Abstract
Background:
Clinical outcomes of percutaneous coronary intervention (PCI) in patients with saphenous vein grafts (SVGs) remain poor despite the use of drug‐eluting stents (DES). There is a disparity in clinical outcomes in SVG PCI based on various registries, and randomized clinical data remain scant. We conducted a meta‐analysis of all existing randomized controlled trials (RCTS) comparing bare‐metal stents (BMS) and DES in SVGPCIs.
Hypothesis:
PCI in patients with SVG disease using DES may reduce need for repeat revascularization without an excess mortality when compared to BMS.
Methods:
An aggregate data meta‐analysis of clinical outcomes in RCTs comparing PCI with DES vs BMS for SVGs reporting at least 12 months of follow‐up was performed. A literature search between Janurary 1, 2003 and September 30, 2011 identified 4 RCTs (812 patients; DES = 416, BMS = 396). Summary odds ratio (OR) and 95% confidence interval (CI) were calculated using the random‐effects model. The primary endpoint was all‐cause mortality. Secondary outcomes included nonfatal myocardial infarction (MI), repeat revascularization, and major adverse cardiac events (MACE). These outcomes were assessed in a cumulative fashion at 30 days, 18 months, and 36 months.
Results:
There were no intergroup differences in baseline clinical and sociodemographic characteristics. At a median follow‐up of 25 months, patients in the DES and BMS group had similar rates of death (OR: 1.63, 95% CI: 0.45–5.92), MI (OR; 0.83, 95% CI: 0.27‐2.60), and MACE (OR: 0.58, 95% CI: 0.25–1.32). Patients treated with DES had lower rates of repeat revascularization (OR: 0.40, 95% CI: 0.22–0.75).
Conclusions:
In this comprehensive meta‐analysis of all RCTs comparing clinical outcomes of PCI using DES vs BMS in patients with SVG disease, use of DES was associated with a reduction in rate of repeat revascularization and no difference in rates of all‐cause death and MI. Clin. Cardiol. 2012 DOI: 10.1002/clc.21984
Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development Service (HSR&D) Career Development Award (CDA‐09‐028), and has research support from Merck and National Football League Charities (all grants to the institution and not individual). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Saphenous venous graft (SVG) interventions account for 6% to 15% of all percutaneous coronary interventions (PCI).1, 2, 3, 4 A review of the American College of Cardiology National Cardiovascular Data Cath PCI Registry found 5.7% of patients who underwent PCI between January 1, 2004 and March 1, 2009 had SVG intervention.4 Practice guidelines are largely based on extrapolation of outcomes from observational studies and a few randomized controlled trials (RCTs). Due to the disparity in clinical outcomes of SVG PCI, actual clinical practice is based on expert opinion for the most part. A recent meta‐analysis of 2 RCTs, a subgroup of an RCT and 26 observational studies comprising a total of 7994 patients, showed that SVG interventions with drug eluting stents (DES) reduced the major adverse cardiac events (MACE) and target vessel revascularization (TVR) rate.2 Several other authors have reported meta‐analyses evaluating the clinical outcomes of PCI in SVG comparing DES and bare‐metal stents (BMS).2, 5, 6, 7, 8, 9, 10, 11, 12 These meta‐analyses were limited by most of their evidence arising from observational studies and the inclusion of 2 small RCTs.13, 14, 15, 16 Late follow‐up of 1 small RCT showed increased all‐cause mortality in patients who underwent PCI with DES.15 Furthermore, since the publication of these meta‐analyses, the largest RCT comparing DES and BMS for SVG PCI was recently reported.17 Similarly, long‐term outcomes of the Stenting of Saphenous Vein Grafts trial (SOS) were also reported.16 We therefore performed a meta‐analysis comparing DES vs BMS in SVG interventions comprised of randomized clinical trials data only as they represent the highest level of evidence.
