Abstract
Background:
Pulmonary arterial hypertension (PAH) is a well‐known complication of systemic sclerosis (SSc). Doppler echocardiographic screening for the detection of PAH (by measuring right ventricular systolic pressure [RVSP]) is therefore recommended for all patients with SSc. However, the validity of RVSP as a predictor of mortality in patients with SSc is not well established.
Hypothesis:
Doppler‐determined PAH identifies a high‐risk subset of patients with SSc with decreased survival.
Methods:
We performed echocardiography in 155 consecutive patients with SSc between May 2005 and July 2006 and tested the value of an RVSP level of ≥36 mm Hg to predict mortality. Cox proportional hazards model was used to examine the individual relationship between each variable and the mortality rate.
Results:
Tricuspid regurgitant jets for RVSP determination were quantified in 129 patients (82.6%), of which 47 (36.4%) had RVSP ≥36 mm Hg. The median follow‐up time was 34 months. The 1‐, 2‐, and 3‐year survival rates were significantly lower among SSc patients with RVSP ≥36 vs ≤36 mm Hg (82%, 78%, and 67% vs 98%, 90%, and 86%, respectively, P < 0.01 by Wilcoxon test). In a multivariate analysis including echocardiographic and clinical variables, only an RVSP ≥36 mm Hg and a New York Heart Association III/IV class were associated with increased mortality; the respective Cox hazard ratios were 2.22 (95% confidence interval [CI]: 1.01–4.89, P = 0.048) and 4.77 (95% CI: 2.09–10.90, P = 0.000).
Conclusions:
Our results indicate that Doppler RVSP identifies a high‐risk subset and supports the use of Doppler RVSP as a screening test in patients with SSc who may warrant early treatment of their PAH. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by microvascular damage and fibrosis in multiple organs leading to significant morbidity and mortality.1, 2 Pulmonary complications, including pulmonary fibrosis and pulmonary artery hypertension, are serious complications in patients with systemic sclerosis.3, 4
Pulmonary arterial hypertension (PAH) in patients with SSc is associated with unfavorable prognosis and survival. The diagnosis of PAH is therefore pivotal because it has a significant impact on the treatment strategy and clinical outcomes.5 The symptoms that suggest PAH, which include fatigue and exertional dyspnea, may not manifest until the pulmonary vascular disease is advanced. Doppler echocardiographic screening for the presence of PAH is therefore recommended for all SSc patients to achieve early detection and plan treatment.6
In the absence of pulmonary valve stenosis or other right ventricular outflow obstruction, right ventricular systolic pressure (RVSP) derived from the tricuspid regurgitant peak velocity using Doppler echocardiography is equivalent (less the right atrial pressure) to pulmonary artery systolic pressure as measured at right heart catheterization. Previous studies demonstrated a good correlation between Doppler echocardiography and right heart catheterization measurements.7, 8, 9, 10 However, the validity of RVSP as an outcome measure in pulmonary arterial hypertension associated with systemic sclerosis (PAH‐SSc) has not been well established. In a systematic review reported by Kowal‐Bielecka et al, echocardiography was found to lack specificity and was not well validated as an outcome measure of PAH in SSc.11 Thus, the objective of the current study was to evaluate the value of RVSP assessed by echocardiography as a predictor of mortality in patients with SSc at risk of PAH.
Methods
Consecutive patients fulfilling the American College of Rheumatology criteria for the diagnosis of systemic sclerosis12 and who had initially undergone complete 2‐dimensional and Doppler echocardiography (without contrast) between May 2005 and July 2006 at Srinagarind Hospital, Khon Kaen University, were enrolled. The data set was locked to analysis in February 2009. The institutional ethics committee approved the study and use of data for this analysis.
The clinical features recorded at the initial presentation to the Scleroderma Clinic included: age, sex, vital parameters, duration of disease, modified Rodnan skin score, functional capacity according to the New York Heart Association (NYHA) classification and grouped as NYHA I‐II or III‐IV, the disease subtype (ie, limited and/or diffuse disease), Raynaud phenomenon, pulmonary fibrosis (determined by high‐resolution computed tomography [CT] scan or abnormal chest x‐ray), and calcium blocker use (yes/no). Death was defined as all causes of death determined from the medical records, phone contact, and/or death certificates.
