Abstract
Background:
Implantable cardioverter‐defibrillator (ICD) therapy for primary prevention is well established in ischemic cardiomyopathy (ICM). Data on the role of ICDs in patients with dilated cardiomyopathy (DCM) and no history of ventricular tachyarrhythmia (VT/VF) are more limited.
Hypothesis:
DCM patients with an impaired left ventricular ejection fraction (LVEF) still represent a low arrhythmic risk subgroup in clinical practice.
Methods:
ICD stored data of DCM patients with an LVEF ≤35% was compared to data of ICM patients meeting Multicenter Automatic Defibrillator Implantation Trial (MADIT) eligibility criteria. VT/VF occurrences and electrical storm (ES) events were analyzed.
Results:
There were 652 patients followed for 50.9 ± 33.9 months. There were 1978 VT and 241 VF episodes analyzed in 66 out of 203 patients (32.5%) with DCM and in 118 out of 449 patients (26.3%, P = 0.209) with ICM. Freedom of appropriate ICD treatment due to VT/VF or ES events did not differ in both patient populations (log‐rank, P>0.05). In patients presenting with VT/VF episodes, mean event rates were comparable in both patient populations (3.2 ± 14.1 for DCM and VT vs 3 ± 13.9 for ICM and VT [P = 0.855], 0.4 ± 1.3 for DCM and VF vs 0.4 ± 1.8 for ICM and VF [P = 0.763], and 0.2 ± 0.7 for DCM and ES vs 0.2 ± 1 for ICM and ES [P = 0.666]).
Conclusions:
DCM patients with prophylactic ICDs implanted due to heart failure and patients fulfilling MADIT criteria reveal comparable patterns of VT/VF/ES events during long‐term follow‐up. Incidence, mean number of events, and time to first event did not differ significantly. Findings support the current guidelines for prophylactic ICD therapy in DCM patients with heart failure. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Sudden cardiac death (SCD) is a common cause of death throughout the world.1, 2 Coronary artery disease (CAD) is responsible for approximately 75% to 80% of all SCDs,3 cardiomyopathies (eg, idiopathic dilated cardiomyopathy [DCM]) and primary electrical abnormalities account for most of the remainder.4 Most of the implantable cardioverter‐defibrillator (ICD) trials for primary prevention of SCD, in which subjects have not experienced a life‐threatening ventricular tachycardia (VT) or ventricular fibrillation (VF), have focused on patients with ischemic cardiomyopathy (ICM).5, 6, 7 Although DCM is known to have an estimated 5‐year mortality of 20% with approximately one third (8%–51%) of deaths occurring due to SCD,8, 9 risk stratification remains difficult in this patient population as data on the role of ICDs are more limited than in ICM patients.7, 10
The purpose of the present study was, therefore, to assess the long‐term arrhythmia burden of patients with DCM and ICDs implanted for primary prevention. Subsequently, event characteristics were compared to those of ICM patients meeting the Multicenter Automatic Defibrillator Implantation Trial‐I and II (MADIT‐I or MADIT‐II) eligibility criteria.11
Methods
Patient Population
The present study is a prospective, longitudinal, single‐center study analyzing the data of 652 DCM and ICM patients with ICDs implanted for primary prevention in conformity with the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines.9 Diagnosis of DCM was established when dilated cardiac chambers were combined with a systolic left ventricular ejection fraction (LVEF) ≤35%. All patients underwent left heart catheterization before ICD implantation. CAD was defined as a stenosis ≥70% in at least 1 major coronary artery. Patients were followed in the outpatient clinic every 3 months or prematurely after receiving ICD shocks. Visits included the assessment of medical history and concomitant medication, a physical examination, a 12‐lead electrocardiogram, and a telemetry device interrogation. Sustained VT/VF events served as the end point of the study. Inappropriate ICD therapies were excluded from analysis. VT/VF and electrical storm (ES) events were excluded from analysis if there was an obvious precipitating cause for the event (eg, acute myocardial ischemia or electrolyte disturbances).
ICD Interrogation
All devices provided extensive data log information and stored endocardial electrograms. Electrograms were analyzed by 2 independent observers to classify the arrhythmia leading to ICD therapy. Classification was based on sudden onset, rate, rate stability, and electrogram morphology of the arrhythmia. VTs with a mean cycle length above 400 ms were defined as slow VTs. Antitachycardia pacing (ATP) therapy success was analyzed for all VT episodes.