Methods
Literature Search
All RCTs and subgroups of RCTs comparing DES and BMS in SVG interventions, conducted between January 1, 2003 and September 30, 2011, were included in this analysis. Comprehensive literature search was performed using MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and conference proceedings of various national and international professional meetings. The key words for the literature search included saphenous vein graft, percutaneous coronary intervention, drug − eluting stent, bare−metal stent, clinical trial, and randomized. We restricted our analysis to RCTs or subgroup analyses from RCTs that met the following inclusion criteria: saphenous vein graft interventions, randomization to DES or BMS, definition of clinical outcomes (as defined below), and a minimum follow‐up of 12 months. The quality of included studies was assessed to minimize bias. Figure 1 shows a flow diagram describing the search methodology.
Figure 1.
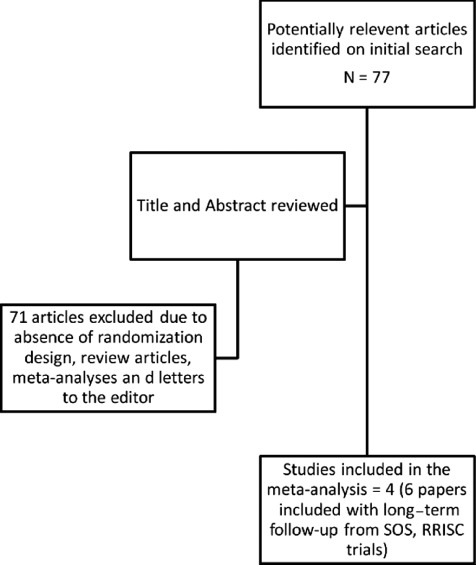
Flow chart showing literature search methodology.
Data Extraction
The included studies were independently reviewed by evaluators (M.A. and S.J.B), and all discrepancies were resolved by consensus. The following outcomes were extracted: all‐cause mortality (primary outcome), nonfatal myocardial infarction (MI), repeat revascularization (including target lesion revascularization [TLR] and target vessel revascularization [TVR]), and MACE (composite endpoint of death, MI, repeat revascularization). The definitions of clinical outcomes in individual studies were reviewed and found to be similar.
Statistical Analysis
An aggregate data meta‐analysis of clinical outcomes in these RCTs comparing PCI with DES vs BMS for SVGs was performed. Review Manager version 5.1 (The Cochrane Collaboration, Oxford, UK) was used for data analyses. Odds ratio (OR) and 95% confidence interval (CI) were used to summarize the effect size for each clinical outcome at the corresponding time point. Systematic bias was assessed using a funnel plot with regard to primary outcome of all‐cause death as shown in Figure 2. A review of the funnel plot shows symmetric distribution of all 4 studies and no publication bias because there are studies at both extremes of outcomes (ie, higher mortality, lower mortality) as well as around the midline (no effect). A 2‐tailed α of 5% was used for hypothesis testing. Primary and secondary outcomes were assessed in cumulative fashion at 30 days, 18 months, and 36 months. Measures of heterogeneity, including Cochran's Q statistic, I 2 index, and the τ 2 tests were estimated. There was evidence of significant heterogeneity for clinical endpoints of all‐cause death, MACE, and nonfatal MI at aggregate follow‐up (36 months). A random‐effects model was used to calculate the summary OR and 95% CI, given variable degrees of data heterogeneity, and given inherent heterogeneity in any systematic review of studies from the published literature. We also performed 2 sensitivity analyses to address for any bias induced in the final results by the inclusion of a subset analysis from the BAsel Stent Kosten Effektivitäts Trial (BASKET)17 and long‐term results of the Death and Events at Long‐term Follow‐up Analysis: Extended Duration of Reduction of Restenosis In Saphenous Vein Grafts With Cypher Sirolimus‐Eluting Stent (DELAYED‐RRISC) and Reduction of Restenosis In Saphenous Vein Grafts with Cypher Stent (RRISC) trials.13, 15
Figure 2.
