Abstract
Background:
A seasonal variation has been reported for occurrence of acute cardiovascular events, such as myocardial infarction, sudden death, and rupture/dissection of aortic aneurysms.
Hypothesis:
The aim of this study was to determine whether a seasonal variation exists for heart failure (HF) hospitalization.
Methods:
The study included all cases of HF admissions to Ferrara Hospital between January 2002 and December 2009. The sample was divided into subgroups by gender, age, cardiovascular risk factors, patients' outcome, and order of ICD‐9 codes (first diagnosis, accessory diagnosis). On the basis of date of admission, cases have been analyzed for seasonal variation and annual frequency. For seasonal analysis, monthly cases were categorized into four 3‐month intervals by seasons. Moreover, monthly admissions have been also adjusted for number of days, and the average number of admissions per month has been used.
Results:
The database included 15 954 patients with the ICD‐9‐CM codes of HF (420–429). Hospital admissions for HF were most frequent in winter (28.4%) and least in summer (20.4%). Chronobiological analysis yielded a significant peak in January for total cases and all subgroups considered. No differences were found for subgroups by gender, age, fatal cases, presence of hypertension and diabetes mellitus, patients' outcome, and order of ICD‐9 codes (first diagnosis, accessory diagnosis).
Conclusions:
A seasonal periodicity for HF hospitalization was demonstrated, with a peak in winter months, independent of gender, age, major cardiovascular risk factor, and patients' outcome. These data could be useful for practitioners to improve causative prevention measures, therapeutic management, and educational strategies. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
The progressive aging of the population, combined with increased survival of patients with cardiovascular disease as a result of therapeutic improvements, have led to an escalating prevalence of heart failure (HF). Consequently, HF has become the most important public health problem in cardiovascular medicine, placing a heavy burden on healthcare systems. In fact, hospitalization of patients with HF consumes 74% of the total healthcare costs.1 Moreover, HF is the most common principal diagnosis among hospitalized adults age 65 years and older.2 Arterial hypertension3 and diabetes mellitus4 play a pivotal role in the genesis of cardiovascular disease in elderly people. In the last 2 decades, it has been shown that major acute cardiovascular events do not occur randomly through time, but exhibit a seasonal variation. A winter peak, in fact, has been reported for myocardial infarction,5, 6 sudden cardiac death,7 stroke and transient ischemic attack,8, 9 rupture/dissection of aortic aneurysms,10, 11 and pulmonary embolism.12
This study aimed to determine whether a seasonal variation exists in HF hospitalizations and deaths and to examine possible contributors to such variability.
Methods
The analysis included all consecutive cases of HF admission at the St. Anna Hospital of Ferrara, Emilia‐Romagna region, Ferrara, Italy, between January 1, 2002 and December 31, 2009. In particular, all cases were included in the database regardless of residential address and nationality (tourists included).
The discharge hospital sheet contains information on each subject: first name and surname, sex, date of birth, date, and department of hospital admission and discharge, main and up to 15 accessory discharge diagnoses, and most important, diagnostic procedures based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). We considered the ICD‐9‐CM codes used for identifying patients with heart failure were 420 to 429, as principal diagnosis or any diagnosis, and in particular:
420–429 Other Forms of Heart Disease
428 Heart failure
428.0 Congestive heart failure, unspecified
428.1 Left heart failure; Acute edema of lung with heart disease NOS or heart failure; Acute pulmonary edema with heart disease NOS or heart failure
428.9 Heart failure, unspecified
The total sample was divided into subgroups by gender, age, presence of major cardiovascular risk factors (arterial hypertension, diabetes mellitus), patients' outcome (dead during hospitalization, discharged alive, transferred to other departments), and order of ICD‐9 codes (first diagnosis, accessory diagnosis). The major cardiovascular risk factors were chosen due to their important causative role and the higher probability of being appropriately expressed by specific codes. Thus, information about the presence of risk factors was taken from discharge diagnosis codes, medical history, and drug prescription. On the basis of date of admission, all cases have been analyzed for seasonal variation and annual frequency. For seasonal analysis, total monthly cases were categorized into four 3‐month intervals (spring: March–June, summer: June–September, autumn: October–December; winter: January–March). The distribution of admissions by seasons was tested for uniformity in all groups by the χ 2 goodness of fit test. Analysis of monthly data was performed by applying a partial Fourier series to the time series data using an internationally validated method (Chronolab software on an Apple Macintosh computer; free download at: http://www.tsc.uvigo.es/BIO/bioing.html).13 This method selects the harmonic or combination of harmonics (cosine waveforms) that best explain the temporal variance of the data. The percentage of the overall variance attributable to the approximated cosine function (percent of rhythm) serves as the estimate of the goodness of fit, with the F‐test statistic applied to the variance accounted by the single or multiple cosine curve approximation vs straight‐line approximation of the time series data to accomplish a test of the null hypothesis of 0 amplitude (ie, absence of significant temporal variation for the given period of the approximated curve function). The parameters calculated for the overall 1‐year period (τ) cosine approximation of the time series data were: the midline estimated statistic of rhythm (MESOR, the rhythm‐adjusted mean for the time period analyzed), amplitude (one half the difference between the absolute maximum and minimum of the fitted approximation), and peak (acrophase) and trough (bathyphase) time referenced to 00:00 hour December 31. Significance levels were assumed for P<0.05.
