Abstract
Background:
Arterial stiffness parameters are commonly used to determine the development of atherosclerotic disease. The independent predictive value of aortic stiffness has been demonstrated for coronary events.
Hypothesis:
The aim of our study was to compare regional and local arterial functional parameters measured by 2 different noninvasive methods in patients with verified coronary artery disease (CAD). We also compared and contrasted these stiffness parameters to the coronary SYNTAX score in patients who had undergone coronary angiography.
Methods:
In this study, 125 CAD patients were involved, and similar noninvasive measurements were performed on 125 healthy subjects. The regional velocity of the aortic pulse wave (PWVao) was measured by a novel oscillometric device, and the common carotid artery was studied by a Doppler echo‐tracking system to determine the local carotid pulse wave velocity (PWVcar). The augmentation index (AIx), which varies proportionately with the resistance of the small arteries, was recorded simultaneously.
Results:
In the CAD group, the PWVao and aortic augmentation index (Alxao) values increased significantly (10.1 ± 2.3 m/sec and 34.2% ± 14.6%) compared to the control group (9.6 ± 1.5 m/sec and 30.9% ± 12%; P < 0.05). We observed similar significant increases in the local stiffness parameters (PWVcar and carotid augmentation index [Alxcar]) in patients with verified CAD. Further, we found a strong correlation for PWV and AIx values that were measured with the Arteriograph and those obtained using the echo‐tracking method (r = 0.57, P < 0.001 for PWV; and r = 0.65, P < 0.001 for AIx values).
Conclusions:
Our results indicate that local and regional arterial stiffness parameters provide similar information on impaired arterial stiffening in patients with verified CAD. © 2011 Wiley Periodicals, Inc.
This study was supported by the Hungarian National Research Foundation (OTKA) No.78480. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
A large volume of evidence is now available on the crucial role of preclinical organ damage in determining cardiovascular (CV) risk in individuals. Arterial stiffness parameters are commonly used for this purpose—to identify structural and functional changes in the arteries in the development of atherosclerotic disease.1, 2 The independent predictive value of aortic stiffness has been demonstrated for fatal stroke, all‐cause and CV mortalities, fatal and nonfatal coronary events in hypertensive, diabetic, end‐stage renal disease, and in elderly patients and in the general population.3, 4, 5, 6 Moreover, carotid‐femoral pulse wave velocity (PWV), a direct measure of aortic stiffness, has become increasingly important for total CV risk estimation, but its application as a routine tool for clinical patient evaluation has been hampered by the absence of reference values, and so the Reference Values for Arterial Stiffness Collaboration Group established reference and normal values for PWV based on a large European population.7
Among stiffness parameters, the aortic PWV and the wave reflection, measured as augmentation index (AIx), were determined. PWV is accepted as the most simple and reproducible method to assess arterial stiffness.1, 8 Recent reports suggested that, in patients with hypertension or diabetes mellitus, the aorta stiffened more than the carotid artery with age and other CV risk factors.9 It also has been proved that the relationship with age and sex of regional aortic stiffness and local carotid stiffness measures are not the same in the age range of 35 to 55 years in healthy subjects.10
New techniques allow us to investigate regional aortic stiffness and local carotid stiffness simultaneously. Carotid stiffness may be of particular interest because in that artery, atherosclerosis is frequent, especially in patients with verified coronary artery disease (CAD). Therefore, new Doppler echo‐tracking techniques were used in most pathophysiologic, pharmacologic and clinical studies to determine local carotid stiffness.11, 12 One important issue is how PWV or arterial stiffness parameters are measured feasibly and routinely in clinical practice, because different methods exist (Complior, Artech Medical, Pantin, France, Sphygmocor, AtCor Medical Inc., Sydney, Australia, Arteriograph [TensioMed, Budapest, Hungary], ultrasound devices), and it is equally important to clarify the importance of regional and local stiffness parameters in patients with different types of atherosclerotic disease.8, 13
The aim of this study, therefore, was to compare regional aortic stiffness and local carotid stiffness parameters measured by 2 different noninvasive methods in patients with verified CAD. Further, we compared and contrasted noninvasive stiffness parameters to the coronary SYNTAX score in patients who had undergone coronary angiography.
