Abstract
Background:
The pulmonary arterial pressure (PAP) response to exercise may provide a tool for the early detection of pulmonary arterial hypertension (PAH). Therefore, an accurate noninvasive method for evaluating exercise‐induced PAH (EIPAH) is desirable.
Hypothesis:
We sought to examine if cardiopulmonary exercise testing (CPET) is able to indicate EIPAH.
Methods:
Fifty‐three patients aged 67.1 ± 1.7 years (37 female, 16 male) with borderline PAH (resting mean PAP 21–24 mm Hg) performed CPET and right heart catheterization at rest and during handgrip testing.
Results:
When comparing patients with an exercise‐induced mean PAP ≥35 mm Hg (group A, n = 24) and subjects with an exercise‐induced mean PAP <35 mm Hg (group B, n = 29), group A had a significantly lower mean aerobic capacity (15.2 ± 1.2 vs 19.7 ± 1.2 mL/min/kg; P = 0.02), higher ventilatory equivalents for oxygen at the anaerobic threshold (34.3 ± 1.5 vs 29.9 ± 1.1; P = 0.02), a widening of the mean alveolar‐arterial oxygen difference (37.8 ± 3.0 vs 26.8 ± 2.4 mm Hg; P = 0.007), an elevated mean functional dead space ventilation (29.5 ± 2.7 vs 21.2 ± 1.7%; P = 0.008), and a higher mean arterial to end‐tidal carbon dioxide gradient at peak exercise (3.7 ± 0.9 vs 0.4 ± 0.8 mm Hg; P = 0.007).
Conclusions:
EIPAH is characterized by a decreased ventilatory efficiency due to ventilation to perfusion inequalities. CPET may be useful for the identification of EIPAH and serve to diagnose PAH at an early stage.
Drs. Schwaiblmair, von Scheidt, and Berghaus conceived and designed the study. Drs. Schwaiblmair, Faul, and Berghaus acquired the study data. Dr. Schwaiblmair performed the statistical analysis and drafted the article. All authors participated in interpreting the data and revised the manuscript for important intellectual content.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
In many patients with pulmonary arterial hypertension (PAH), the disease is not diagnosed until pulmonary arterial pressure (PAP) is markedly elevated and right ventricular dysfunction has begun.1, 2, 3 Similarly, PAP measured only at rest fails to detect a significant percentage of patients with exercise‐induced PAH (EIPAH). EIPAH is a poorly understood entity.4, 5, 6, 7, 8, 9, 10, 11, 12 Some believe that EIPAH is an early and more treatable phase that precedes resting PAH, whereas others suggest that it may be a stable variant of the disease.5, 6, 13, 14, 15 To our knowledge, there have been surprisingly few invasive studies of EIPAH.10, 14, 15, 16
Early identification of PAH may favorably alter disease management. Although the gold standard for measuring PAP remains right heart catheterization (RHC), noninvasive alternatives are needed that correlate well with invasive methods. In patients with PAH, the initial symptoms are usually related to exertion, and this is reflected in reduced maximum oxygen (O2) consumption on incremental exercise testing.17 To test the hypothesis that EIPAH produces these symptoms, attempts have been made to look for evidence of pulmonary hypertension (PH) occurring during exercise.16, 18 In healthy individuals, PAP has been reported to remain unchanged during exercise or increase slightly19; on the other side, it is recognized that exercise produces abnormally large increases in PAP in patients with pulmonary vascular disease.
Cardiopulmonary exercise testing (CPET) is suitable in describing the exercise capacity by examining the underlying physiologic abnormalities associated with the decreased perfusion of the pulmonary vascular bed.20 PH patients usually present with reductions in peak O2 uptake, anaerobic threshold, O2 pulse, and ventilatory efficiency.17, 20, 21, 22, 23 It has been speculated that these variables might serve as potential noninvasive surrogate markers for the PAP response to exercise. To test this hypothesis, we measured PAP by RHC at rest and during exercise to detect the development of EIPAH in a group of patients with borderline PAH (defined as a mean PAP between 21 and 24 mm Hg at rest24). Then we compared these results with CPET parameters to determine the accuracy of noninvasive CPET for the detection of EIPAH.