Moreover, data of monthly admissions were adjusted for the different number of days in the months using the average number of admissions per month. This analysis is available using the method by Barnettt and Dobson14 (free download at: http://cran.r‐project.org/web/packages/season/season.pdf).
Results
During the analyzed period, the hospital database contained the records of 15 954 patients (mean age, 77.7 ± 10.5 years) with the ICD‐9‐CM codes of HF (420–429). Of these patients, 7733 were male (48.5%) and 8221 were female (51.5%). The mean ± standard deviation age was 76.8 ± 11.5 years (75.9 ± 10.3 and 79.4 ± 10.4 years for males and females, respectively, t = 15514, P<0.001).
As for season, hospital admissions for heart failure were most frequent in winter (28.4%) and least in summer (20.4%) (Figure 1). No differences were found considering the subgroups by gender, age, fatal cases, presence of hypertension or diabetes mellitus, patients' outcome (dead during hospitalization, discharged alive, transferred to other department), and order of ICD‐9 codes (first diagnosis, accessory diagnosis) (Table 1). Chronobiological analysis yielded a significant peak in January for total cases (Figure 2), and all subgroups considered (Table 2).
Figure 1.
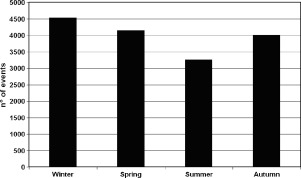
Seasonal distribution of heart failure hospital admissions (total cases) at the Ferrara Hospital, years 2002–2009.
Table 1.
Seasonal Distribution of Heart Failure Hospital Admissions for Total Subjects and Analyzed Subgroups
| Winter (%) | Spring (%) | Summer (%) | Autumn (%) | χ 2 Test for Nonuniformity | P | |
|---|---|---|---|---|---|---|
| All cases (n = 15 954) | 4536 (28.4) | 4145 (26.0) | 3261 (20.4) | 4012 (25.1) | 214.16 | <0.001 |
| Males (n = 7733) | 2235 (28.9) | 2034 (26.3) | 1587 (20.5) | 1877 (24.3) | 116.01 | <0.001 |
| Females (n = 8221) | 2301 (28.0) | 2111 (25.7) | 1674 (20.4) | 2135 (26.0) | 104.73 | <0.001 |
| Age < 60 yrs (n = 894) | 263 (29.4) | 236 (26.4) | 170 (19.0) | 225 (25.2) | 20.55 | <0.001 |
| Age 60–69 yr (n = 2042) | 570 (27.9) | 513 (25.1) | 429 (21.0) | 530 (26.0) | 20.73 | <0.001 |
| Age 70–79 yr (n = 5269) | 1532 (29.1) | 1352 (25,7) | 1088 (20.6) | 1297 (24.6) | 76.15 | <0.001 |
| Age > 79 yrs (n = 7749) | 2171 (28.0) | 2044 (26.4) | 1574 (20.3) | 1960 (25.3) | 102.48 | <0.001 |
| Diabetes (n = 3061) | 870 (28.4) | 773 (25.3) | 603 (19.7) | 815 (26.6) | 52.07 | <0.001 |
| No diabetes (n = 12 893) | 3666 (28.4) | 3372 (26.2) | 2658 (20.6) | 3197 (24.8) | 167.03 | <0.001 |
| Hypertension (n = 5464) | 1560 (28.6) | 1459 (26.7) | 1090 (19.9) | 1355 (24.8) | 89.74 | <0.001 |
| No hypertension (n = 10 490) | 2976 (28.4) | 2686 (25.6) | 2171 (20.7) | 2657 (25.3) | 127.4 | <0.001 |
| Dead during hospitalization (n = 2129) | 627 (29.5) | 517(24.3) | 450 (21.1) | 535 (25.1) | 30.04 | <0.001 |
| Discharged alive (n = 12 558) | 3544 (28.2) | 3310 (26.4) | 2541 (20.2) | 3163 (25.2) | 175.68 | <0.001 |
| Transferred to another department (n = 1267) | 365 (28.8) | 318 (25.1) | 270 (21.3) | 314 (24.8) | 14.31 | 0.003 |
| First diagnosis code ICD‐9 428 (n = 7278) | 2087 (28.7) | 1936 (26.6) | 1388 (19.1) | 1867 (25.7) | 150.4 | <0.001 |
| No first code ICD‐9 428 (n = 8676) | 2449 (28.2) | 2209 (25.5) | 1873 (21.6) | 2145 (24.7) | 77.54 | <0.001 |
Figure 2.