Device Settings
Devices were uniformly programmed using 2 detection zones. Three ATP attempts (84%) followed by shock were programmed in a single VT zone. The lowest VT detection boundary was programmed based on the rate of inducible VT at the electrophysiologic study or by programming empirical detection rates between 130 and 180 bpm. The average VT detection rate was 167 bpm. VF detection was uniformly programmed at 214 bpm.
Definition of ES
ES was defined as ≥3 separate VT/VF events in ≤24 hours.12, 13 ES recurrence (ES‐R) was considered to be completed if patients experienced ≥3 new distinct VT/VF episodes in ≤24 hours, with the first VT/VF episode of the ES‐R occurring at least 24 hours later than the last episode of the previous ES event. If multiple appropriate ICD therapies were delivered to terminate a single episode of VT/VF, these were considered to be part of 1 event.
Statistical Analysis
Absolute and relative frequencies are reported for categorical variables. Continuous variables are expressed as mean ± standard deviation. Continuous variables were analyzed by Student t test if normally distributed. The Mann‐Whitney test was used for variables with skewed distribution. The χ 2 test was used for discrete variables. Freedom of appropriate ICD treatment was calculated according to the Kaplan‐Meier method. Differences between groups were calculated with the log‐rank (Mantel‐Cox) test. A 2‐tailed P value <0.05 was considered statistically significant. For data storage and analysis, SPSS version 17.0.0 (SPSS, Inc., Chicago, IL) was used.
Results
Baseline Characteristics
There were 652 patients followed for a mean of 50.9 ± 33.9 months (2766 patient years). There were 203 patients (31.1%) who received their ICD for DCM and 449 (68.9%) for ICM. When comparing baseline characteristics (Table 1), patients with ICM more often suffered from atrial fibrillation (40.8% vs 25.6%, P<0.001) or diabetes mellitus (41.2% vs 25.6%, P<0.001), whereas patients with DCM were significantly more often classified in New York Heart Association [NYHA] class III heart failure (51.2% vs 34.5%, P<0.001). Additionally, patients with DCM revealed a significantly lower mean LVEF (26.4 ± 5.3% vs 29.7 ± 4.9%, P<0.001), were significantly more often treated with digitalis glycosides (45.3% vs 34.1%, P = 0.007) and diuretics (80.3% vs 67.7%, P = 0.001) than patients with ICM, whereas statin intake was more often present in patients with ICM (78.4% vs 34%, P<0.001). However, when comparing patients with ICM or DCM with or without VT/VF episodes on or off statin, digitalis glycosides, or diuretic therapy with univariate analysis, no significant differences were determined between the groups (P>0.05). Additionally, log‐rank tests computed to determine freedom of VT/VF events in patients with DCM and ICM corrected for statin (P = 0.692), digitalis glycoside (P = 0.079), and diuretics intake (P = 0.067) revealed no significant differences between groups.
Table 1.
Baseline Demographics and Clinical Characteristics of the Study Populations
| Baseline Characteristics | ICD Patients With DCM and LVEF ≤35% (n = 203) | MADIT Patients (n = 449) | P Value |
|---|---|---|---|
| Age (y) | 66.2 ± 12.5 | 70.6 ± 9.7 | <0.001 |
| Male gender | 157 (77.3) | 380 (84.6) | 0.024 |
| Mean follow‐up (mo) | 47.8 ± 34.5 | 52.2 ± 33.5 | 0.125 |
| Single‐chamber ICD | 117 (57.6) | 304 (67.7) | 0.884 |
| Dual‐chamber ICD | 46 (22.7) | 118 (26.3) | 0.487 |
| Biventricular ICD | 40 (19.7) | 27 (6) | <0.001 |
| Clinical parameters | |||
| LVEF (%) | 26.4 ± 5.3 | 29.7 ± 4.9 | <0.001 |
| NYHA III | 104 (51.2) | 155 (34.5) | <0.001 |
| History of myocardial infarction | 0 (0) | 290 (64.6) | <0.001 |
| History of PCI | 2 (1) | 230 (51.2) | <0.001 |
| Ventricular aneurysm | 0 (0) | 144 (32.1) | <0.001 |
| History of syncope | 40 (19.7) | 104 (23.2) | 0.484 |
| Diabetes mellitus | 52 (25.6) | 185 (41.2) | <0.001 |