Echocardiography was performed routinely in all patients with systemic sclerosis, and the initial echocardiographic parameters were used for this analysis. Complete 2‐dimensional and Doppler echocardiograms were recorded using a 2.5‐ or 3.5‐MHz phased array imaging transducer (Hewlett‐Packard Sonos 5500; Hewlett‐Packard Co., Palo Alto, CA). M‐mode recordings from parasternal long axis were used to determine the dimension of the left atrium and aorta. The right ventricular diameter was measured at parasternal long axis view. Left ventricular mass and ejection fraction were calculated using standard formulae.13 Pericardial effusion was carefully sought in all patients. The severity of valvular regurgitation was assessed on the basis of the color flow imaging from multiple views. The size and respiratory response of the inferior vena cava was not routinely obtained.
RVSP was measured by continuous wave Doppler echocardiography using the modified Bernoulli equation (p = 4 v2+ right atrial pressure, where v = the peak tricuspid regurgitant velocity and right atrial pressure was assumed to be 10 mm Hg). The apical 4‐chamber and short axis views were obtained to ascertain and record optimal tricuspid flow signals. Pulmonary hypertension was defined as an RVSP ≥ 36 mm Hg (tricuspid regurgitant velocity >2.5 m/s) and used in the analysis as a predictor of mortality.6 Doppler mitral valve (MV) inflow and left ventricular outflow velocities were recorded in the 4‐chamber view, with the sample volume placed at the level of the MV leaflet tips and immediately below the aortic valve, respectively. The right and left ventricular myocardial performance indices (MPI) were performed as previously reported.14, 15, 16 All echocardiographic parameters were made in triplicate then averaged.
Doppler measurements of 10 patients with SSc were performed by 2 independent observers (S.K. and C.W.) on 2 separate occasions to determine interpretative variability. The interobserver differences were calculated as the absolute value of the differences between 2 observations divided by the mean of the observations and were expressed in percentages.17
Continuous variables are presented as mean ± standard deviation, and categorical variables are described as frequencies and percentages. Differences between the patient groups for categorical variables were examined using the χ 2 or Fisher exact test or the z test. Differences in the continuous variables between groups were assessed using the Student t test, Mann‐Whitney U test, or Wilcoxon rank sum test, where appropriate. Eight clinical and echocardiographic variables were tested in a univariate analysis; these included age, NYHA class, type of scleroderma, RVSP, pericardial effusion, modified Rodman skin score, right ventricular MPI, and the presence of pulmonary fibrosis. A univariate Cox proportional hazards model was used to examine the individual relationship between each variable and the mortality rate.
Variables that achieved a P value <0.25 were selected for further testing in a multivariable model. HRs and 95% confidence intervals (CIs) were used to illustrate the association between potential risk factors and mortality. The Kaplan‐Meier method was used to estimate the survival probability. A 2‐sided P value <0.05 was required for statistical significance. All analyses were performed by using Stata version 10.0 (Stata Corp., College Station, TX).
Results
A total of 155 patients with systemic sclerosis were initially evaluated, of whom 26 were excluded because of an inadequate tricuspid regurgitant velocity signal. Thus, 129 patients satisfied the study protocol and were retained for analysis. Table 1 summarizes the clinical characteristics of all of the patients and of each group. The study population was largely female (73%) and middle‐aged (48 years); 77% had diffuse SSc, 69% Raynaud phenomenon, and 74% showed evidence of pulmonary fibrosis from either a high‐resolution CT scan (n = 30) or a chest x‐ray (n = 65). The majority of patients (77%) had a diffuse type of SSc, and the mean Rodnan skin score was 14.4. One hundred nine patients (84%) had an NYHA class of I/II, and 29 patients had an NYHA class of III/IV. The majority of patients (90%) received a calcium antagonist to control the Raynaud symptoms. Pulmonary vasodilators, including phosphodiesterase V inhibitor, endothelin receptor antagonist, and prostacyclin analog were rarely prescribed.
Table 1.