Methods
Study Population
Fifty‐three patients with borderline PAH as defined by the European Society of Cardiology/European Respiratory Society guidelines24 were prospectively investigated in the study. This study included patients who were referred to our institute to confirm or to exclude PH by RHC. Exclusion criteria were: age younger than 18 years, left ventricular diastolic or systolic dysfunction (assessed by echocardiography), impaired renal function (glomerular filtration rate <60 mL/min), significant restrictive (total lung capacity <80% of predicted) or obstructive (forced expiratory volume in 1 second <70% of predicted) lung disease. Moreover, all patients showing signs of acute right heart pressure or volume overload were excluded.
All procedures adhered to the institutional and ethical guidelines, and written informed consent was obtained from every patient.
Cardiopulmonary Exercise Testing
CPET was performed using a standardized protocol.25 Briefly, work rate was continuously increased by 5 to 15 W/min to a maximum tolerated level on an electromagnetically braked cycle ergometer (ViaSprint 150 p; Ergoline, Windhagen, Germany). Blood gas analysis was done at rest and during maximal exercise. Heart rate was monitored continuously and blood pressure was taken noninvasively every 2 minutes. O2 uptake (VO2), minute ventilation (Ve), and carbon dioxide (CO2) output (VCO2) were measured breath by breath wearing an adult facemask (Vmax spectra 229 D, Sensormedics, Yorba Linda, CA). Peak VO2, O2 pulse (O2 pulse), alveolar‐arterial O2 difference (AaDO2), and functional dead space ventilation (Vd/Vt) were calculated as described previously.25 The anaerobic threshold (AT) was chosen at the peak VO2 at which the ventilatory equivalent for O2 (Ve/VO2) increased and the ventilatory equivalent for CO2 (Ve/VCO2) decreased or remained constant. Peak VO2 was defined as the value of averaged data during the final 15 seconds of exercise. The Ve/VCO2 slope was determined as the linear regression slope of Ve and VCO2 from the start of exercise until the respiratory compensation point (the time point at which ventilation is stimulated by acidemia and the end‐tidal CO2 (etCO2) begins to decrease).
Right Heart Catheterization
RHC was performed in all study participants. Patients received no medication on the morning of the procedure. A True Size Thermodilution S‐tip catheter (Edwards Lifesciences, Irvine, CA) was inserted via the right femoral vein. Hemodynamic measurements were performed in supine position including heart rate, pressure in wedge position (PCWP), PAP, and right atrium pressure (RAP). O2 saturation (SO2) was measured in mixed venous blood samples. Cardiac index (CI) was obtained using the thermodilution method (COM‐2 Cardiac Output Computer; Edwards Lifesciences). CI and pulmonary vascular resistance (PVR) were calculated using standard formulas (PVR = [mean PAP‐PCWP]/Q).
Handgrip Testing
During the preparatory phase of the catheterization procedure all patients were instructed how to perform the handgrip testing. The stress test was performed in accordance to the study of Lind and McNicol.26 After hemodynamic measurements at rest, patients were asked to sustain voluntary handgrip contractions at both arms for 5 minutes with a load of 50 kPa with 60 contractions per minute in supine body position. Cardiac output was measured during the 3rd to 5th minute of the handgrip testing. During the stress test, EIPAH was defined as mean PAP ≥ 30 mm Hg, PCWP < 20 mm Hg, and PVR ≥ 80 dyn*s*cm‐5.10
Statistical Analysis
The data were presented as mean ± standard error of mean. A statistical software package (SPSS version 12.0 for Windows; SPSS, Inc., Chicago, IL) was used for analysis. According to earlier studies,10, 27 patients were assigned to 2 different subgroups: in group A the mean PAP increased ≥35 mm Hg during exercise, whereas in group B the exercise‐induced mean PAP remained <35 mm Hg. Variables of both groups were compared using the Student t test for unpaired probes. Correlation analysis using the Pearson correlation index was performed to compare RHC data with CPET parameters. All results were tested for 2‐sided significance. In general, P values <0.05 were considered to be statistically significant.