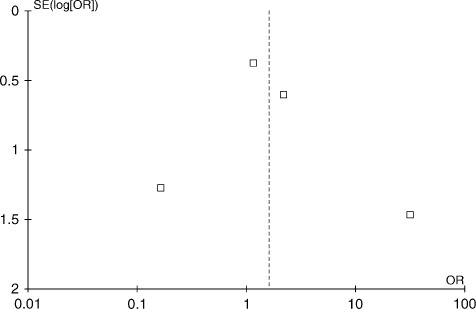
Funnel plot to assess systematic bias using primary outcome of all‐cause mortality. Funnel plot using 4 included studies shows a near symmetric distribution of effect sizes from individual studies. There does not appear to be publication bias as studies at both the extremes of outcomes as well as studies without any difference in outcomes are reported. Abbreviations: OR, odds ratio; SE, standard error.
Results
Search Results
A total of 77 studies (Figure 1) were identified using and initial keyword search. Further review of the titles and abstracts of these studies identified a total of 6 articles reporting 4 studies that met the inclusion criteria as outlined in Methods section.13, 14, 15, 16, 17, 18 One of these studies provided a subgroup analysis of patients with SVG interventions from a larger main study population included in the BASKET trial, which was not primarily randomized for patients with SVG interventions.17 Table 1 provides basic details of these 4 studies included in the meta‐analysis. These RCTs enrolled 812 patients, of whom 416 and 396 underwent PCI of SVGs with DES or BMS, respectively. Overall, there were no intergroup differences in baseline clinical and sociodemographic characteristics. A majority of the patients in these trials were men, and only 12.9% and 13.1% of patients in the DES and BMS arms, respectively, were women. The most common comorbid conditions included dyslipidemia (88.5% vs 86.9%), hypertension (73.6% vs 73.7%), diabetes mellitus (39.4% vs 36.9%), and smoking (10.8% vs 7.8%), respectively, in the DES and BMS groups of patients. At clinical presentation, an acute coronary syndrome (ACS) was diagnosed in 42.3% and 43.9% of patients in the DES and BMS groups, respectively. The average age of the SVGs at the time of the index procedure was 13.4 ± 1.4 and 13.3 ± 0.8 years in the DES and BMS groups, respectively. Table 3 provides sociodemographic, clinical, and procedure‐specificdetails of the patient population included in this meta‐analysis.
Table 1.
Details of Studies Included in the Meta‐Analysis
| ISAR‐CABG18 | SOS14, 16 | BASKET17 | RRISC13, 15 | |||||
|---|---|---|---|---|---|---|---|---|
| DES | BMS | DES | BMS | DES | BMS | DES | BMS | |
| Year | 2011 | 2011 | 2009 | 2009 | 2009 | 2009 | 2006 | 2006 |
| No. | 303 | 307 | 41 | 39 | 34 | 13 | 38 | 37 |
| Age, y | 71.4 ± 9.0 | 71.5 ± 9.3 | 66 ± 9 | 67 ± 9 | 71 ± 8 | 71 ± 8 | 73 ± 7 | 72 ± 8 |
| Follow‐up, mo | 12 | 12 | 35 | 35 | 18 | 18 | 36 | 36 |
| Female, no. | 40 | 48 | 0 | 0 | 7 | 0 | 7 | 4 |
| Diabetes mellitus, no. | 111 | 107 | 18 | 17 | 29 | 17 | 6 | 5 |
| Smoking, no. | 25 | 18 | 12 | 9 | 6 | 0 | 2 | 4 |
| Hypertension, no. | 216 | 223 | 38 | 37 | 30 | 11 | 22 | 21 |
| Dyslipidemia, no. | 268 | 264 | 40 | 37 | 27 | 12 | 33 | 31 |
| LVEF, % | 49.2 ± 12.2 | 49.5 ± 13.8 | 20 (≥50%)a | 22 (≥50%)a | NR | NR | 68 ± 18 | 72 ± 12 |
| Graft age, y | 13.8 ± 5.5 | 13.5 ± 5.1 | 11 ± 6 | 12 ± 6 | NR | NR | 12.4 ± 4.6 | 12.6 ± 5.9 |
| ACS, no. | 115 | 124 | 26 | 22 | 12 | 9 | 23 | 19 |
| EPD use, no. (%) | <5% | <5% | 29(51) | 31(56) | NR | NR | 37 (78.7) | 41 (83.7) |
| Mean stent length, mm | 26.8 ± 15.4 | 27.5 ± 17.7 | 20 ± 1 | 21 ± 9 | 41 ± 2 | 46 ±3 | 29.9 ± 15.6 | 25.2 ± 11.9 |
Abbreviations: ACS, acute coronary syndrome; BASKET, BAsel Stent KostenEffektivitäts Trial; BMS, bare‐metal stent; DES, drug‐eluting stent, EPD, embolic protection device; ISAR‐CABG, Is Drug‐Eluting Stenting Associated With Improved Results in Coronary Artery Bypass Grafts; LVEF, left ventricular ejection fraction; NR, not reported; RRISC, Reduction of Restenosis In Saphenous Vein Grafts with Cypher Stent; SOS, Stenting of Saphenous Vein Grafts.