Overall Characteristics of Patients With Scleroderma
| Overall (n = 129) | RVSP < 36 mm Hg (n = 82) | RVSP ≥ 36 mm Hg (n = 47) | Pa | |
|---|---|---|---|---|
| Age, y | 48.8 ± 11.3 | 47.5 ± 11.7 | 50.9 ± 10.5 | 0.095 |
| Sex, females, n (%) | 94 (72.9) | 65 (69.1) | 29 (30.8) | 0.031 |
| Systolic BP | 115 ± 13.8 | 114.4 ± 13.4 | 118.6 ± 14.3 | 0.097 |
| Type of scleroderma | ||||
| Diffuse type, n (%) | 99 (76.7) | 60 (60.6) | 39 (39.4) | 0.209 |
| Limited type, n (%) | 30 (23.3) | 22 (72.4) | 8 (27.6) | |
| Rodnan skin score | 14.7 ± 7.7 | 13.9 ± 7.5 | 15.2 ± 8.1 | 0.308 |
| Pulmonary fibrosis, n (%) | 95 (73.6) | 58 (61.0) | 37 (38.9) | 0.321 |
| Disease duration (mo) | 46.5 ± 39.3 | 47.4 ± 40.9 | 44.8 ± 36.6 | 0.722 |
| Raynaud, n (%) | 90 (70.0) | 57 (63.3) | 33 (36.7) | 0.985 |
| Medication | ||||
| Calcium channel blocker | 115 (89.1) | 70 (60.8) | 45 (39.1) | 0.068 |
| Other vasodilator therapy | 14 (10.9) | 13 (92.8) | 2 (14.2) | |
| NYHA | ||||
| Class I,II | 109 (84.5) | 73 (66.9) | 36 (33.1) | 0.061 |
| Class III,IV | 20 (15.5) | 9 (45.0) | 11 (55.0) | |
| Death | 26 (20.2) | 11 (42.3) | 15 (57.7) | 0.011 |
Abbreviations: BP, blood pressure; NYHA, New York Heart Association; RVSP, right ventricular systolic pressure.
Comparisons between RVSP <36 mm Hg vs ≥36 mm Hg.
Echocardiographic data are presented in Table 2. Overall, 47 patients (36.4%) had an RVSP >36 mm Hg, 11 (23.4%) of whom were in NYHA class III/IV (Table 2). The right ventricular dimension in patients with RVSP ≥ 36 mm Hg were larger than those with RVSP <36 mm Hg (2.7 mm ± 0.4 mm vs 2.3 mm ± 0.4 mm, P < 0.000). Twenty‐eight patients (59.6%) had evidence of left ventricular diastolic dysfunction (E/A ratio <1) and 12 (9.3%) had a left ventricular ejection fraction <50%. The right and left ventricular myocardial performance indices were both similar in those with and without pulmonary hypertension. Minimal pericardial effusion (<10 mm) was detected in 32 patients (24.8%); the presence of pericardial effusion was no different in patients with RVSP ≥ 36 mm Hg vs those with RVSP <36 mm Hg.
Table 2.
Echocardiographic Findings
| Overall (n = 129) | RVSP <36 mm Hg (n = 82) | RVSP ≥36 mm Hg (n = 47) | Pa | |
|---|---|---|---|---|
| RV diameter (mm) | 2.49 ± 0.43 | 2.37 ± 3.41 | 2.69 ± 0.40 | <0.000 |
| LA diameter | 3.48 ± 2.99 | 3.38 ± 3.04 | 3.65 ± 2.92 | 0.628 |
| Aorta diameter | 2.90 ± 1.81 | 2.69 ± 0.30 | 3.27 ± 2.97 | 0.081 |
| IVS (d) (mm) | 0.94 ± 0.27 | 0.94 ± 0.18 | 0.94 ± 0.38 | 0.892 |
| LVD (d) (mm) | 4.68 ± 0.60 | 4.68 ± 0.59 | 4.69 ± 0.63 | 0.912 |
| PW (d) (mm) | 0.90 ± 0.14 | 0.91 ± 0.16 | 0.90 ± 0.11 | 0.758 |
| IVS (s) (mm) | 1.20 ± 0.22 | 1.20 ± 0.21 | 1.20 ± 0.21 | 0.890 |
| LVD (s) (mm) | 3.11 ± 0.54 | 3.11 ± 0.51 | 3.11 ± 0.59 | 0.996 |
| PW (s) (mm) | 1.29 ± 0.22 | 1.30 ± 0.25 | 1.26 ± 0.27 | 0.313 |
| LVEF (%) | 61.51 ± 9.29 | 61.43 ± 8.62 | 61.64 ± 10.53 | 0.945 |
| LV mass index | 101.09 ± 27.83 | 103.02 ± 29.21 | 97.76 ± 29.63 | 0.303 |
| MV E velocity (cm/s) | 73.39 ± 18.01 | 74.77 ± 17.01 | 70.87 ± 20.05 | 0.244 |