| Renal dysfunction | 54 (26.6) | 154 (34.3) | 0.051 |
| COPD | 31 (15.3) | 100 (22) | 0.08 |
| Electro‐ and echocardiographic parameters | |||
| Atrial fibrillation | 52 (25.6) | 183 (40.8) | <0.001 |
| Heart rate at rest (/min) | 72.2 ± 13.1 | 70.5 ± 12.8 | 0.131 |
| LVEDD (mm) | 65.8 ± 9.2 | 59.9 ± 8.2 | <0.001 |
| Left atrial size (mm) | 46.7 ± 7.3 | 45.3 ± 6.7 | 0.038 |
| Septum (mm) | 10.4 ± 2.1 | 11.2 ± 2.2 | 0.001 |
| Posterior wall (mm) | 10.5 ± 1.7 | 10.7 ± 1.8 | 0.231 |
| Diastolic dysfunction | 174 (85.7) | 364 (80.5) | 0.166 |
| Concomitant medication | |||
| β‐blockers | 180 (88.7) | 400 (89.1) | 0.834 |
| ACE‐inhibitors and/or ARBs | 186 (91.6) | 405 (90.2) | 0.427 |
| Digitalis glycosides | 92 (45.3) | 153 (34.1) | 0.007 |
| Diuretics | 163 (80.3) | 304 (67.7) | 0.001 |
| Statins | 69 (34) | 352 (78.4) | <0.001 |
| Amiodarone | 15 (7.4) | 46 (10.2) | 0.246 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; DCM, idiopathic dilated cardiomyopathy; ICD, implantable cardioverter‐defibrillator; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; MADIT, Multicenter Automatic Defibrillator Implantation Trial; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Data are presented as the mean value ± standard deviation for continuous variables and number (%) for categorical variables.
Incidence and Characteristics of Ventricular Tachyarrhythmia
There were 1978 VT and 241 VF episodes that occurred in 66 out of 203 DCM (32.5%) and in 118 out of 449 ICM patients (26.3%, P = 0.209), which comprises an overall annual VT/VF event rate of 0.8 events per patient. Mean number of VT (3.2 ± 14.1 vs 3 ± 13.9, P = 0.855), VF (0.4 ± 1.3 vs 0.4 ± 1.8, P = 0.763) and ES events (0.2 ± 0.7 vs 0.2 ± 1, P = 0.666) did not differ significantly within DCM and ICM patients. Mean event rates in symptomatic patients remained comparable in both patient populations (9.8 ± 23.6 for DCM and VT vs 11.3 ± 25.4 for ICM and VT [P = 0.694], 1.2 ± 2 for DCM and VF vs 1.4 ± 3.3 for ICM and VF [P = 0.778]). The mean cycle length of all VT episodes was 331.3 ± 34 ms (range, 260–480 ms). Further evaluation revealed a trend to faster mean VT cycle lengths in DCM compared to ICM patients (325.2 ± 27.7 ms vs 334.8 ± 36.8 ms, P = 0.098). Slow VT was detected in 10 out of 184 patients (5.4%) presenting with VTs. Three out of 10 patients (30%) with slow VTs experienced a second VT morphology with faster VT cycle lengths (295 ms/330 ms/350 ms) during follow‐up. Twenty‐eight out of 203 DCM patients (13.8%) experienced ≥1 VF episode, which represents a trend to a higher incidence of VF in DCM patients, as only 40 out of 449 patients (8.9%) with ICM experienced ≥1 VF episode during follow‐up (P = 0.059).
Device Therapy of Ventricular Tachycardia
In both patient groups, 87% of all VT events were successfully terminated by ATP therapy (P = 0.761). Three ineffective ATP attempts leading to shock therapy or acceleration of VT via ATP triggering shock therapy occurred in 5 out of 55 DCM patients (9.1%) and in 12 out of 104 ICM patients (11.5%, P = 0.824). Primary shock therapy due to VT detection in the VF detection zone was delivered in 2 out of 55 DCM patients (3.6%) and in 1 out of 104 ICM patients (1%, P = 0.912).
Incidence of Electrical Storms
There were 111 ES episodes (range, 0–12) that occurred in 14 out of 203 DCM patients (6.9%) and in 26 out of 449 ICM patients (5.8%, P = 0.575). Overall, DCM patients developed an average of 0.2 ± 0.7 and ICM patients an average of 0.2 ± 1 ES episodes during follow‐up (P = 0.666). DCM patients symptomatic for ES developed an average of 2.1 ± 1.6 ES episodes during follow‐up compared to 3.1 ± 2.8 ES episodes in ICM patients (P = 0.233). ES‐R occurred in 7 out of 14 DCM patients (50%) and in 17 out of 26 ICM patients (65.4%, P = 0.841). DCM patients with ES‐R developed an average of 3.3 ± 1.5 ES episodes compared to 4.2 ± 2.8 ES events in ICM patients (P = 0.415).