Monthly distribution of average number of hospital admission for heart failure (total cases) at the Ferrara Hospital, years 2002–2009.
Table 2.
Chronobiologic Analysis of Annual Frequency of Hospital Discharges for Heart Failure
| No. | Amplitude ± SE | Acrophase (peak), d | P | |
|---|---|---|---|---|
| Total | 15954 | 219.20 ± 27.91 | 24, January | <0.001 |
| Females | 8221 | 108.48 ± 16.43 | 17, January | <0.001 |
| Males | 7733 | 112.18 ± 13.50 | 30, January | <0.001 |
| Age < 60 yr | 894 | 14.98 ± 4.33 | 30, January | 0.022 |
| Age 60–69 yr | 2042 | 27.14 ± 7.74 | 8, January | 0.021 |
| Age 70–79 yr | 5269 | 78.86 ± 12.15 | 27, January | <0.001 |
| Age > 79 yr | 7749 | 104.01 ± 13.55 | 23, January | <0.001 |
| Diabetes | 3061 | 47.94 ± 5.59 | 16, January | <0.001 |
| No diabetes | 12893 | 171.83 ± 24.32 | 26, January | <0.001 |
| Hypertension | 5464 | 81.45 ± 10.18 | 27, January | <0.001 |
| No hypertension | 10490 | 137.99 ± 20.31 | 22, January | <0.001 |
| Dead during hospitalization | 2129 | 33.98 ± 5.19 | 11, January | <0.001 |
| Discharged alive | 12558 | 170.64 ± 25.56 | 28, January | <0.001 |
| Transferred to another department | 1267 | 16.60 ± 4.84 | 6, January | <0.001 |
Amplitude is one half the difference between the absolute maximum and minimum of the fitted curve, expressed in degrees. Acrophase (peak) is the absolute maximum value during the observed period, expressed in days and month.
Abbreviations: SE, standard error.
Discussion
Congestive heart failure (CHF) is the end stage of many cardiac disease processes. Coronary heart disease and hypertension (either singly or together) account for the vast majority of cases of CHF within the developed world.15 A patient with CHF has little physiological reserve to deal with an increase in cardiac workload.
The present study showed the existence of a seasonal periodicity for HF deaths and hospitalization, characterized by a winter peak independent of gender, age, major cardiovascular risk factor, and outcome.
These result are in agreement with various studies performed either in the Northern Hemisphere15, 16, 17, 18, 19, 20, 21, 22, 23 and in the Southern Hemisphere.24, 25, 26, 27, 28, 29 These similarities occurred despite the fact that there can be considerable differences between climates due to on‐site variability or between sites. It is possible that increased deaths from CHF may depend mainly on a certain individual threshold of temperature level rather than that of absolute temperature values.