| MV A velocity (cm/s) | 70.93 ± 18.96 | 70.11 ± 18.20 | 72.42 ± 20.40 | 0.514 |
| MV E/A ratio | 1.09 ± 0.41 | 1.18 ± 0.39 | 1.06 ± 0.44 | 0.406 |
| LVET (msec) | 0.28 ± 0.03 | 0.28 ± 0.03 | 0.28 ± 0.03 | 0.896 |
| LV MPI index | 0.47 ± 0.19 | 0.48 ± 0.18 | 0.47 ± 0.20 | 0.755 |
| TV E velocity (cm/s) | 52.84 ± 12.75 | 54.26 ± 14.32 | 50.29 ± 18.38 | 0.094 |
| TV A velocity (cm/s) | 49.01 ± 15.86 | 48.17 ± 18.32 | 50.51 ± 18.38 | 0.430 |
| TV E/A ratio | 1.16 ± 0.39 | 1.19 ± 0.36 | 1.11 ± 0.45 | 0.245 |
| RVET (msec) | 0.28 ± 0.05 | 0.28 ± 0.03 | 0.28 ± 0.06 | 0.839 |
| RV MPI index | 0.40 ± 0.16 | 0.38 ± 0.14 | 0.42 ± 0.17 | 0.199 |
| Pericardial effusion, n (%) | 32 (24.8) | 17 (53.1) | 15 (46.8) | 0.321 |
Abbreviations: IVS, interventricular septum; LA, left atrium; LV, left ventricle; LVD (d), left ventricular end‐diastolic dimension; LVD (s), left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; LVET, left ventricular ejection time; MPI, myocardial performance index; MV, mitral valve; PW, posterior wall; RV, right ventricle; RVET, right ventricular ejection time; RVSP, right ventricular systolic pressure; TV, tricuspid valve.
Comparisons between RVSP <36 mm Hg vs ≥36 mm Hg.
During the median observational period of 34 months (range, 1–45 months), 26 patients (20.2%) died. The causes of death were as follows; respiratory failure (n = 14, 54%), heart failure (n = 4, 15%), renal failure (n = 3, 12%), sepsis (n = 3, 12%), and malignancy (n = 2, 7%). Overall, the average 1‐, 2‐, and 3‐year survival rate was 93%, 86%, and 79%, respectively. The respective 1‐, 2‐ and 3‐year survival of patients with RVSP ≥36 mm Hg vs <36 mm Hg was 82%, 78%, and 67% vs 98%, 90%, and 86%, respectively, P < 0.01 (Figure 1).
Figure 1.
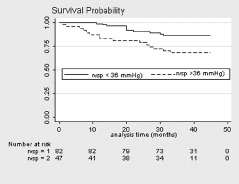
Kaplan‐Meier survival graph comparing patients with systemic sclerosis at right ventricular systolic pressure (RVSP) <36 mm Hg (RVSP = 1) and ≥36 mm Hg (RVSP = 2).
The univariate analyses of the clinical and echocardiographic variables are presented in Table 3. NYHA, RVSP, modified Rodman skin score, pulmonary fibrosis, diffuse systemic sclerosis, and pericardial effusion achieved a P value <0.25. Left ventricular ejection fraction (per 1% increment) was not a significant predictor of mortality in the univariate analysis with a Cox HR of 0.98 (95% CI: 0.98–1.02, P = 0.319).
Table 3.
Univariable Model for Risk Factors for Mortality in Patients With Scleroderma
| HR (95% CI) | P | |
|---|---|---|
| Age by 10 years | 0.99 (0.71–1.39) | 0.990 |
| Functional class III‐IV | 5.45 (2.49–11.89) | <0.000 |
| Diffuse systemic sclerosis | 8.15 (1.10–60.21) | 0.040 |
| Modified Rodman skin score | 1.08 (1.03–1.13) | 0.001 |
| Pulmonary fibrosis | 0.47 (0.16–1.38) | 0.175 |
| RVSP ≥36 mm Hg | 2.53 (1.25–5.95) | 0.011 |
| Pericardial effusion | 0.56 (0.25–1.27) | 0.169 |
| Increased RV MPI by 0.1 | 1.01 (0.79–1.29) | 0.919 |
| Left ventricular ejection fraction by 1% | 0.97 (0.98–1.02) | 0.319 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MPI, myocardial performance index; RV, right ventricle; RVSP, right ventricular systolic pressure.