Results
Study Population
Sixteen male (30.2%) and 37 female (69.8%) participants were included in the trial. The mean age of the study population was 67.1 ± 1.7 years (Table 1). Fourteen out of 53 patients (26.4%) suffered from connective tissue diseases. Nine study participants (17.0%) had a history of recurrent venous thromboembolism. Five patients (9.4%) were known to have concomitant hematological disorders. Sleep‐disordered breathing was present in 3 patients (5.7%).
Table 1.
Clinical Characteristics, Hemodynamics, and Cardiopulmonary Exercise Parameters of the Study Population
| Characteristics | Value | |
|---|---|---|
| Age, y | 67.1 ± 1.7 | |
| Female/male, n | 37/6 | |
| BMI, kg/qm | 27.1 ± 0.8 | |
| Hemodynamics | At Rest | During Exercise |
| mPAP, mm Hg | 22.1 ± 1.0 | 34.7 ± 1.5 |
| CI, L*min‐1*m‐2 | 2.78 ± 0.11 | 3.47 ± 0.15 |
| PVR, dynes*s*cm‐5 | 209.1 ± 15.4 | 286.1 ± 21.6 |
| mRAP, mm Hg | 5.3 ± 0.4 | 8.2 ± 0.9 |
| PCPW, mm Hg | 9.7 ± 0.7 | 11.9 ± 0.8 |
| SO2, % | 66.8 ± 0.9 | 60.9 ± 1.6 |
| Cardiopulmonary Exercise Testing | Value | |
| W, watts | 74.4 ± 5.7 | |
| W, % | 73.7 ± 4.1 | |
| VO2, mL/min | 1343 ± 76 | |
| VO2, % | 90.5 ± 3.7 | |
| VO2, mL/min/kg | 18.0 ± 0.9 | |
| AT, mL/min/kg | 11.4 ± 0.5 | |
| O2 pulse, mL/min/beat | 12.2 ± 0.6 | |
| Ve, L/min | 51.2 ± 2.5 | |
| Ve/VO2 | 31.8 ± 1.0 | |
| Ve/VCO2 | 37.7 ± 1.0 | |
| AaDO2, mm Hg | 31.5 ± 2.0 | |
| Vd/Vt, % | 24.8 ± 1.6 | |
| a‐etCO2, mm Hg | 1.87 ± 0.6 | |
Abbreviations: AaDO2, alveolar‐arterial oxygen difference at peak exercise; a‐etCO2, arterial‐end‐expiratory carbon dioxide difference at peak exercise; AT, anaerobic threshold; BMI, body mass index; CI, cardiac index; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; O2 pulse, oxygen pulse; PCPW, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; SO2, mixed venous oxygen saturation; Ve, peak minute ventilation; Ve/VCO2, carbon dioxide equivalent at anaerobic threshold; Vd/Vt, functional dead space ventilation at peak exercise; Ve/VO2, oxygen equivalent at anaerobic threshold; VO2, peak oxygen uptake, W, work capacity. Data are expressed as means ± standard error of mean.
Hemodynamics
Values for mean PCWP were normal at rest in all subjects (9.7 ± 0.7 mm Hg). Values for mean PAP were elevated with 22.1 ± 1.0 mm Hg in average. The mean RAP was measured with 5.3 ± 0.4 mm Hg at the upper level of normal. During exercise, mean PAP raised to 34.7 ± 1.5 mm Hg in average, whereas the mean PCWP stayed normal (11.9 ± 0.8 mm Hg). Mean CI increased from 2.78 ± 0.11 to 3.47 ± 0.15 L/min/m2. Consequently, the mean PVR increased from 209.1 ± 15.4 to 286.1 ± 21.6 dynes*s*cm‐5 (Table 1).
