average of 89 ms for those age ≥40 years, with little awake‐to‐sleep variation. There was a similar constancy in the value of %LVST with the age >40 years, also with little awake‐to‐sleep change. Voutilainen et al21 studied circadian variation of LV function in healthy people. They found that systolic function was less dependent on the time of day, but that diastolic function did include a nocturnal decrease and a daytime increase in the rate of LV relaxation that they attributed to sympathoadrenal activity. The findings in this study on electromechanical activation time and %LVST support the conclusion of less variation from day to night in systolic function in asymptomatic subjects, whereas there was more significant variation in the detected S4 from awake to sleep, reflecting a decrease in diastolic function during sleep.
Study Limitations
This study was performed on an asymptomatic and ambulatory population with limited information acquired on their compliance to medications, use of herbal and lifestyle drugs, blood pressure, and other factors that may have impacted the findings of this study. The population was predominantly Caucasian and geographically confined to the northwestern area of the United States. Overall, 93% of the data were of sufficient quality to allow analysis (4% did not have sufficient ECG quality for analysis and 3% did not have sufficient heart‐sound quality for analysis).
Conclusion
In an ambulatory and asymptomatic population, systolic time intervals that reflect LV systolic function demonstrate less change with age and less circadian/postural variation than diastolic heart sounds (S3 and S4). The S3 after the age of 40 years is a relatively uncommon finding and therefore should be a specific sign of cardiac disease. The S4 increases in prevalence with age and is likely a result of changes in diastolic filling patterns with increasing age (specifically, impaired relaxation).7 Continuous monitoring of diastolic heart sounds and systolic time intervals is possible using acoustic cardiography.
References
- 1. Collins SP, Arand P, Lindsell CJ, et al. Prevalence of the third and fourth heart sound in asymptomatic adults. Congest Heart Fail. 2005;11:242–247. [DOI] [PubMed] [Google Scholar]
- 2. Erne P. Beyond auscultation—acoustic cardiography in the diagnosis and assessment of cardiac disease. Swiss Med Wkly. 2008;138:439–452. [DOI] [PubMed] [Google Scholar]
- 3. Moyers B, Shapiro M, Marcus GM, et al. Performance of phonoelectrocardiographic left ventricular systolic time intervals and B‐type natriuretic peptide levels in the diagnosis of left ventricular dysfunction. Ann Noninvasive Electrocardiol. 2007;12:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roos M, Toggweiler S, Jamshidi P, et al. Non‐invasive detection of left‐ventricular systolic dysfunction by acoustic cardiography in cardiac failure patients. J Card Fail. 2008;14:310–319. [DOI] [PubMed] [Google Scholar]
- 5. Shapiro M, Moyers B, Marcus GM, et al. Diagnostic characteristics of combining phonocardiographic third heart sound and systolic time intervals for the prediction of left ventricular dysfunction. J Card Fail. 2007;13:18–24. [DOI] [PubMed] [Google Scholar]
- 6. Roos M, Toggweiler S, Zuber M, et al. Acoustic cardiographic parameters and their relationship to invasive hemodynamic measurements in patients with left ventricular systolic dysfunction. Congest Heart Fail. 2006;12(suppl 1):19–24. [DOI] [PubMed] [Google Scholar]
- 7. Shah , SJ , Nakamura K, Marcus GM, et al. Association of the fourth heart sound with increased left ventricular end‐diastolic stiffness. J Card Fail. 2008;14:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah SJ, Michaels AD. Hemodynamic correlates of the third heart sound and systolic time intervals. Congest Heart Fail. 2006;12(suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 9. Kupari M, Koskinen P, Virolainen J, et al. Prevalence and predictors of audible physiological third heart sound in a population sample aged 36 to 37 years. Circulation. 1994;89:1189–1195. [DOI] [PubMed] [Google Scholar]
- 10. Drazner MH, Rame JE, Stevenson LW, et al. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–581. [DOI] [PubMed] [Google Scholar]
- 11. Marcus GM, Gerber IL, McKeown BH, et al. Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. JAMA. 2005;293:2238–2244. [DOI] [PubMed] [Google Scholar]
- 12. Rodin P, Tabatznik B. The effect of posture on added heart sounds. Br Heart J. 1963;25:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spodick DH, Quarry VM. Prevalence of the fourth heart sound by phonocardiography in the absence of cardiac disease. Am Heart J. 1974;87:11–14. [DOI] [PubMed] [Google Scholar]
- 14. Erikssen J, Rasmussen K. Prevalence and significance of the fourth heart sound (S4) in presumably healthy middle‐aged men, with particular relation to latent coronary heart disease. Eur J Cardiol. 1979;9:63–75. [PubMed] [Google Scholar]
- 15. Swistak M, Mushlin H, Spodick DH. Comparative prevalence of the fourth heart sound in hypertensive and matched normal persons. Am J Cardiol. 1974;33:614–616. [DOI] [PubMed] [Google Scholar]
- 16. Harris IS, Lee E, Yeghiazarians Y, et al. Phonocardiographic timing of third and fourth heart sounds during acute myocardial infarction. J Electrocardiol. 2006;39:305–309. [DOI] [PubMed] [Google Scholar]
- 17. Kuznetsova T, Herbots L, Lopez B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. [DOI] [PubMed] [Google Scholar]
- 18. Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–116. [DOI] [PubMed] [Google Scholar]
- 19. Zuber M, Erne P. Non‐invasive assessment of diastolic cardiac perfusion time through acoustic cardiography. Paper presented at: European Society of Cardiology Congress; August 30–September 3, 2008 Munich, Germany. Abstract 82953.
- 20. Kazuo E, Satoshi H, Joji I, et al. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens. 2009;27:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voutilainen S, Kupari M, Hippelainen M, et al. Circadian variation of left ventricular diastolic function in healthy people. Heart. 1996;75:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