In the multivariate analysis (Table 4) only RVSP ≥ 36 mm Hg and a NYHA class of III/IV were associated with increased mortality, with a Cox HR of 2.22 (95% CI: 1.01–4.89, P = 0.048) and 4.77 (95% CI: 2.09–10.90, P < 0.000), respectively.
Table 4.
Multivariable Model for Risk Factors for Mortality in Patients With Scleroderma
| HR (95% CI) | P | |
|---|---|---|
| Functional class III,IV | 4.77 (2.09–10.90) | <0.000 |
| Diffuse systemic sclerosis | 6.02 (0.79–45.89) | 0.083 |
| Modified Rodman skin score | 1.04 (0.99–1.09) | 0.097 |
| Pulmonary fibrosis | 0.59 (0.20–1.73) | 0.338 |
| RVSP ≥36 mm Hg | 2.22 (1.01–4.89) | 0.048 |
| Pericardial effusion | 0.76 (0.33–1.79) | 0.540 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RVSP, right ventricular systolic pressure.
There was an acceptable, nonsignificant 10.4% ± 9.5% difference in the RVSP value obtained by 2 observers (P = 0.41); the correlation between the 2 observers was excellent (r = 0.91, P < 0.001). The interobserver variability for the right ventricular index of myocardial performance was 16.4% ± 10.3%.
Discussion
The principle finding of this study was that pulmonary artery pressure measured noninvasively identifies a high‐risk subset; specifically, a Doppler‐determined RVSP ≥36 mm Hg is independently associated with a decreased survival rate in patients with SSc.
The prevalence of PAH defined by Doppler echocardiography in the present study was 36%, which is comparable with the 32% prevalence of Doppler‐detected PAH reported by Kumar et al.18 However, MacGregor et al,19 using a single pressure reading of ≥30 mm Hg in 930 patients with SSc, reported that the cumulative prevalence of PAH was 13%. Moreover, Hachulla et al20 found that only 18 of 33 (54.5%) patients with suspected PAH, based on Doppler echocardiography, were later confirmed to have PAH by right heart catheterization. Although these data suggest that the prevalence of PAH assessed by echocardiography tends to be overestimated compared to right heart catheterization, different populations and bias, nonsimultaneous acquisition, and methodological variability may explain part of this discrepancy. Nevertheless, the validity of echocardiography as an outcome measure in scleroderma‐related pulmonary hypertension was found to lack specificity in a systematic review by Kowal‐Bielecka et al.11 It should be noted that the specificity of echocardiography for the diagnosis of PAH increased with a higher RVSP threshold, achieving 97% for RVSP ≥45 mm Hg.
Although the definition of clinically significant PAH using the estimation of right ventricular systolic pressure by Doppler‐echocardiography is not precisely defined, it has become an important screening tool for the presence of PAH in patients with SSc. An RVSP of approximately 36 mm Hg is commonly used as the definition for PAH,20, 21, 22 and this was our prespecified cutoff point. Statistical significance was lost when RVSP was treated as a continuous variable (HR = 1.02 for every mm Hg RVSP, 95% CI: 0.99–1.04, P = 0.132); this may be due to a nonlinear dose response of pressure or to a skewed distribution of pressures. An important limitation of Doppler‐determined RVSP is that not all patients have a sufficient tricuspid regurgitation jet needed for its calculation. Notably, in the study by Borgeson et al,7 Doppler echocardiography examination by experienced sonographers yielded tricuspid regurgitation signals in only 74% of cases. More recently, Ristow et al were able to measure tricuspid regurgitation gradients in 80% of cases.23 By comparison, in the current study quantifiable tricuspid regurgitation signals were obtained in 83.2% (129/155) of patients. Despite the limitations of Doppler echocardiography, this study demonstrated that RVSP ≥36 mm Hg can be used as a predictor of survival and indicator of scleroderma patients who may warrant early treatment of PAH.