A patient with CHF has little physiological reserve to deal with an increase in cardiac workload. Temperature reduction can cause physiological changes leading to HF decompensation and increased hospitalization rates (eg, overload secondary to increased heart rate and total peripheral resistance, changes of total extracellular volume secondary to decrease in water loss by transpiration and perspiration, increased blood pressure values, and arrhythmias).15, 18 Moreover, higher rates of infectious diseases in winter, particularly respiratory tract infections, may play a role.30, 31 Again, C‐reactive protein levels, a well‐recognized marker of the potential risk of cardiovascular events, shows a seasonal variation as well, characterized by a winter peak.32
Blood pressure levels are higher during winter months.33 When the temperature falls, a compensatory vasoconstrictive response, particularly to the skin, is observed. This is associated with an increased after‐load for the failing heart, and is achieved by upregulation of the neurohumoral cascade and increased levels of vasoconstrictors. Therefore, cardiac work increases to overcome the rise in after‐load, and at the end‐stage the failing heart is unable to cope with this increased demand.34, 35, 36, 37 A study on a large Austrian database quantified the contribution of seasonal risk factor variation in mortality from coronary heart disease.38 In fact, the authors found that total cholesterol levels, blood pressure, and body mass index showed pronounced seasonal variations with average levels significantly higher during the winter months, independent of age and gender groups, giving an estimated increase in score risk of 6.8% in men and 3.6% in women.38
The known winter increase in caloric intake may result in an increase in sodium intake, although a modest weight gain over the winter holiday season was found.39 Vitamin D deficiency could precipitate heart failure. People with preexisting HF often have a sedentary lifestyle, which may result in few hours outdoors with minimal exposure to sunlight. For these people a vitamin D supplementation may reduce their event risk, although a specific trial of this intervention needs to be carried out before supplementation can be recommended.27 A potential increase in alcohol consumption during winter can decrease contractility and induce atrial fibrillation.40 Variations in factors such as atmospheric pollution, other climatic factors (atmospheric pressure or rainfall/sun exposure), and use of drugs may also be involved.15 A comprehensive view of multiple proposed possible mechanisms are summarized in Figure 3.
Figure 3.

Hypotheses about pathways between winter and heart failure (HF). Abbreviation: BMI, body mass index.
It has been hypothesized that lower summer admissions compared with winter may be attributable to bed closures during the summer, a common practice in many hospitals, and the concomitant reduction of local population for summer vacations.21 However, these considerations cannot apply for our study. The city of Ferrara, Italy, every summer has a large increase of summer tourist population. The attractiveness of its historical and architectural heritage, the close proximity to the sea and white sand beaches, along with the high level of local healthcare organizations, greatly favors the arrival of elderly tourists. Indeed, a possible strength of this study may derive just from inclusion of total cases, regardless of residential address or nationality. The use of tourists, in fact, may overcome the criticism leveled at these studies, that the seasonal differences are due to the movement of tourists.
This study, however, has a number of limitations that require comment, most of them common to retrospective studies based on ICD‐9 coding. In fact, the use of ICD 9‐CM code may be biased by the physicians' habits with regard to assigning a diagnosis. Moreover, information on the severity of illness is not available. Our regional database, maybe the best in Italy, is far from collecting the series of information of U.S. databases. In particular, for example, there is no information on pulmonary and cardiovascular risk factors or possible precipitating factors for relationship between season and CHF. Thus, we decided to use raw indicators of outcome, death during hospitalization, or discharge alive, and we limited to the primary cause of death or discharge diagnosis. Again, caution must be used in the interpretation of hospitalization data. These data, unlike the mortality information, describe episodes of care. Any given individual may experience several episodes of hospital care. Therefore, hospitalization data are not person based. Also, the hospitalization data do not reflect information on outpatient department use, a much more frequent source of care of mild to moderate CHF.
Conclusion
The circannual seasonal periodicity of CHF deaths and hospitalizations, characterized by a peak incidence in winter months, might have important clinical implications. For example, healthcare systems should adjust the availability of emergency services and other hospital resources to the most vulnerable periods. Susceptible patients should be informed of the increased risk during winter, and the demonstration of a higher‐risk period could be useful for general practitioners to improve causative prevention measures, therapeutic management, and educational strategies.
Acknowledgements
The authors thank Dr. Nicola Napoli and Dr. Franco Guerzoni, Centre for Health Statistics, Hospital of Ferrara, Italy, for their precious and valuable collaboration.