Methods
Patients
We studied a total of 250 subjects, age 40 to 84 years. Exclusion criteria were arrhythmia, valvular heart disorders, and heart failure (New York Heart Association criteria III–IV). We performed elective coronary angiography in 125 consecutive patients who were referred to the Department of Invasive Cardiology of our hospital. All patients had previous concordant noninvasive findings for CAD and had experienced angina pectoris. Determining arterial stiffness parameters was undertaken for 125 CAD patients (mean age, 62 ± 10 years) and 125 age‐ and sex‐matched and apparently healthy control subjects. All patients in the CAD group received appropriate medical treatment (β‐blockers, aspirin, statins, and angiotensin‐converting enzyme inhibitors) according to the relevant guidelines.7, 14 The patients' characteristics are shown in the Table 1. The protocol of this clinical study was reviewed and approved by the local institutional ethics committee. Written informed consent was obtained from all patients who participated in the study. The investigation conforms to the principles outlined in the Declaration of Helsinki.
Table 1.
Characteristics of the Patients With Verified Coronary Artery Disease and Healthy ControlSubjects
| Variable | Control Group (n = 125) | CAD Group (n = 125) | P Value |
|---|---|---|---|
| Age, y | 62 ± 10 | 62 ± 10 | – |
| Male, n (%) | 97 (78) | 97 (78) | – |
| Weight, kg | 82.1 ± 15.3 | 84.4 ± 15.2 | 0.020 |
| Height, cm | 171 ± 9 | 170 ± 8 | 0.379 |
| BMI, kg/m2 | 28.2 ± 4.4 | 29.3 ± 4.3 | <0.01 |
| SBP, mm Hg | 139 ± 18 | 135 ± 21 | 0.056 |
| DBP, mm Hg | 83 ± 10 | 80 ± 14 | 0.034 |
| MAP, mm Hg | 101 ± 12 | 98 ± 15 | 0.029 |
| HR, beat/min | 73 ± 12 | 70 ± 12 | <0.01 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure.
Data are presented as mean ± standard deviation.
Noninvasive measurements were taken by the same investigator in a temperature‐controlled room (22°C), unaffected by external environmental influences in accordance with the international guidelines.13 All measurements were done simultaneously using a carotid color Doppler echo‐tracking system (Aloka SSD‐5500, Tokyo, Japan) and oscillometric occlusive equipment (Arteriograph, TensioMed, Budapest; Hungary).
Carotid Stiffness (Echo Tracking)
The Aloka color Doppler system with a 7.5‐MHz linear array probe and an echo‐tracking subsystem was used for recording the wave‐intensity data. The data were updated with a frequency of 1 kHz, and the steering angle of the ultrasound beam never exceeded ±20 degrees for any recording. Blood pressure was simultaneously measured with a cuff‐type manometer applied to the upper arm as a required input to the Aloka system. The maximal and minimal values of changes in diameter of the artery were calibrated by systolic and diastolic blood pressure. The wave intensity was averaged over a minimum of 4 heartbeats at the same site in the artery. The relationship between pressure and diameter waveforms in human carotid artery was previously confirmed to be relatively linear throughout the cardiac cycle (goodness of fit r 2 ≥ 0.97).15, 16, 17 The diameter and wall motion of the right common carotid artery were measured 2 cm below the carotid bifurcation. This Doppler echo‐tracking system allows the determination of local carotid PWV (PWVcar) and AIx (AIxcar) data using online 1‐point measurements.18, 19, 20, 21
Aortic Stiffness (Arteriograph)
The simultaneous measurements of aortic AIx (AIxao), aortic PWV (PWVao), and brachial blood pressure were performed within 3 to 4 minutes with the oscillometric, occlusive device, the Arteriograph. This invasively validated method is based on the complete occlusion of the brachial artery by a simple cuff, which allows the recording and separation of pronounced early (forward) and late (reflected) systolic waves.22 The time elapsed between the early and late systolic wave peaks equals the travel time of the forward aortic pulse wave to the bifurcation and its backward reflection to the observational site. The sternal notch/pubic bone distance (an acceptable estimate of the aortic length and close to the magnetic resonance imaging–determined arterial path length) was used to determine the PWVao.23 The augmentation index (AIxao) was calculated taking the differences between amplitudes of the forward and reflected systolic waves; the resulting value was divided by the pulse pressure and finally multiplied by 100. The measurements were taken in a supine position and were accepted if the quality indicator of the recordings was within the acceptable range (i.e., if the standard deviation [SD] of the beat‐to‐beat measured PWVao values was less than 1.1 m/sec).