Several studies have identified predictors of mortality in patients with scleroderma.24, 25, 26 Although elevated mean pulmonary artery pressure assessed by right heart catheterization and the NYHA class are strongly associated with high mortality in patients with SSc, the significance of interstitial lung disease and pulmonary fibrosis on the survival rate is controversial. For example, Mathai et al,26 reported that interstitial lung disease was associated with increased mortality in SSc patients with PAH using data from right heart catheterization. Nadrous et al reported that pulmonary hypertension (systolic pulmonary artery pressure >50 mm Hg) assessed by Doppler cardiography was a significant predictor of mortality in patients with idiopathic pulmonary fibrosis.27 In contrast, Chang et al21 found no difference in survival between patients with or without interstitial lung disease. In the current study, pulmonary fibrosis determined by high‐resolution CT scan or abnormal chest x‐ray was not independently associated with mortality in the multivariate analysis. The lack of correlation of pulmonary fibrosis with pulmonary hypertension in this study is unexpected. The differences may result in part by the variable definitions of PAH (and its severity) and interstitial lung disease and pulmonary fibrosis.
The right ventricular (RV) MPI is a measure of combined RV systolic and diastolic function associated with mortality in patients with pulmonary hypertension. In 1 study, a 0.1 increase in the index resulted in a 1.3 times greater risk of death.28 Yeo et al found that RV MPI was a prognostic factor for cardiac death and lung transplantation.29 By contrast, in our study, an increase of MPI by 0.1 was not associated with mortality in patients with scleroderma. The reasons for this discrepancy are unclear, and the application of RV MPI in patients with scleroderma warrants further study.
Although the administration of pulmonary vasodilators, including phosphodiesterase V inhibitor, endothelin receptor antagonist, and prostacyclin analogs improves survival of patients with PAH,30, 31 most patients in the current study were undertreated with these types of medications. For example, the 1‐year survival rate of SSc‐PAH patients who received bosentan was 85.9% compared to 82% among those who did not receive it.32
Conclusion
Our results indicate that Doppler RVSP identifies a high‐risk subset and supports the use of Doppler RVSP as a screening test in patients with SSc who may warrant early treatment of their PAH.
Study Limitations
There are several limitations that have to be taken into consideration when interpreting the results from this study. First, retrospective observational study designs are subject to bias. Second, confirmatory right heart catheterization measurements were not available, limiting the ability to extrapolate the correlation of RVSP derived from echocardiography and mean pulmonary artery pressure. The right atrial pressure was assumed to be 10 mm Hg, which may influence RVSP estimates, particularly at relatively low pulmonary arterial pressures. Third, estimates of right ventricular tissue Doppler and strain were not available. Fourth, echocardiographic measurements were unidimensional M‐modes, although ventricles were of uniform geometry. Finally, other significant predictors of mortality in patients with SSc, for example carbon monoxide diffusing capacity and 6‐minute walk (55% of patients in the current study had joint deformities that precluded a 6‐minute walk) were not recorded. Despite these limitations, this study provides strong support for Doppler echocardiographic screening of individuals with SSc at risk for pulmonary arterial hypertension.
Acknowledgements
We thank the Department of Medicine and the Faculty of Medicine, Khon Kaen University for their support, and Mr. Bryan Roderick Hamman for assistance with the English‐language presentation of the manuscript.