References
- 1. Ahmed A, Allman RM, Fonarow GC, et al. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity‐matched study. J Card Fail. 2008;14:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rich MW. Heart failure in the elderly: undertreated or understudied? Am J Geriatr Cardiol. 2002;11:285–287. [DOI] [PubMed] [Google Scholar]
- 3. Webb‐Peploe KM, MacGregor GA. Hypertension in the elderly. Am J Geriatr Cardiol. 2000;9:130–137. [DOI] [PubMed] [Google Scholar]
- 4. Wilson PWF, Kannel WB. Obesity, diabetes, and risk of cardiovascular disease in elderly. Am J Geriatr Cardiol. 2002;11:119–123. [DOI] [PubMed] [Google Scholar]
- 5. Spencer FA, Goldberg RJ, Becker RC, et al. Seasonal distribution of acute myocardial infarction in the second national registry of myocardial infarction. J Am Coll Cardiol. 1998;31:1226–1233. [DOI] [PubMed] [Google Scholar]
- 6. Manfredini R, Manfredini F, Boari B, et al. Seasonal and weekly patterns of hospital admissions for nonfatal and fatal myocardial infarction. Am J Emerg Med. 2009;27:1096–1102. [DOI] [PubMed] [Google Scholar]
- 7. Arntz HR, Willich SN, Schreiber C, et al. Diurnal, weekly and seasonal variation of sudden death. Population‐based analysis of 24061 consecutive cases. Eur Heart J. 2000;21:315–320. [DOI] [PubMed] [Google Scholar]
- 8. Manfredini R, Gallerani M, Portaluppi F, et al. Chronobiological patterns of onset of acute cerebrovascular diseases. Thromb Res 1997;88:451–463. [DOI] [PubMed] [Google Scholar]
- 9. Manfredini R, Manfredini F, Boari B, et al. Temporal patterns of hospital admissions for transient ischemic attack. A retrospective population‐based study in the Emilia‐Romagna region of Italy. Clin Appl Thromb Hemost. 2010;16:153–160. [DOI] [PubMed] [Google Scholar]
- 10. Mehta HR, Manfredini R, Hassan F, et al. Chronobiological patterns of acute aortic dissection. Circulation. 2002;106:1110–1115. [DOI] [PubMed] [Google Scholar]
- 11. Manfredini R, Boari B, Manfredini F, et al. Seasonal variation in occurrence of aortic diseases: the database of hospital discharge data of the Emilia‐Romagna region, Italy. J Thorac Cardiovasc Surg. 2008;135:442–444. [DOI] [PubMed] [Google Scholar]
- 12. Gallerani M, Boari B, Smolensky MH, et al. Seasonal variation in occurrence of pulmonary embolism: analysis of the database of the Emilia‐Romagna region. Chronobiol Int. 2007;24:143–160. [DOI] [PubMed] [Google Scholar]
- 13. Mojon A, Fernandez JR, Hermida RC. Chronolab: an interactive software package for chronobiologic time series analysis written for the Macintosh Computer. Chronobiol Int. 1992;9:403–412. [DOI] [PubMed] [Google Scholar]
- 14. Barnett AG,Dobson AJ. Analysing Seasonal Health Data. Heidelberg, Germany: Springer; 2010. [Google Scholar]
- 15. Boulay F, Berthier F, Sisteron O, et al. Seasonal variation in chronic heart failure hospitalizations and mortality in France. Circulation. 1999;100:280–286. [DOI] [PubMed] [Google Scholar]
- 16. Allegra JR, Cochrane DG, Biglow R. Monthly, weekly, and daily patterns in the incidence of congestive heart failure. Acad Emerg Med. 2001;8:682–685. [DOI] [PubMed] [Google Scholar]
- 17. Montes Santiago J, Rey Garcia G, Mediero Dominguez A, et al. Seasonal changes in hospitalization and mortality resulting from chronic heart failure in Vigo [in Spanish]. An Med Interna. 2001;18:578–581. [PubMed] [Google Scholar]
- 18. Martinez‐Selles M, Garcia Robles JA, Prieto L, et al. Annual rates of admission and seasonal variations in hospitalizations for heart failure. Eur J Heart Fail. 2002;4:779–786. [DOI] [PubMed] [Google Scholar]
- 19. Stewart S, McIntyre K, Capewell S, et al. Heart failure in a cold climate. Seasonal variation in heart failure‐related morbidity and mortality. J Am Coll Cardiol. 2002;39:760–766. [DOI] [PubMed] [Google Scholar]
- 20. Tepper D. Frontiers in congestive heart failure: heart failure in a cold climate. Seasonal variation in heart failure‐related morbidity and mortality. Congest Heart Fail. 2002;8:90. [PubMed] [Google Scholar]