Time to VT/VF and ES Events
In univariate analysis, DCM patients trended to shorter mean time intervals to first VT/VF event after ICD implantation (18 ± 30.9 vs 20 ± 24.9 months for first VT [P = 0.669] and 13.7 ± 14.4 vs 16 ± 15.4 months for first VF [P = 0.544]). ICM patients suffered from earlier ES events (23.4 ± 29.8 vs 27.6 ± 34.4 months, P = 0.693) and ES‐R (26.5 ± 30.2 vs 34.6 ± 43.5 months, P = 0.253). However, none of these findings were statistically significant. Kaplan‐Meier plots analyzing time from ICD implantation to first VT, VF, or ES event and time to first ES‐Recurrence again yielded no significant differences between DCM and ICM patients, but confirmed a trend to earlier VF episodes in DCM patients (Figures 1, 2, 3, 4).
Figure 1.
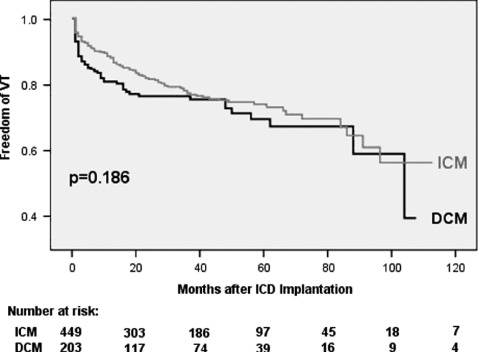
Freedom of first ventricular tachycardia (VT). Abbreviations: DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy.
Figure 2.
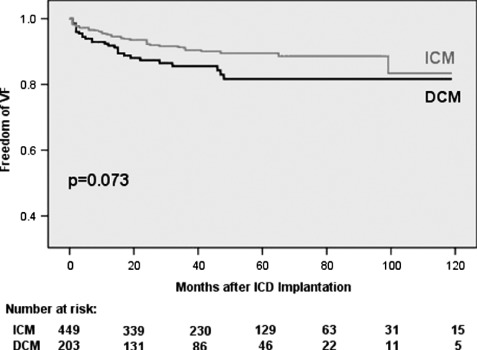
Freedom of first ventricular fibrillation (VF). Abbreviations: DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy.
Figure 3.
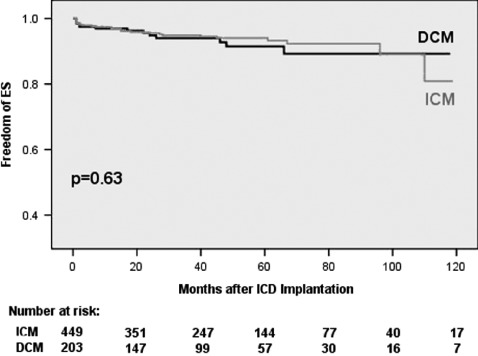
Freedom of first electrical storm (ES). Abbreviations: DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy.
Figure 4.
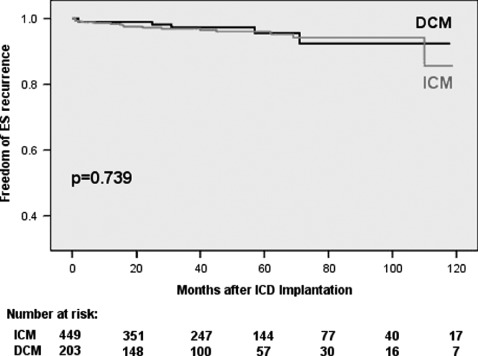
Freedom of first electrical storm recurrence. Abbreviations: DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy.
Discussion
Main Findings
This long‐term follow‐up analysis compared incidence and characteristics of spontaneous VT/VF and ES events in a large population of patients with ICDs implanted for primary prevention, either due to idiopathic dilated cardiomyopathy with a reduced ejection fraction ≤35% or ischemic cardiomyopathy meeting the MADIT eligibility criteria. Contrary to expectations, the incidence, mean number, and the timing of VT/VF and ES events as well as the efficacy of ATP therapy for VTs did not differ significantly in these real‐world patient populations. Overall, 32.5% of the DCM patients and 26.3% of the ICM patients (P = 0.209) developed at least 1 successfully treated VT/VF episode during a mean follow‐up of 50.9 months, which might have been fatal in the absence of an ICD.