Cardiopulmonary Exercise Testing
Most patients showed a reduction in work capacity of 73.7 ± 4.1% of predicted with a VO2 of 18.0 ± 0.9 mL/min/kg, an O2 pulse of 12.2 ± 0.6 mL/min/beat, and slightly elevated Ve/VO2 of 31.8 ± 1.0 and a Ve/VCO2 of 37.7 ± 1.0 at the AT of 11.54 ± 0.5 mL/min/kg. Furthermore, we observed an elevated AaDO2 (31.5 ± 2.0 mm Hg) and a normal Vd/Vt of 24.8 ± 1.6% during peak exercise with an elevated a‐etCO2 gradient of 1.87 ± 0.60 mm Hg (Table 1).
Correlation of Exercise‐Induced Hemodynamics With Parameters of CPET
Mean PAP during exercise significantly correlated with AaDO2 (r = 0.31; P = 0.042), Vd/Vt (r = 0.32; P = 0.032), and with the a‐etCO2 gradient (r = 0.36; P = 0.019). Mean PAP during exercise inversely correlated significantly with VO2 (r = −0.36; P = 0.015). Mean RAP during exercise showed a highly significant negative correlation only with VO2 (r = −0.55; P = 0.005) (Figure 1). Furthermore, our analysis revealed positive correlations of the PVR during exercise with Vd/Vt (r = 0.42; P = 0.004) (Figure 2) and the a‐etCO2 difference (r = 0.37; P = 0.013), as well as negative correlations with work capacity (r = −0.34; P = 0.024) and VO2 (r = −0.37; P = 0.014). SO2 significantly correlated with VO2 (r = 0.39; P = 0.012), and there was a negative correlation with AaDO2 (r = −0.42; P = 0.008) and Vd/Vt (r = −0.36; P = 0.021) (Table 2).
Figure 1.
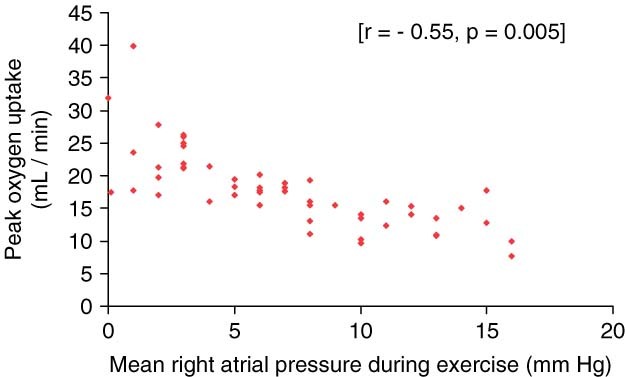
Significant negative correlation between peak oxygen uptake and mean right atrial pressure during exercise.
Figure 2.
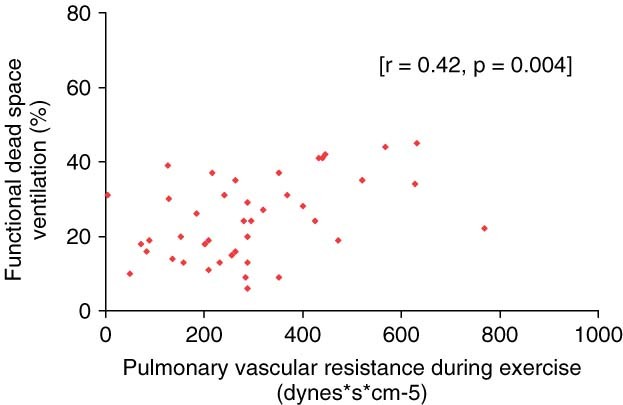
Significant positive correlation between functional dead space ventilation and pulmonary vascular resistance during exercise.
Table 2.