Diagnosis of CAD and Calculation of the SYNTAX Score
In the present study, CAD was verified by coronary angiography. All patients underwent routine coronary angiography (using the Judkins technique) on digitized coronary angiography equipment (Integris; Philips Medical Systems, Best, The Netherlands). Coronary angiograms were computerized and assessed by 3 experienced angiographers who were blinded to the results of arterial stiffness measurements. For this study, we defined significant CAD as showing at least 50% or greater stenosis or at least 75% or greater flow reduction in 1 coronary artery. All patients in the CAD group received appropriate medical treatment (β‐blockers, aspirin, statins, and angiotensin‐converting enzyme inhibitors) according to the relevant guidelines.13
The SYNTAX score was calculated with an interactive question‐based computer program. The algorithm consists of 12 main questions referring to the coronary anatomy and total number and extent of coronary artery lesions. In our study, the SYNTAX score was calculated for each coronary lesion producing a ≥50% luminal obstruction in vessels with a diameter of 1.5 mm or more. Patients were randomized according to 2‐year rates of major adverse coronary events as low (0–22), intermediate (23–32), and high (≥33) SYNTAX score groups. In our study, 64 patients were in low, 18 were in intermediate, and 43 were in high SYNTAX score groups.24, 25 After these individual lesions were scored, the total SYNTAX score was determined and correlated to regional and local arterial stiffness parameters.
Data Analysis
The values were expressed as mean ± SD. We used simple regression to evaluate linear association between aortic and carotid stiffness parameters. The correlation coefficient was defined as r according to Spearman. Differences between control subjects and subjects with CAD were tested with the 2‐tailed t test. P < 0.05 was taken as the level of statistical significance.
Results
Control patients were examined at the outpatient department of our hospital. All control patients were referred by their general practitioner for regular CV screening checkup without any complaints or medication. The characteristics of the CAD patients and control subjects are summarized in the Table. As the Table shows, CAD patients proved to be overweight compared to the control group (BMI, 29 ± 4.3 kg/m2 vs 28.1 ± 4.4 kg/m2 for CAD and control patients, P < 0.01). In patients with CAD, most patients reached the target systolic and diastolic blood pressure (130/80 mm Hg), and so these patients exhibited lower systolic and diastolic blood pressure values compared to the control group. The most plausible reason for the lower blood pressure in the CAD group could be the active and adequate blood pressure–lowering therapy in the CAD group; in the control group, asymptomatic, apparently healthy subjects were included, without known CV disease and having no medical treatment. We also found that heart‐rate values decreased significantly in CAD patients because of β‐receptor blocker treatment (70 ± 12 beat/min vs 73 ± 12 beat/min for CAD and control group, respectively).
Comparative measurements of regional arterial stiffness parameters were taken in 125 CAD patients and 125 age‐ and sex‐matched (apparently healthy) control subjects. Figure 1A illustrates the results of regional PWVao, which was measured by the occlusive oscillometric method. We found a significant increase in regional PWVao for the CAD patients compared to the control subjects (10.1 ± 2.3 m/sec vs 9.6 ± 1.5 m/sec; P = 0.019). Similarly, significant differences were observed between the 2 groups when AIxao values were compared (34.2% ± 14.6% vs 30.9% ± 12% for the CAD and control groups, P = 0.05) (Figure 1B).
Figure 1.