No. of patients with LVEF ≥50%.
Table 3.
Sensitivity Analysis Using Random Effects Model Excluding BASKET17
| Outcome | Follow‐up | Event Rate | Odds Ratio (95% CI) | Qa | P | I 2 b | τ 2 c | |
|---|---|---|---|---|---|---|---|---|
| DES, No. With Events/Total | BMS, No. With Events/Total | |||||||
| All‐cause death | 0–30 days | 2/382 | 3/383 | 0.67 (0.11‐ 4.06) | NA | NA | NA | NA |
| 0–18 months | 26/382 | 16/383 | 1.86 (0.66–5.26) | 2.91 | 0.23 | 31.0 | 0.31 | |
| 0–36 months | 37/382 | 14/383 | 2.41 (0.66–8.77) | 5.64 | 0.06 | 65.0 | 0.78 | |
| MACEd | 0–30 days | 21/382 | 25/383 | 0.98 (0.28–3.43) | 5.88 | 0.05 | 66.0 | 0.78 |
| 0–18 months | 68/382 | 98/383 | 0.62 (0.43–0.88) | 0.38 | 0.83 | 00.0 | 0.00 | |
| 0–36 months | 91/382 | 103/383 | 0.76 (0.33–1.72) | 7.26 | 0.03 | 72.0 | 0.38 | |
| Repeat revascularization | 0–30 days | 1/382 | 1/383 | 0.95 (0.06–15.7) | NA | NA | NA | NA |
| 0–18 months | 26/382 | 59/383 | 0.31 (0.13–0.77) | 3.52 | 0.17 | 43.0 | 0.30 | |
| 0–36 months | 35/382 | 67/383 | 0.43 (0.21–0.91) | 4.07 | 0.13 | 51.0 | 0.22 | |
| MId | 0–30 days | 19/382 | 21/383 | 0.89 (0.46–1.70) | 5.48 | 0.06 | 64.0 | NA |
| 0–18 months | 21/382 | 31/383 | 0.64 (0.36–1.15) | 1.75 | 0.42 | 00.0 | NA | |
| 0–36 months | 27/382 | 38/383 | 0.76 (0.21–2.72) | 8.32 | 0.02 | 76.0 | 0.93 | |
Abbreviations: BASKET, BAsel Stent Kosten Effektivitäts Trial; BMS, bare‐metal stent; DES, drug‐eluting stent; CI, confidence interval; MACE, major adverse cardiac events; MI, myocardial infarction; NA, Not Applicable.
Cochran Q score for heterogeneity.
I 2 index for degree of heterogeneity.
τ 2 measure of heterogeneity.
Composite end point of death, nonfatal myocardial infarction, and repeat revascularization.
Nonfatal myocardial infarction.