- 21. Feldman DE, Platt R, Dery V, et al. Seasonal congestive heart failure mortality and hospitalisation trends, Quebec 1990‐1998. J Epidemiol Community Health. 2004;58:129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogawa M, Tanaka F, Onoda T, et al; the Northern Iwate Heart Disease Registry Consortium . A community based epidemiological and clinical study of hospitalization of patients with congestive heart failure in Northern Iwate, Japan. Circ J. 2007;71:455–459. [DOI] [PubMed] [Google Scholar]
- 23. Oktay C, Luk JH, Allegra JR, Kusoglu L. The effect of temperature on illness severity in emergency department congestive heart failure patients. Ann Acad Med Singapore. 2009;38:1081–1084. [PubMed] [Google Scholar]
- 24. Isezuo SA. Seasonal variation in hospitalisation for hypertension‐related morbidities in Sokoto, north‐western Nigeria. Int J Circumpolar Health. 2003;62:397–409. [DOI] [PubMed] [Google Scholar]
- 25. Diaz A, Ferrante D, Badra R, et al. Seasonal variation and trends in heart failure morbidity and mortality in a South American community hospital. Congest Heart Fail. 2007;13:263–266. [DOI] [PubMed] [Google Scholar]
- 26. Ansa VO, Ekott JU, Essien IO, et al. Seasonal variation in admission for heart failure, hypertension and stroke in Uyo, South‐Eastern Nigeria. Ann Afr Med. 2008;7:62–66. [DOI] [PubMed] [Google Scholar]
- 27. Barnett AG, de Looper M, Fraser JF. The seasonality in heart failure deaths and total cardiovascular deaths. Aust N Z J Public Health. 2008;32:408–413. [DOI] [PubMed] [Google Scholar]
- 28. Inglis SC, Clark RA, Shakib S, et al. Hot summers and heart failure: seasonal variations in morbidity and mortality in Australian heart failure patients (1994–2005). Eur J Heart Fail. 2008;10:540–549. [DOI] [PubMed] [Google Scholar]
- 29. Jorge JE, Cagy M, Mesquita ET, et al. Seasonal variation in hospitalizations due to heart failure in Niteroi city, Southeastern Brazil. Rev Saude Publica. 2009;43:555–557. [DOI] [PubMed] [Google Scholar]
- 30. Sandoval C, Walter SD, Krueger P, et al. Comparing estimates of influenza‐associated hospitalization and death among adults with congestive heart failure based on how influenza season is defined. BMC Public Health. 2008;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yap FH, Ho PL, Lam KF, et al. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73:617–623. [DOI] [PubMed] [Google Scholar]
- 32. Sung KC. Seasonal variation of C‐reactive protein in apparently healthy Koreans. Int J Cardiol. 2006;107:338–342. [DOI] [PubMed] [Google Scholar]
- 33. Brennan PJ, Greenberg G, Miall WE, et al. Seasonal variation in arterial blood pressure. BMJ. 1982;285:919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilmshurst P. Temperature and cardiovascular mortality. BMJ 1994;309:1029–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnett A, Dobson A, Salomaa V, et al; for the WHO MONICA Project . The effect of temperature on systolic blood pressure. Blood Press Monit. 2007;12:195–203. [DOI] [PubMed] [Google Scholar]
- 36. White M, Blanchet M, Ducharme A, et al. Beta‐adrenergic blockers attenuate exercise‐induced adrenergic activation and cold‐induced impairment in exercise performance in patients with congestive heart failure. J Am Coll Cardiol. 2001;37(suppl A): 281A. [Google Scholar]
- 37. Izzo JL, Larrabee PS, Sander E, et al. Hemodynamics of seasonal adaptation. Am J Hypertens. 1990;3:405–407. [DOI] [PubMed] [Google Scholar]
- 38. Ulmer H, Kelleher C, Diem G, et al. Estimation of seasonal variations in risk factor profiles and mortality from coronary heart disease. Wien Klin Wochenschr. 2004;116:662–668. [DOI] [PubMed] [Google Scholar]
- 39. Yanovski JA, Yanovski SZ, Sovik KN, et al. A prospective study of holiday weight gain. N Engl J Med. 2000;342:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kupari M, Koskinen P. Seasonal variation in occurrence of acute atrial fibrillation and relation to air temperature and sale of alcohol. Am J Cardiol. 1990;66:1519–1520. [DOI] [PubMed] [Google Scholar]