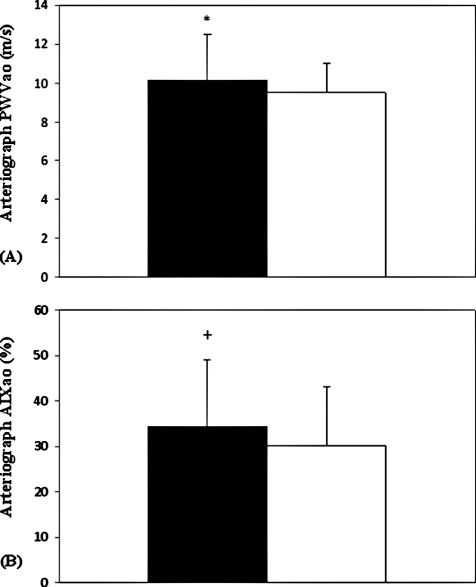
(A) Comparison of regional (aortic) pulse wave velocity (PWVao) in patients with verified coronary artery disease (CAD) with age‐ and sex‐matched apparently healthy control subjects. (B) Comparison of regional (aortic) augmentation index (AIxao) in patients with verified CAD with age‐ and sex‐matched apparently healthy control subjects. These measurements were carried out with occlusive, oscillometric device (Arteriograph). Data are presented as mean ± standard deviation.  = CAD group (n = 125).
= CAD group (n = 125).  = control group (n = 125). *
P < 0.05; +P = 0.05.
= control group (n = 125). *
P < 0.05; +P = 0.05.
In 35 of the 125 CAD patients, simultaneous measurements were taken by the carotid echo‐tracking method to determine local arterial stiffness parameters. We found a significant increase of the local PWVcar for the CAD patients compared to the control subjects (7.4 ± 1.3 m/sec vs 6.5 ± 1.1 m/sec; P < 0.01) (Figure 2A). Further, the CAD patients exhibited elevated AIxcar values compared to the control group. (19.4% ± 10.7% vs 5.1% ± 9.8%) for CAD and control group, P < 0.01; (Figure 2B). Figure 3 shows the correlation between regional (aortic) and local (carotid) arterial stiffness parameters in patients with verified CAD. In regard to PWV, we found a significant positive correlation between PWVao values that were measured by Arteriograph and PWVcar values that were determined by echo‐tracking method (r = 0.57, P < 0.001) (Figure 3A). Similar correlations were observed between regional (AIxao) and local (AIxcar) AIx values (Figure 3B) (r = 0.65, P < 0.001).
Figure 2.
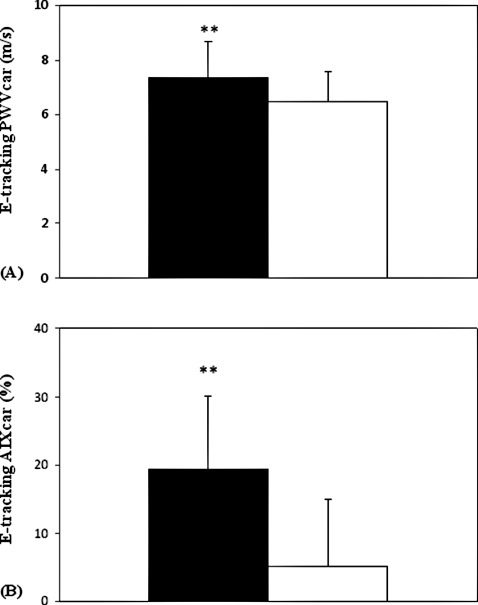
(A) Comparison of local (carotid) pulse wave velocity (PWVcar) in patients with verified coronary artery disease (CAD) with age‐ and sex‐matched apparently healthy control subjects. (B) Comparison of local (carotid) augmentation index (AIxcar) in patients with verified CAD with age and sex‐matched apparently healthy control subjects. These measurements were carried out with Doppler echo‐tracking method. Data are presented as mean ± standard deviation.  = CAD group (n = 35).
= CAD group (n = 35).  = control group (n = 35). **
P < 0.01.
= control group (n = 35). **
P < 0.01.
Figure 3.