Incidence and Type of Ventricular Tachyarrhythmia
Limited data is available describing VT/VF characteristics in DCM patients with ICDs implanted for primary prevention, as most of the prior studies predominately consisted of secondary preventive patient populations.14, 15, 16, 17, 18 Two mortality‐driven studies in DCM patients with prophylactic ICDs published data regarding specific VT/VF characteristics. The Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial reported that 17.9% of the patients treated with standard oral medical therapy and ICDs received appropriate shocks during a mean follow‐up of 29 ± 14.4 months.19 Lower event rates in DEFINITE compared to those found in the present routine clinical practice study are explained by higher VT detection boundaries (180 bpm) and the shorter follow‐up duration (29 ± 14.4 months) of DEFINITE. The Sudden Cardiac Death in Heart Failure trial (SCD‐HeFT) enrolled NYHA class II and III patients with a LVEF ≤35% and CAD or nonischemic cardiomyopathy.20 In contrast to the present study, only single‐lead, shock‐only ICDs with a detection rate at 187 bpm were implanted. Again, the conservative device programming serves as an explanation for lower event rates observed in SCD‐Heft (21.4% vs 27.8%).
However, some issues remain unclear even after publication of SCD‐HeFT. As mentioned by Packer et al, SCD‐HeFT was unique with regard to the mechanism of event adjudication by both electrophysiologists and heart failure cardiologists, but the main limitation of the study was the inherent difficulty in defining causality and the associated classification. This limitation was particularly relevant to the category of cardiac mortality resulting from VT/VF. Although such deaths were by definition sudden, some events classified as VT/VF may have been due to other causes, including bradyarrhythmias, pulseless electrical activity, myocardial infarction, and noncardiac causes. Information from ICD interrogations was, in contrast to the present study, purposely not made available because it might have affected the events committee's decision using data not available in the amiodarone or placebo treatment groups.21
Some other recently published studies described the characteristics of VT/VF events in SCD‐HeFT‐like and MADIT‐II–like patients.22 Authors counted appropriate ICD therapies in 54% of SCD‐HeFT–like patients and in 56% of MADIT‐II–like patients during a mean follow‐up of 33 ± 19 months, but in contrast to the present study following 203 patients with DCM, only 7 out of 47 patients (14.9%) with VT/VF episodes in the SCD‐HeFT–like patient population suffered from DCM. However, no significant differences regarding incidence and time to first VT/VF and ES event—at least the latter are known to be associated with increased mortality rates12, 23—were detected in the present study when comparing DCM and ICM patients with prophylactic ICDs and sufficient heart failure medication. Even a trend to earlier VF occurrence in DCM patients was observed. These findings are remarkable, as 2 different heart diseases with mayor differences in VT/VF initiation and maintenance mechanisms were compared.24
Implications of the Present Study
In general, the indications for ICDs have rapidly expanded over the past decade. Clinical trial data have quickly been implemented into guidelines, even with emerging evidence that ICD therapy has inherent risks, including procedural complications, inadequate shocks, and other device malfunctions.25 Regardless of this and of the fact that DCM patients with an impaired LVEF still seem to represent a low arrhythmic risk subgroup in clinical practice,7 event rates comparable to those of MADIT patients were observed in the DCM patient population. Therefore, the results of the present study support the current guidelines for ICD therapy in DCM patients with heart failure. Nevertheless, more precise risk stratification models have to be developed in clinically relevant DCM subgroups to improve the benefit that has been achieved with the use of ICDs by now.
Conclusion
DCM patients with prophylactic ICDs implanted due to heart failure and patients fulfilling MADIT criteria reveal comparable patterns of VT/VF and ES events during long‐term follow‐up. Incidence, mean number of treated VT/VF and ES events, and time to first event did not differ significantly within the groups. Findings support the current guidelines for prophylactic ICD therapy in DCM patients with heart failure.