Correlations Between Exercise‐Induced Hemodynamics and Cardiopulmonary Exercise Testing
| mPAP (mm Hg) | PVR (Dynes*s*cm‐5) | mRAP (mm Hg) | SO2 (%) | |
|---|---|---|---|---|
| W, watts | −0.28 (ns) | −0.34a | −0.24 (ns) | 0.31 (ns) |
| VO2, mL/min | −0.36a | −0.37a | −0.55b | 0.39a |
| AT, mL/min/kg | −0.24 (ns) | −0.16 (ns) | −0.40 (ns) | 0.19 (ns) |
| O2 pulse, mL/min/beat | −0.09 (ns) | −0.16 (ns) | 0.04 (ns) | 0.05 (ns) |
| Ve/VO2 | 0.13 (ns) | 0.16 (ns) | −0.04 (ns) | −0.16 (ns) |
| AaDO2, mm Hg | 0.31a | 0.21 (ns) | 0.16 (ns) | −0.42b |
| Vd/Vt, % | 0.32a | 0.42b | 0.17 (ns) | −0.36a |
| a‐etCO2, mmHg | 0.36a | 0.37a | 0.02 (ns) | −0.30 (ns) |
Abbreviations: AaDO2, alveolar‐arterial oxygen difference at peak exercise; a‐etCO2, arterial‐endexpiratory carbon dioxide difference at peak exercise; AT, anaerobic threshold; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; ns, P > 0.05; O2 pulse, oxygen pulse; PVR, pulmonary vascular resistance; SO2, mixed venous oxygen saturation; Vd/Vt, functional dead space ventilation at peak exercise; Ve/VO2, oxygen equivalent at anaerobic threshold; VO2, peak oxygen uptake; W, work capacity.
P < 0.05.
P < 0.01.
Evaluation of Hemodynamics and CPET in Patients With Increasing Mean Pulmonary Artery Pressure During Exercise
Significant differences between group A (n = 24, mean PAP during exercise ≥35 mm Hg) and group B (n = 29, mean PAP during exercise <35 mm Hg) existed in the following CPET parameters: peak VO2 (1175 ± 92 vs 1472 ± 109 mL/min; P = 0.04 and 15.6 ± 1.2 vs 19.7 ± 1.2 mL/min/kg; P = 0.02), Ve/VO2 at the AT (34.3 ± 1.5 vs 29.9 ± 1.1; P = 0.02), AaDO2 during peak exercise (37.8 ± 3.0 vs 26.8 ± 2.4 mm Hg; P = 0.007), Vd/Vt during peak exercise (29.5 ± 2.7 vs 21.2 ± 1.7%; P = 0.008), and the a‐etCO2 gradient (3.7 ± 0.9 vs 0.4 ± 0.8 mm Hg; P = 0.007) (Table 3). The 2 groups did not differ in any anthropometric data or in resting hemodynamics.
Table 3.
Comparison of Hemodynamics and Cardiopulmonary Exercise Testing in Patients With mPAP During Exercise ≥35 mm Hg (Group A) and in Patients With mPAP During Exercise <35 mm hg (Group b)
| Group A | Group B | P | |
|---|---|---|---|
| Hemodynamics | |||
| ΔmPAP, mm Hg | 17.4 ± 1.7 | 8.7 ± 0.6 | ≤0.001 |
| ΔPVR, dynes*s*cm‐5 | 113.7 ± 33.4 | 55.0 ± 11.6 | ns |
| CPET | |||
| W, watts | 62.8 ± 5.2 | 83.2 ± 8.8 | ns |
| W, % | 66.8 ± 5.7 | 78.9 ± 35.6 | ns |
| VO2, mL/min | 1175 ± 92 | 1472 ± 109 | 0.04 |
| VO2, mL/min/kg | 15.6 ± 1.2 | 19.7 ± 1.2 | 0.02 |
| AT, mL/min/kg | 10.8 ± 0.7 | 12.0 ± 0.7 | ns |
| O2 pulse, mL/min/beat | 11.5 ± 1.0 | 12.7 ± 0.8 | ns |
| Ve/VO2 | 34.3 ± 1.5 | 29.9 ± 1.1 | 0.02 |
| AaDO2, mm Hg | 37.8 ± 3.0 | 26.8 ± 2.4 | 0.007 |
| Vd/Vt, % | 29.5 ± 2.7 | 21.2 ± 1.7 | 0.008 |
| a‐etCO2, mm Hg | 3.7 ± 0.9 | 0.4 ± 0.8 | 0.007 |
Data are expressed as means ± SEM. Abbreviations: ΔmPAP, mean pulmonary artery pressure (mPAP) during exercise (mPAP at rest); ΔPVR, pulmonary vascular resistance (PVR) during exercise (PVR at rest); AaDO2, alveolar‐arterial oxygen difference at peak exercise; a‐etCO2, arterial–end‐expiratory carbon dioxide difference at peak exercise; AT, anaerobic threshold; CPET, cardiopulmonary exercise testing; ns, not significant (P < 0.05) O2 pulse, oxygen pulse; Vd/Vt, functional dead space ventilation at peak exercise; Ve/VO2, oxygen equivalent at anaerobic threshold; VO2, peak oxygen uptake; W, work capacity.