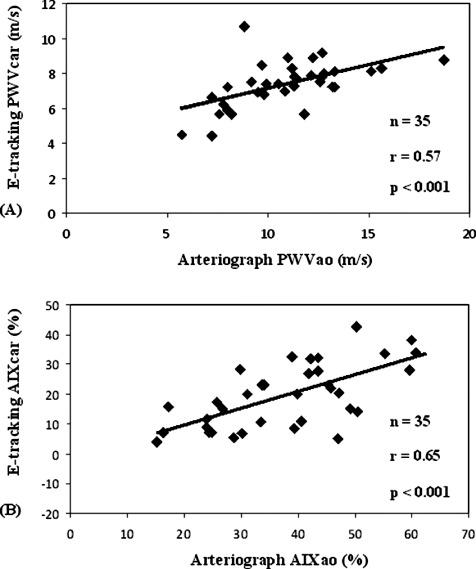
(A) Relation between regional (aortic) and local (carotid) pulse wave velocity (PWV) parameters in patient with verified coronary artery disease (CAD). The aortic PWV (PWVao) was measured by the occlusive, oscillometric device (Arteriograph). The carotid PWV (PWVcar) was determined by the Doppler echo‐tracking (e‐tracking) method. (B) Relation between regional (aortic) and local (carotid) augmentation index (AIx) parameters in patient with verified CAD. The aortic AIx (AIxao) was measured by the occlusive, oscillometric device (Arteriograph). The carotid AIx (AIxcar) was determined by the Doppler e‐tracking method.
In patients with verified CAD, the individual coronary lesions were scored, and the total SYNTAX score was correlated to the regional (PWVao and AIxao) and local (PWVcar and AIxcar) arterial stiffness parameters. We did not find any significant correlation between the SYNTAX score and regional arterial stiffness parameters (Figure 4, panel A, B). Similarly, the coronary SYNTAX score did not correlate with the carotid stiffness parameters (PWVcar and AIxcar; data not shown).
Figure 4.
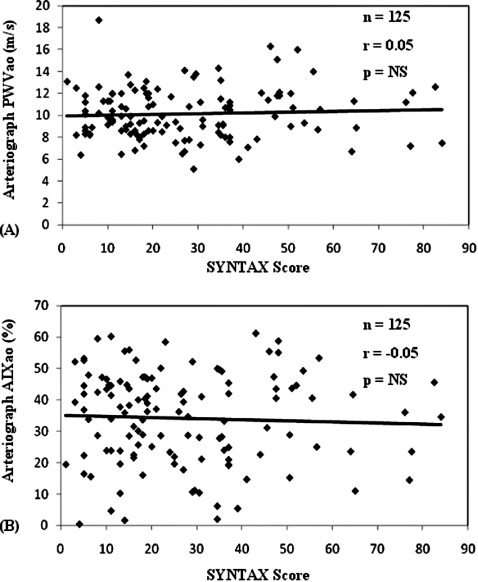
Relation between (A) coronary SYNTAX score and aortic pulse wave velocity (PWVao) and (B) aortic augmentation index (AIxao) in patients with verified coronary artery disease who underwent coronary angiography. NS = nonsignificant.
Discussion
In this study, we first aimed to evaluate the correlation between regional (aortic) and local (carotid) arterial stiffness in patients with verified CAD. We also contrasted arterial stiffness parameters that were obtained by 2 different noninvasive methods to the coronary SYNTAX score in patients who had undergone coronary angiography.
Lekakis et al applied the modified Gensini score to investigate whether arterial wave reflection may detect atherosclerosis of peripheral arteries in patients with documented CAD.26, 27 Radial artery applanation tonometry and pulse wave analysis was performed in 184 patients with documented CAD at coronary angiography; central blood pressures and AIx were measured. Although AIx is a marker of extensive extracoronary atherosclerosis in patients with CAD, in their study no relation was found between AIx and Gensini score or for the number of diseased coronary vessels.4 These observations are concordant with our findings with respect of aortic PWV and AIx, although in our study the coronary SYNTAX score was applied for grading of CAD.14, 24, 25
Increased arterial stiffness is one of the key factors associated with CV disease.28, 29 There are only a few studies reporting a comparison in the association of CV disease with stiffness of different arterial segments. Pannier et al30 measured PWV of the aorta and arteries in the upper and lower extremities. They found that only PWV of the aorta significantly predicted death from CV disease in 305 hemodialysis patients. The results of the Rotterdam Study indicated that aortic PWV predicted the occurrence of CAD and stroke, but carotid distensibility did not; thus, aortic stiffness may have more important roles in stroke than stiffness of other arterial segments.6
Paini et al found that carotid‐femoral PWV and carotid stiffness provided similar information on the impact of aging on large‐artery stiffness in normal subjects; this was not the case for high blood pressure and diabetes. In these cases, the aorta stiffened more than carotid artery with age and other CV risk factors.9
In the Second Manifestations of Arterial Disease (SMART) study, the authors investigated whether carotid stiffness is related to risk of new vascular events in patients with manifest arterial disease. In this large‐scale cohort study, common carotid distension was measured at baseline by ultrasonography. The major finding was that carotid artery stiffness is not an independent risk factor for vascular events in patients with manifest arterial disease. However, in patients with low systolic blood pressure, decreased carotid stiffness may indicate a decreased risk of vascular events.31
The principal contribution of the present study is that we found a significant increase of both aortic and carotid PWV and AIx values determined by occlusive oscillometric device and carotid echo‐tracking method in patients with verified CAD. These observations clearly indicated that PWVao and PWVcar provide similar information on impaired arterial stiffening in CAD patients. A further important observation of our clinical study is that there is a strong correlation between aortic and carotid stiffness parameters measured by 2 different noninvasive methods.