Outcomes
At an aggregate follow‐up up to 36 months (range, 12–36 months), there was no difference in the primary outcome of all‐cause mortality between DES and BMS groups at 30 days (OR: 0.67, 95% CI: 0.11–4.06), 18 months (OR: 1.41, 95% CI: 0.43–4.65), and 36 months (OR: 1.63, 95% CI: 0.45–5.92). There was, however, a lower incidence of MACE in the DES group at 18 months (OR: 0.53, 95% CI: 0.34–0.83), which was no longer significant at 36 months aggregate follow‐up (OR: 0.58, 95% CI: 0.25–1.32). The 2 groups of patients had similar clinical outcomes in terms of nonfatal MI at 30 days, 18 months, and 36 months as shown in Table 2. However, the rates of repeat revascularization were lower in the DES arm compared to the BMS arm at 18 months (OR: 0.33, 95% CI: 0.17–0.64) and 36 months (OR: 0.40, 95% CI: 0.22–0.75). Table 2 provides details of the meta‐analysis outcomes at various follow‐up periods. Overall, the cumulative rate of repeat revascularization at 36 months was 41/416 (9.86%) in the DES arm compared to 73/396 (18.43%) in the BMS arm. This difference translated into 60% relative risk reduction (RRR) and 8.6% absolute risk reduction(number needed to treat [NNT] 12 patients) in repeat revascularization with implantation of DES in SVG interventions. Similarly, overall rates of MACE at 36 months was 98/416 (23.6%) in the DES group compared to 121/416 (30.6%) in the BMS group (RRR = 19.0%, NNT = 18 patients). Figure 3 shows forest plots comparing all‐cause mortality (3A) and repeat revascularization (3B) in the DES and BMS arms. Because 1 of these studies was a subset analysis of a larger RCT,17 we performed a sensitivity analysis by excluding this study from the analysis. This analysis is shown in Table 3 and essentially shows similar clinical outcomes at all time periods. In addition, a second sensitivity analysis was performed after excluding the only RCT that showed increased all‐cause mortality in the DES group at longer‐term follow‐up.13, 15 The exclusion of this study reduced the heterogeneity seen in the earlier analysis. However, there were no major differences in the clinical outcomes at all intervals except in addition to lower MACE rates at 36 months, and we also observed a reduction in MACE at 18 months (OR: 0.51, 95% CI: 0.28–0.95). This analysis is shown in Table 4. Importantly, both of these sensitivity analyses showed no increase in all‐cause mortality and a significant reduction in MACE and repeat revascularization in the DES group compared to BMS group.
Table 2.
Meta‐Analysis Outcomes Using Random Effects Model
| Outcome | Follow‐up | Event Rate | Odds Ratio (95% CI) | Qa | P | I 2 b | τ 2 c | |
|---|---|---|---|---|---|---|---|---|
| DES, No. With Events/Total | BMS, No. With Events/ Total | |||||||
| All‐cause death | 0–30 days | 2/382 | 3/383 | 0.67 (0.11–4.06) | NA | NA | NA | NA |
| 0–18 months | 27/416 | 18/396 | 1.41 (0.43–4.65) | 5.65 | 0.13 | 47.0 | 0.67 | |
| 0–36 months | 38/416 | 21/396 | 1.63 (0.45–5.92) | 8.61 | 0.03 | 65.0 | 1.00 | |
| MACEd | 0–30 days | 21/382 | 25/383 | 0.98 (0.28–3.43) | 5.88 | 0.05 | 66.0 | 0.78 |
| 0–18 months | 75/416 | 106/396 | 0.53 (0.34–0.83) | 3.69 | 0.30 | 19.0 | 0.04 | |
| 0–36 months | 98/416 | 121/396 | 0.58 (0.25–1.32) | 11.3 | 0.01 | 73.0 | 0.50 | |
| Repeat revascularization | 0–30 days | 1/382 | 1/383 | 0.95 (0.06–15.7) | NA | NA | NA | NA |
| 0–18 months | 32/416 | 65/396 | 0.33 (0.17–0.64) | 3.97 | 0.26 | 24.0 | 0.12 | |
| 0–36 months | 41/416 | 73/396 | 0.40 (0.22–0.75) | 4.8 | 0.19 | 37.0 | 0.15 | |
| MIe | 0–30 days | 19/382 | 21/383 | 0.89 (0.46–1.70) | 5.48 | 0.06 | 64.0 | NA |
| 0–18 months | 23/416 | 31/396 | 0.67 (0.38–1.19) | 2.28 | 0.52 | 00.0 | NA | |
| 0–36 months | 29/416 | 38/396 | 0.83 (0.27–2.60) | 8.87 | 0.03 | 66.0 | 0.80 | |
Abbreviations: BMS, bare‐metal stent; DES, drug‐eluting stent; MACE, major adverse cardiac events; MI, myocardial infarction; CI, confidence interval; NA, Not Applicable.