Limitations of the Study
This study was nonrandomized and mortality was not an end point. Additionally, slow monomorphic VT events entered analysis. Considering that most slow VT episodes do not lead to a life‐threatening situation, their inclusion into analysis may have introduced bias estimating the potential benefit from ICD treatment. Furthermore, it has to be considered that lower LVEF values and higher NYHA functional classes in patients with DCM could have played an important role in increasing VT/VF burden in this cohort. However, differences in heart failure characteristics between real‐world ICM and DCM patients could reflect the difficulty in identifying patients with DCM and early NYHA class II heart failure symptoms in routine clinical practice, as diagnosis of DCM is frequently not established until heart failure symptoms exceed NYHA functional class II.
References
- 1. Priori SG, Aliot E, Blomstrom‐Lundqvist C, et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J. 2001;22:1374–1450. [DOI] [PubMed] [Google Scholar]
- 2. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. [DOI] [PubMed] [Google Scholar]
- 3. Spooner PM, Albert C, Benjamin EJ, et al. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a national heart, lung, and blood institute workshop, part I. Circulation. 2001;103:2361–2364. [DOI] [PubMed] [Google Scholar]
- 4. Sukhija R, Mehta V, Leonardi M, et al. Implantable cardioverter defibrillators for prevention of sudden cardiac death. Clin Cardiol. 2007;30:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 7. Dickstein K, Cohen‐Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 8. Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–1575. [DOI] [PubMed] [Google Scholar]
- 9. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J. 2006;27:2099–2140. [DOI] [PubMed] [Google Scholar]
- 10. Cevik C, Nugent K, Perez‐Verdia A, et al. Prophylactic implantation of cardioverter defibrillators in idiopathic nonischemic cardiomyopathy for the primary prevention of death: a narrative review. Clin Cardiol. 2010;33:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moss AJ. MADIT‐I and MADIT‐II. J Cardiovasc Electrophysiol. 2003;14(9 suppl):S96–S98. [DOI] [PubMed] [Google Scholar]
- 12. Exner DV, Pinski SL, Wyse DG, et al. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001;103:2066–2071. [DOI] [PubMed] [Google Scholar]
- 13. Hohnloser SH, Al‐Khalidi HR, Pratt CM, et al. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006;27:3027–3032. [DOI] [PubMed] [Google Scholar]
- 14. Bansch D, Bocker D, Brunn J, et al. Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol. 2000;36:566–573. [DOI] [PubMed] [Google Scholar]
- 15. Fazio G, Veltri EP, Tomaselli G, et al. Long‐term follow‐up of patients with nonischemic dilated cardiomyopathy and ventricular tachyarrhythmias treated with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1991;14(11 pt 2): 1905–1910. [DOI] [PubMed] [Google Scholar]
- 16. Grimm W, Hoffmann JJ, Muller HH, et al. Implantable defibrillator event rates in patients with idiopathic dilated cardiomyopathy, nonsustained ventricular tachycardia on Holter and a left ventricular ejection fraction below 30%. J Am Coll Cardiol. 2002;39:780–787. [DOI] [PubMed] [Google Scholar]
- 17. Grimm W, Marchlinski FE. Shock occurrence and survival in 49 patients with idiopathic dilated cardiomyopathy and an implantable cardioverter‐defibrillator. Eur Heart J. 1995;16:218–222. [DOI] [PubMed] [Google Scholar]
- 18. Rankovic V, Karha J, Passman R, et al. Predictors of appropriate implantable cardioverter‐defibrillator therapy in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;89: 1072–1076. [DOI] [PubMed] [Google Scholar]
- 19. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 20. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 21. Packer DL, Prutkin JM, Hellkamp AS, et al. Impact of implantable cardioverter‐defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kreuz J, Balta O, Liliegren N, et al. Incidence and characteristics of appropriate and inappropriate therapies in recipients of ICD implanted for primary prevention of sudden cardiac death. Pacing Clin Electrophysiol. 2007;30(suppl 1):S125–S127. [DOI] [PubMed] [Google Scholar]
- 23. Verma A, Kilicaslan F, Marrouche NF, et al. Prevalence, predictors, and mortality significance of the causative arrhythmia in patients with electrical storm. J Cardiovasc Electrophysiol. 2004;15:1265–1270. [DOI] [PubMed] [Google Scholar]
- 24. Attin M, Ideker RE, Pogwizd SM. Mechanistic insights into ventricular arrhythmias from mapping studies in humans. Heart Rhythm. 2008;5(6 suppl):S53–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter‐defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52: 1111–1121. [DOI] [PubMed] [Google Scholar]