Discussion
In this study we demonstrated that EIPAH in patients with “borderline” PAH at rest is associated with significantly reduced exercise capacity and peak VO2 in combination with elevated AaDO2 and a‐eCO2 differences at peak exercise. The exercise‐induced hemodynamics significantly correlated with peak VO2. Furthermore patients with exercise‐induced PAP ≥35 mm Hg were characterized by a diminished functional capacity, higher Ve/VO2 ratios, elevated AaDO2, and a‐etCO2 differences at peak exercise compared to subjects with exercise‐induced PAP <35 mm Hg.
Our data support the notion that EIPAH may represent an early physiological stage of PAH,28 as we were able to demonstrate that CPET parameters observed in EIPAH patients were similar to those formerly described in PAH. Subjects with EIPAH showed a reduced peak VO2 in our study. Peak VO2 has been used as an excellent marker of exercise capacity as it integrates maximal cardiac output, ventilatory capacity and the potential of the exercising muscle to extract O2.20 Peak VO2 reflects the inability of pulmonary blood flow to increase adequately in PAH patients.29 In PAH, PAP and PVR are further increasing during exercise, owing to impaired pulmonary vasodilatation and recruitment of pulmonary vessels.30 As a result maximum exercise capacity is decreased.22 In addition, subjects with EIPAH showed a decline in SO2 during exercise in our study. This decline might indicate increased O2 extraction, likely due to an inadequate cardiac output in response to the peripheral circulatory demands. Our findings are in accordance with the work of Tolle et al who demonstrated that maximum systemic O2 extraction is impaired in PAH and might contribute to the limitation in exercise capacity.31
In agreement with another report, the exercise‐induced mean PAP was negatively correlated with peak VO2 in our study.27 Kovacs and co‐workers demonstrated that elevated mean PAP and PVR during moderate exercise were associated with decreased exercise capacity. Possible explanations are the loss of pulmonary vasodilatatory capacity, reduced distensibility, and inadequate or excessive vasoconstriction.32 In long‐standing PH intimal proliferation or fibrosis, medial hypertrophy and in situ thrombosis characterize the pathological findings in the pulmonary vessels. These changes and the upstream sequelae such as right ventricular dysfunction are time dependent and might result in progressive symptoms and impairment of exercise tolerance.20 Thus, if EIPAH was an early phase of PAH, screening and early detection would potentially prevent progression to resting PAH by early initiation of treatment.