In our study, we demonstrated that in patients with clinical organ damage, AIx significantly increased, clearly indicating an impaired arterial function. These results are in good correspondence with earlier findings: central AIx proved to be an independent predictor of mortality in hypertension, in end‐stage renal disease, and in patients undergoing percutaneous coronary intervention.11, 12 Our measurements also indicate a strong correlation of AIx data simultaneously conducted through oscillometric and echo‐tracking techniques. There are numerous articles in the literature that can prove the relationship of carotid intima‐media thickness and PWV in patients with different types of atherosclerosis. In addition, Matsushima et al found a relationship of carotid intima‐media thickness, PWV, and ankle brachial index to the severity of coronary artery atherosclerosis.32 In our study, we applied the Doppler echo‐tracking system to determine local carotid PWVcar and AIxcar. This novel method provides valuable data about local arterial stiffness, which are different from those parameters that we can obtain from the intima‐media thickness measurements.
Although the local carotid and central aortic stiffness parameters correlated significantly, they are not identical for several reasons. Concerning the PWV, the interchangeability does not stand because both vessels are basically different in their characteristics. However, not only morphologic differences cause the lack of interchangeability between local (carotid) and regional stiffness. Until today, only the aortic PWV proved to be an independent predictor of hard outcomes. Consequently, as far as the local carotid PWV is concerned, this parameter cannot be regarded as a more suitable parameter, as compared to central aortic PWV.33
In contrast, we did not find any significant correlation when local and systemic arterial stiffness parameters were compared to the coronary SYNTAX score in patients who underwent coronary angiography. From this observation we concluded that although the increase of local and regional stiffness parameters correlated significantly with the impaired arterial function in patients with CAD, stiffness parameters cannot provide any information about the arterial damage in the coronary vessels. Furthermore, arterial stiffness represents the function of the inner lining of the endothelium and therefore is very hard to correlate to the severity and extent of the coronary heart disease.
In the present study, we found a strong correlation between the stiffness parameters measured with the Arteriograph and those obtained using the echo‐tracking method. Accordingly, previous studies demonstrated that these stiffness parameters closely match those values measured with other, more sophisticated technologies.34 Our findings encourage the implementation of regional and local arterial stiffness and function measurements in daily clinical routine in patients suspected of having CAD.