References
- 1. Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford). 2009;48(suppl 3): iii45–iii48. [DOI] [PubMed] [Google Scholar]
- 2. Hinchcliff M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician. 2008;78:961–968. [PubMed] [Google Scholar]
- 3. Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, et al. Mortality in systemic sclerosis: an international meta‐analysis of individual patient data. Am J Med. 2005;118:2–10. [DOI] [PubMed] [Google Scholar]
- 4. Wells AU, Steen V, Valentini G. Pulmonary complications: one of the most challenging complications of systemic sclerosis. Rheumatology (Oxford). 2009;48(suppl 3):iii40–iii44. [DOI] [PubMed] [Google Scholar]
- 5. McLaughlin V, Humbert M, Coghlan G, et al. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford). 2009;48(suppl 3): iii25–iii31. [DOI] [PubMed] [Google Scholar]
- 6. Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):40S–47S. [DOI] [PubMed] [Google Scholar]
- 7. Borgeson DD, Seward JB, Miller FA, et al. Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr. 1996;9:832–837. [DOI] [PubMed] [Google Scholar]
- 8. Chotivittayatarakorn P, Pathmanand C, Thisyakorn C, et al. Doppler echocardiographic predictions of pulmonary artery pressure in children with congenital heart disease. J Med Assoc Thai. 1992;75:79–84. [PubMed] [Google Scholar]
- 9. Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler‐catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. [DOI] [PubMed] [Google Scholar]
- 10. Denton CP, Cailes JB, Phillips GD, et al. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol. 1997; 36:239–243. [DOI] [PubMed] [Google Scholar]
- 11. Kowal‐Bielecka O, Avouac J, Pittrow D, et al. Echocardiography as an outcome measure in scleroderma‐related pulmonary arterial hypertension: a systematic literature analysis by the EPOSS group. J Rheumatol. 2010;37:105–115. [DOI] [PubMed] [Google Scholar]
- 12. Preliminary criteria for the classification of systemic sclerosis (scleroderma) . Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. [DOI] [PubMed] [Google Scholar]
- 13. Devereaux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 14. Mugerwa JA, Kiatchoosakun S, Restivo J, et al. The myocardial performance index in patients with aortic stenosis. Echocardiography. 2002;19:267–272. [DOI] [PubMed] [Google Scholar]
- 15. Dujardin KS, Tei C, Yeo TC, et al. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic‐dilated cardiomyopathy. Am J Cardiol. 1998;82:1071–1076. [DOI] [PubMed] [Google Scholar]
- 16. Gullulu S, Kaderli AA, Ekbul A, et al. Tissue Doppler echocardiography and myocardial performance index in patients with scleroderma. J Int Med Res. 2005;33:417–424. [DOI] [PubMed] [Google Scholar]
- 17. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1: 307–310. [PubMed] [Google Scholar]
- 18. Kumar U, Ramteke R, Yadav R, et al. Prevalence and predictors of pulmonary artery hypertension in systemic sclerosis. J Assoc Physicians India. 2008;56:413–417. [PubMed] [Google Scholar]
- 19. MacGregor AJ, Canavan R, Knight C, et al. Pulmonary hypertension in systemic sclerosis: risk factors for progression and consequences for survival. Rheumatology. 2001;40:453–459. [DOI] [PubMed] [Google Scholar]
- 20. Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005; 52:3792–3800. [DOI] [PubMed] [Google Scholar]
- 21. Chang B, Wigley FM, White B, et al. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol. 2003;30:2398–2405. [PubMed] [Google Scholar]
- 22. Schachna L, Wigley FM, Chang B, et al. Age and risk of pulmonary arterial hypertension in scleroderma. Chest. 2003;124: 2098–2104. [DOI] [PubMed] [Google Scholar]
- 23. Ristow B, Ali S, Ren X, et al. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease. J Am Coll Cardiol. 2007;49:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung L, Krishnan E, Chakravarty EF. Hospitalizations and mortality in systemic sclerosis: results from the Nationwide Inpatient Sample. Rheumatology. 2007;46:1808–1813. [DOI] [PubMed] [Google Scholar]
- 25. D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 26. Mathai SC, Hummers LK, Champion HC, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum. 2009; 60:569–577. [DOI] [PubMed] [Google Scholar]
- 27. Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with pulmonary fibrosis. Chest. 2005;128: 2393–2399. [DOI] [PubMed] [Google Scholar]
- 28. Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. [DOI] [PubMed] [Google Scholar]
- 29. Yeo TC, Dujardin KS, Tei C, et al. Value of a Doppler‐derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998; 81:1157–1161. [DOI] [PubMed] [Google Scholar]
- 30. McLaughlin VV. Survival in patients with pulmonary arterial hypertension treated with first‐line bosentan. Eur J Clin Invest. 2006;36(suppl 3):iii10–iii15. [DOI] [PubMed] [Google Scholar]
- 31. Denton CP, Pope JE, Peter HH, et al. Long‐term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Ann Rheum Dis. 2008;67:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Denton CP, Humbert M, Rubin L, et al. Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open‐label extensions. Ann Rheum Dis. 2006;65:1336–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]