Minute ventilation was significantly greater in patients with a mean PAP increase ≥35 mm Hg compared to patients with a mean PAP increase <35 mm Hg during peak exercise. This was accompanied by greater Ve/VO2 ratios, lower end‐tidal CO2 values, and greater a‐etCO2 gradients. Taken together with the fall in SO2, these findings imply decreased ventilatory efficiency. Several factors may have accounted for this: (1) Lactic acidosis may have developed at a low work rate as a result of an impaired right ventricular output response. This would drive ventilation and reduce the arterial CO2 set‐point. In turn, greater Ve would be necessary to achieve the same CO2 output. (2) Shunting of pulmonary arterial blood may occur, either through a patent foramen ovale or intrapulmonary channels. Such right‐left shunting would result in a fall in the partial pressure of O2 in arterial blood and stimulate the carotid chemoreceptors, resulting in an increased ventilatory drive.33 (3) A true increase in Vd/Vt may occur as a result of excessive hyperventilation caused by an increased stimulation of receptors in the pulmonary vessels that are responsive to blood perfusion.34, 35, 36 In addition, lung tissue may remain underperfused in EIPAH as a result of an impaired recruitment of the capillary bed during exercise.37 Therefore, measurements of the Ve/VO2 ratio and the a‐etCO2 gradient during exercise may be useful in identifying individuals at risk of developing PH.16
We acknowledge that our study has limitations. In our trial, subjects underwent a resistive rather than a dynamic type of exercise testing. Isometric handgrip testing may have affected systemic blood pressure and may have resulted in an increase in intrathoracic pressure, which per se might have increased PAP due to increased extravascular resistive forces or additional pulmonary vasoconstriction. As a consequence, the percentage of patients with EIPAH might have been overestimated in our cohort.
Conclusion
Despite these limitations, we conclude that CPET might be useful in evaluating patients with suspected EIPAH. The clinical significance of a hypertensive PAP response to exercise is currently unsettled, but EIPAH should remain a strongly observed clinical condition. Further studies are mandatory to clarify whether EIPAH reflects an enhanced risk for the development of PAH.
References
- 1. Gruenig E, Weissmann S, Ehlken N, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension. Circulation. 2009;119:1747–1757. [DOI] [PubMed] [Google Scholar]
- 2. Runo J, Loyd J. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. [DOI] [PubMed] [Google Scholar]
- 3. Markowitz D, Systrom D. Diagnosis of pulmonary vascular limit to exercise by cardiopulmonary exercise testing. J Heart Lung Transplant. 2004;23:88–95. [DOI] [PubMed] [Google Scholar]
- 4. Bossone E, Bodini B, Mazza A, et al. Pulmonary arterial hypertension: the key role of echocardiography. Chest. 2005;127:1836–1843. [DOI] [PubMed] [Google Scholar]
- 5. Gruenig E, Janssen B, Mereles D, et al. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation. 2000;102:1145–1150. [DOI] [PubMed] [Google Scholar]
- 6. McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence‐based clinical practice guidelines. Chest. 2004;126:14S–34S. [DOI] [PubMed] [Google Scholar]
- 7. Huez S, Naeije R. Exercise stress tests for detection and evaluation of pulmonary hypertension. Eur Heart J. 2007;9:H17–H21. [DOI] [PubMed] [Google Scholar]
- 8. Kiencke S, Bernheim A, Maggiorini M, et al. Exercise induced pulmonary arterial hypertension: a rare finding. J Am Coll Cardiol. 2008;29:513–518. [DOI] [PubMed] [Google Scholar]
- 9. Kovacs G, Berghold A, Scheidl S, et al. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. [DOI] [PubMed] [Google Scholar]
- 10. Tolle J, Waxman A, van Horn T, et al. Exercise‐induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vachiery J, Pavelescu A. Exercise echocardiography in pulmonary hypertension. Eur Heart J. 2007;9:H48–H53. [Google Scholar]
- 12. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107:216–223. [DOI] [PubMed] [Google Scholar]
- 13. Proudman S, Stevens W, Sahhar J, et al. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37:485–494. [DOI] [PubMed] [Google Scholar]