Cochran Q score for heterogeneity.
I2 index for degree of heterogeneity.
Tτ 2 measure of heterogeneity.
Composite end point of death, nonfatal myocardial infarction, and repeat revascularization.
Nonfatal myocardial infarction.
Figure 3.
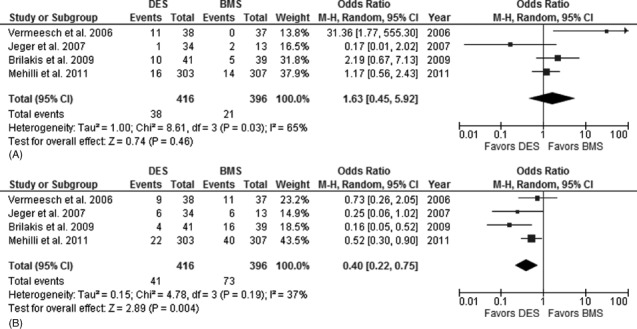
(A) Forest plot showing cumulative rates of all‐cause death in patients with saphenous vein graft (SVG) percutaneous intervention (PCI) with drug‐eluting stent (DES) vs. bare‐metal stent (BMS) use (0–36 months). (B) Forest plot showing cumulative rates of repeat revascularization in patients with SVG PCI with DES vs BMS use (0–36 months). Forest plots of primary outcome all‐cause death (A) and secondary outcome of repeat revascularization (B) show no difference in all‐cause mortality and reduction in repeat revascularization with use of DES in saphenous vein graft interventions. Abbreviations: CI, confidence interval, M‐H = Mantel‐Haenszel Odds Ratio.
Table 4.
| Outcome | Follow‐up | Event Rate | Odds Ratio (95% CI) | Qa | P | I 2 b | τ 2 c | |
|---|---|---|---|---|---|---|---|---|
| DES, No. With Events/Total | BMS, No. With Events/Total | |||||||
| All‐cause death | 0–30 days | 2/344 | 3/346 | 0.67 (0.11–4.06) | NA | NA | NA | NA |
| 0–18 months | 22/378 | 18/359 | 1.08 (0.37–3.11) | 3.16 | 0.21 | 37.0 | 0.36 | |
| 0–36 months | 27/378 | 21/359 | 1.15 (0.45–2.94) | 3.41 | 0.18 | 41.0 | 0.29 | |
| MACEd | 0–30 days | 10/344 | 20/346 | 0.49 (0.22–1.07) | 0.49 | 0.48 | 00.0 | 0.00 |
| 0–18 months | 69/378 | 95/359 | 0.51 (0.28–0.95) | 3.49 | 0.17 | 43.0 | 0.13 | |
| 0–36 months | 76/378 | 106/359 | 0.41 (0.20–0.86) | 4.39 | 0.11 | 54.0 | 0.23 | |
| Repeat revascularization | 0–30 days | 1/344 | 1/346 | 0.95 (0.06–15.7) | NA | NA | NA | NA |
| 0–18 months | 30/378 | 57/359 | 0.34 (0.15–0.75) | 3.24 | 0.20 | 38.0 | 0.21 | |
| 0–36 months | 32/378 | 62/359 | 0.32 (0.15–0.71) | 3.69 | 0.16 | 46.0 | 0.23 | |
| MIe | 0–30 days | 8/344 | 16/346 | 0.49 (0.21–1.16) | 0.50 | 0.48 | 00.0 | 0.50 |
| 0–18 months | 21/378 | 30/359 | 0.63 (0.35–1.13) | 1.46 | 0.48 | 00.0 | NA | |
| 0–36 months | 22/378 | 36/359 | 0.51 (0.20–1.31) | 0.31 | 0.16 | 46.0 | 0.31 | |
Abbreviations: BMS, bare‐metal stent; DES, drug‐eluting stent; CI, confidence interval; MACE, major adverse cardiac events; MI, myocardial infarction; NA, Not Applicable; RRISC, Reduction of Restenosis In Saphenous Vein Grafts with Cypher Stent.