References
- 1. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 2. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 4. Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 5. Blacher J, London G, Safar B, et al. Influence of age and end‐stage renal disease on the stiffness of carotid wall material in hypertension. J Hypertens. 1999;17:237–244. [DOI] [PubMed] [Google Scholar]
- 6. Mattace‐Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 7. Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancia G, de Backer G, Dominiczak A, et al. 2007. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 9. Paini A, Boutouyrie P, Calvet D, et al. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006;47: 371–376. [DOI] [PubMed] [Google Scholar]
- 10. Vermeersch SJ, Rietzschel ER, De Bruyere ML, et al. Age and gender related patterns in carotid‐femoral PWV and carotid and femoral stiffness in a large healthy, middle‐age population. J Hypertens. 2008;26:1408–1416. [DOI] [PubMed] [Google Scholar]
- 11. London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end‐stage renal failure. Hypertension. 2001;38:434–438. [DOI] [PubMed] [Google Scholar]
- 12. Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. [DOI] [PubMed] [Google Scholar]
- 13. Van Bortel LM, Duprez D, Starmans‐Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–452. [DOI] [PubMed] [Google Scholar]
- 14. European Association for Percutaneous Cardiovascular Interventions , Wijns W, Kolh P, et al. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501–2555. [DOI] [PubMed] [Google Scholar]
- 15. Steinvil A, Sadeh B, Arbel Y, et al. Prevalence and predictors of concomitant carotid and coronary artery atherosclerotic disease. J Am Coll Cardiol. 2011;57:779–783. [DOI] [PubMed] [Google Scholar]
- 16. Evans MR, Escalante A, Battafarano DF. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Am Coll Rheum. 2011;63:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freitas WM, Quaglia LA, Santos SN, et al. Association of systemic inflammatory activity with coronary and carotid atherosclerosis in the very elderly. Atherosclerosis. 2011;216:212–216. [DOI] [PubMed] [Google Scholar]
- 18. Niki K, Sugawara M, Chang D, et al. A new noninvasive measurement system for wave intensity: evaluation of carotid arterial wave intensity and reproducibility. Heart Vessels. 2002;17:12–21. [DOI] [PubMed] [Google Scholar]
- 19. Sugawara M, Niki K, Furuhata H, et al. Relationship between the pressure and diameter of the carotid artery in humans. Heart Vessels. 2000;15:49–51. [DOI] [PubMed] [Google Scholar]
- 20. Harada A, Okada T, Niki K, et al. On‐line noninvasive one‐point measurements of pulse wave velocity. Heart Vessels. 2002;17:61–68. [DOI] [PubMed] [Google Scholar]
- 21. Antonini‐Canterin F, Rosca M, Beladan CC, et al. Echo‐tracking assessment of carotid artery stiffness in patients with aortic valve stenosis. Echocardiography. 2009;26:823–831. [DOI] [PubMed] [Google Scholar]
- 22. Horvath IG, Nemeth A, Lenkey Z, et al. Invasive validation of a new oscillometric device (arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–2075. [DOI] [PubMed] [Google Scholar]
- 23. Sugawara J, Hayashi K, Yokoi T, et al. Age‐associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–748. [DOI] [PubMed] [Google Scholar]
- 24. van Gaal WJ, Ponnuthurai FA, Selvanayagam J, et al. The Syntax score predicts peri‐procedural myocardial necrosis during percutaneous coronary intervention. Int J Cardiol. 2009;135:60–65. [DOI] [PubMed] [Google Scholar]
- 25. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 26. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. [DOI] [PubMed] [Google Scholar]
- 27. Lekakis JP, Ikonomidis I, Protogerou AD, et al. Arterial wave reflection is associated with severity of extracoronary atherosclerosis in patients with coronary artery disease. Eur J Cardiovasc Prev Rehab. 2006;13:236–242. [DOI] [PubMed] [Google Scholar]
- 28. Kingwell BA, Waddell TK, Medley TL, et al. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:773–779. [DOI] [PubMed] [Google Scholar]
- 29. Hatsuda S, Shoji T, Shinohara K, et al. Regional arterial stiffness associated with ischemic heart disease in type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13:114–121. [DOI] [PubMed] [Google Scholar]
- 30. Pannier B, Guerin AP, Marchais SJ, et al. Stiffness of capacitive and conduit arteries: prognostic significance for end‐stage renal disease patients. Hypertension. 2005;45:592–596. [DOI] [PubMed] [Google Scholar]
- 31. Dijk JM, Algra A, van der Graaf Y, et al. Carotid stiffness and the risk of new vascular events in patients with manifest cardiovascular disease: the SMART study. Eur Heart J. 2005;26:1213–1220. [DOI] [PubMed] [Google Scholar]
- 32. Matsushima Y, Kawano H, Koide Y, et al. Relationship of carotid intima‐media thickness, pulse wave velocity, and ankle brachial index to the severity of coronary artery atherosclerosis. Clin Cardiol. 2004;27:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Rourke MF, Kelly RP, Avolio AP. What is the pulse? In: The Arterial Pulse. 17th ed. Philadelphia, PA: Lea & Febiger, 1992.. [Google Scholar]
- 34. Boutouyrie P, Revera M, Parati G. Obtaining arterial stiffness indices from simple arm cuff measurements: the holy grail? J Hypertens. 2009;27:2159–2161. [DOI] [PubMed] [Google Scholar]