- 14. Raeside D, Chalmers G, Clelland J, et al. Pulmonary artery pressure variation in patients with connective tissue disease: 24 hour ambulatory pulmonary artery pressure monitoring. Thorax. 1998;53:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James K, Maurer J, Wolski K, et al. Exercise hemodynamic findings in patients with exertional dyspnea. Tex Heart Inst J. 2000;27:100–105. [PMC free article] [PubMed] [Google Scholar]
- 16. Raeside D, Smith A, Brown A, et al. Pulmonary artery pressure measurement during exercise testing in patients with suspected pulmonary hypertension. Eur Respir J. 2000;16:282–287. [DOI] [PubMed] [Google Scholar]
- 17. Riley M, Porszasz J, Engelen M, et al. Gas exchange responses to continuous incremental cycle ergometry exercise in primary pulmonary hypertension in humans. Eur J Appl Physiol. 2000;83:63–70. [DOI] [PubMed] [Google Scholar]
- 18. Alkotob L, Soltani P, Sheatt M, et al. Reduced exercise capacity and stress‐induced pulmonary hypertension in patients with scleroderma. Chest. 2006;130:176–181. [DOI] [PubMed] [Google Scholar]
- 19. Raeside D, Brown A, Patel K, et al. Ambulatory pulmonary artery pressure monitoring during sleep and exercise in normal individuals and patients with COPD. Thorax. 2002;57:1050–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun X, Hansen J, Oudiz R, et al. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104:429–435. [DOI] [PubMed] [Google Scholar]
- 21. Oudiz R, Barst R, Hansen J, et al. Cardiopulmonary exercise testing and six‐minute walk correlations in pulmonary arterial hypertension. Am J Cardiol. 2006;997:123–126. [DOI] [PubMed] [Google Scholar]
- 22. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlated and prognostic significance of six‐minute walk test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–492. [DOI] [PubMed] [Google Scholar]
- 23. Wensel R, Opitz C, Anker S, et al. Assessment of survival in patients with primary pulmonary hypertension. Importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–324. [DOI] [PubMed] [Google Scholar]
- 24. ESC/ERS Guidelines. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–2537. [DOI] [PubMed] [Google Scholar]
- 25. Wasserman K, Hansen J, Sue D, et al. Principles of Exercise Testing and Interpretation. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2004.. [Google Scholar]
- 26. Lind A, McNicol G. Circulatory responses to sustained hand‐grip contractions performed during other exercise, both rhythmic and static. J Physiol. 1967;192:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kovacs G, Maier R, Aberer E, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180:881–886. [DOI] [PubMed] [Google Scholar]
- 28. Steen V, Shanmugam V, Mathias M, et al. Exercise‐induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest. 2008;134:146–151. [DOI] [PubMed] [Google Scholar]
- 29. Riley M, Porszasz J, Engelen M, et al. Responses to constant work rate bicycle ergometry exercise in primary pulmonary hypertension: the effect of inhaled nitric oxide. J Am Coll Cardiol. 2000;36:547–556. [DOI] [PubMed] [Google Scholar]
- 30. Blumberg F, Riegger C, Pfeifer M. Hemodynamic effects of aerosolized iloprost in pulmonary hypertension at rest and during exercise. Chest. 2002;121:1566–1571. [DOI] [PubMed] [Google Scholar]
- 31. Tolle J, Waxman A, Systrom D. Impaired systemic oxygen extraction at maximum exercise in pulmonary hypertension. Med Sci Sports Exerc. 2008;40:3–8. [DOI] [PubMed] [Google Scholar]
- 32. Reeves J, Linehan J, Stenmark K. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:419–425. [DOI] [PubMed] [Google Scholar]
- 33. Yasunobu Y, Oudiz R, Sun X, et al. End‐tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127:1637–1646. [DOI] [PubMed] [Google Scholar]
- 34. Sun X, Hansen J, Oudiz R. Gas exchange detection of exercise‐induced right‐to‐left shunt in patients with primary pulmonary hypertension. Circulation. 2002;105:54–60. [DOI] [PubMed] [Google Scholar]
- 35. Holverda S, Gan C, Marcus J. Impaired stroke volume response to exercise in pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:1732–1733. [DOI] [PubMed] [Google Scholar]
- 36. Velez‐Roa S, Ciarka A, Najem B. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 37. Nootens M, Wolfkiel C, Chomka E, et al. Understanding right and left ventricular systolic function and interactions at rest and with exercise in primary pulmonary hypertension. Am J Cardiol. 1995;75:374–375. [DOI] [PubMed] [Google Scholar]