Cochran Q score for heterogeneity.
I2 index for degree of heterogeneity.
τ 2 measure of heterogeneity.
Composite end point of death, nonfatal myocardial infarction, and repeat revascularization.
Nonfatal myocardial infarction.
Discussion
The results of our meta‐analysis showed that the use of DES in revascularization of saphenous vein grafts is associated with a significant reduction in risk of repeat revascularization (NNT = 12)and MACE (NNT = 18) when compared to BMS. In addition, there were no differences in the incidence of all‐cause death and nonfatal MI between the 2 groups.
Several prior meta‐analyses have reported a lower all‐cause mortality with implantation of DES compared to BMS in SVG disease in the setting of data derived largely from nonrandomized clinical reports.2, 5, 6, 7, 9, 10, 11 These findings were, however, not confirmed in our meta‐analysis, which included all of the available randomized clinical data. Our analysis confirms the previously observed findings in the setting of randomized clinical data.13, 14, 15, 16, 17, 18 All 4 clinical trials in our analysis showed decreased rates of repeat revascularization in the DES arm.4, 5, 6, 7, 8, 9 At longer‐term follow‐up in the RRISC trial, the DES benefit of reduced TVR seen at 6 months was no longer significant, and a higher mortality was observed among patients who received a DES.15 However, in our pooled analysis of all available randomized controlled trials, we did not observe any difference in the risk of all‐cause mortality at intermediate‐term follow‐up. However, our meta‐analysis at an overall follow‐up (up to 36 months) confirms durable reduction in repeat revascularization with a much larger number of patients. Therefore, it is reasonable to infer that use of DES is safe and effective for SVG lesions.
The conclusions from our meta‐analysis should be interpreted in view of the limitations due to an overall low number of events, inherent heterogeneity in the included studies, and lack of long‐term follow‐up. We attempted to reduce heterogeneity in the reported studies by conducting 2 sensitivity analyses (Tables 3 and 4) and found consistent results across the primary and secondary clinical outcomes. In addition, different types of DES were used in these clinical trials and may induce bias from treatment effects. However, our meta‐analysis reports the largest number of patients in the setting of randomized clinical data. Similarly, there are a significant number of patients in both DES and BMS arms with ACS; therefore, our findings can also be extrapolated to patients with SVG disease presenting with ACS.
Conclusion
In this comprehensive meta‐analysis of all reported randomized clinical trials comparing clinical outcomes of PCI using DES vs BMS in patients with SVG disease, we conclude that use of DES is associated with significant reduction in the rate of repeat revascularization and MACE, without an increase in rates of nonfatal MI or all‐cause death.
References
- 1. Coolong A, Baim DS, Kuntz RE, et al. Saphenous vein graft stenting and major adverse cardiac events: a predictive model derived from a pooled analysis of 3958 patients. Circulation. 2008;117:790–797. [DOI] [PubMed] [Google Scholar]
- 2. Hakeem A, Helmy T, Munsif S, et al. Safety and efficacy of drug eluting stents compared with bare metal stents for saphenous vein graft interventions: a comprehensive meta‐analysis of randomized trials and observational studies comprising 7,994 patients. Catheter CardiovascInterv. 2011;77:343–355. [DOI] [PubMed] [Google Scholar]
- 3. Nwasokwa ON. Coronary artery bypass graft disease. Ann Intern Med. 1995. 1;123:528–545. [DOI] [PubMed] [Google Scholar]
- 4. Brilakis ES, Wang TY, Rao SV, et al. Frequency and Predictors of drug‐eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data Cath PCI registry. JACC Cardio vascInterv. 2010;3:1068–1073. [DOI] [PubMed] [Google Scholar]
- 5. Mamas MA, Foley J, Nair S, et al. A comparison of drug‐eluting stents versus bare metal stents in saphenous vein graft PCI outcomes: a meta‐analysis. J IntervCardiol. 2011;24:172–180. [DOI] [PubMed] [Google Scholar]
- 6. Wiisanen ME, Abdel‐Latif A, Mukherjee D, et al. Drug‐eluting stents versus bare‐metal stents in saphenous vein graft interventions: a systematic review and meta‐analysis. JACC Cardio vascInterv. 2010;3:1262–1273. [DOI] [PubMed] [Google Scholar]
- 7. Lupi A, Navarese EP, Lazzero M, et al. Drug‐eluting stents vs. bare metal stents in saphenous vein graft disease. Insights from a meta‐analysis of 7,090 patients. Circ J. 2011;75:280–289. [DOI] [PubMed] [Google Scholar]
- 8. Paradis JM, Bélisle P, Joseph L, et al. Drug‐eluting or bare metal stents for the treatment of saphenous vein graft disease: a Bayesian meta‐analysis. CircCardiovascInterv. 2010;3:565–576. [DOI] [PubMed] [Google Scholar]
- 9. Testa L, Agostoni P, Vermeersch P, et al. Drug eluting stents versus bare metal stents in the treatment of saphenous vein graft disease: a systematic review and meta‐analysis. EuroIntervention. 2010;6:527–536. [DOI] [PubMed] [Google Scholar]
- 10. Meier P, Brilakis ES, Corti R, et al. Drug‐eluting versus bare‐metal stent for treatment of saphenous vein grafts: a meta‐analysis. PLoS One. 2010;5:e11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanchez‐Recalde A, Jiménez Valero S, Moreno R, et al. Safety and efficacy of drug‐eluting stents versus bare‐metal stents in saphenous vein grafts lesions: a meta‐analysis. EuroIntervention. 2010;6:149–160. [PubMed] [Google Scholar]
- 12. Lee MS, Yang T, Kandzari DE, et al. Comparison by meta‐analysis of drug‐eluting stents and bare metal stents for saphenous vein graft intervention. Am J Cardiol. 2010;105:1076–1082. [DOI] [PubMed] [Google Scholar]
- 13. Vermeersch P, Agostoni P, Verheye S, et al. Randomized double‐blind comparison of sirolimus‐eluting stent versus bare‐metal stent implantation in diseased saphenous vein grafts: six‐month angiographic, intravascular ultrasound, and clinical follow‐up of the RRISC Trial. J Am CollCardiol. 2006;48:2423–2431. [DOI] [PubMed] [Google Scholar]
- 14. Brilakis ES, Lichtenwalter C, de Lemos JA, et al. A randomized controlled trial of a paclitaxel‐eluting stent versus a similar bare‐metal stent in saphenous vein graft lesions the SOS (Stenting of Saphenous Vein Grafts) trial. J Am CollCardiol. 2009;53:919–928. [DOI] [PubMed] [Google Scholar]
- 15. Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus‐eluting stents versus bare‐metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am CollCardiol. 2007;50:261–267. [DOI] [PubMed] [Google Scholar]
- 16. Brilakis ES, Lichtenwalter C, Abdel‐karim AR, et al. Continued benefit from paclitaxel‐eluting compared with bare‐metal stent implantation in saphenous vein graft lesions during long‐termfollow‐up of the SOS (Stenting of Saphenous Vein Grafts) trial. JACC Cardio vascInterv. 2011;4:176–182. [DOI] [PubMed] [Google Scholar]
- 17. Jeger RV, Schneiter S, Kaiser C, et al. Drug‐eluting stents compared with bare metal stents improve late outcome after saphenous vein graft but not after large native vessel interventions. Cardiology. 2009;112:49–55. [DOI] [PubMed] [Google Scholar]
- 18. Mehilli J, Pache J, Abdel‐Wahab M, et al. Is Drug‐Eluting‐Stenting Associated with Improved Results in Coronary Artery Bypass Grafts? (ISAR‐CABG) Investigators. Drug‐eluting versus bare‐metal stents in saphenous vein graft lesions (ISAR‐CABG): a randomised controlled superiority trial. Lancet. 2011;378:1071–1078. [DOI] [PubMed] [Google Scholar]


