Abstract
Background:
The difference in underlying pathophysiology in different congenital heart disease (CHD) may have an influence on clinical outcome. It remains unclear whether the effect of sildenafil on pulmonary arterial hypertension (PAH) varies in different types of CHD.
Hypothesis:
The potential effect of sildenafil on pulmonary arterial hypertension related to CHD may be associated with shunt location.
Methods:
In this 12‐week, prospective, open label, multicenter trial, 55 patients with CHD were divided into the 3 groups: atrial septal defects group (ASD, n = 15), ventricular septal defects group (VSD, n = 24), and patent ductus arteriosus group (PDA, n = 16). Exercise capacity, hemodynamic parameters, and arterial oxygen saturation were assessed at baseline and after sildenafil therapy (25 mg, 3 times daily).
Results:
Six‐minute walk distance significantly increased from 377.2 ± 68.7 m to 436.0 ± 70.4 m in patients with ASD, from 371.2 ± 66.0 m to 413.7 ± 83.1 m in VSD, and from 384.3 ± 90.2 m to 440.9 ± 71.8 m in PDA (P<0.01, respectively). Moreover, sildenafil also improved the pulmonary vascular resistance and pulmonary blood flow index in the 3 groups, whereas no significant changes in systemic vascular resistance and systemic arterial pressure were observed. However, arterial oxygen saturation was significantly improved in the ASD group only. The incidence of adverse events was similar among the 3 groups.
Conclusions:
Sildenafil therapy seems to be effective and safe for PAH secondary to ASD, VSD, and PDA, although some clinical and hemodynamic parameters were changed in a different manner among the 3 groups. © 2011 Wiley Periodicals, Inc.
This study was partly supported by National Grant from The Ministry of Science and Technology (2006BAI01A07) and Capital Development Scientific Fund (2005‐1018). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Study Group participants are listed in the Supporting Information Appendix.
Introduction
Pulmonary arterial hypertension (PAH) is a common, long‐term complication in congenital heart disease (CHD),1, 2 which may persist even after shunt closure by surgical or percutaneous means. Simple CHD can be categorized as pretricuspid such as atrial septal defects (ASD) and post‐tricuspid such as ventricular septal defects (VSD), or patent ductus arteriosus (PDA) lesions.1 Severity of PAH is related to the location of the intracardiac defect.2 In addition, because the direction and magnitude of flow across the shunt in post‐tricuspid lesions is largely dependent on the ratio between pulmonary vascular resistance and systemic vascular resistance, the use of disease‐targeting therapies, which may potentially lower systemic vascular resistance more than pulmonary vascular resistance, may be at risk of aggravating the right‐to‐left shunt.3, 4 Thus, the difference in underlying pathophysiology in the different CHDs may have a potential influence on treatment outcome.3, 5 This highlights the importance of evaluating the safety and efficacy of disease‐targeting therapies in the different types of CHD.
A previous study has suggested that a treatment effect of bosentan in patients with PAH related to Eisenmenger syndrome was independent of the location of septal defect.4 Some studies have demonstrated that sildenafil is a safe and effective treatment for PAH related to CHD and/or Eisenmenger syndrome.6, 7, 8, 9, 10 However, no study has specifically assessed its effect in the different types of CHD, and it remains unclear whether the effect of sildenafil on pulmonary arterial hypertension varies in different types of CHD. The objective of the present study was, therefore, to evaluate efficacy and safety of oral sildenafil in patients with PAH secondary to ASD, VSD, or PDA, respectively.
Methods
Study Population
Patients with PAH associated with simple CHD were included if they had persistent PAH at 5 years after surgical or interventional repair (ASD group, n = 1; VSD group, n = 2; PDA group, n = 1), or if they were not eligible for surgical or interventional treatment because of Eisenmenger syndrome (ASD group, n = 12; VSD group, n = 20; PDA group, n = 15), or economic problems (ASD group, n = 2; VSD group, n = 2). The exclusion criteria were the following: (1) a positive acute vasodilator response; (2) current treatment with PAH‐specific therapy; (3) inability to perform a 6‐minute walk test or with a 6‐minute walk distance (6MWD) of <100 m or >500 m; (4) previously diagnosed heart diseases such as serious cardiac arrhythmias, unstable angina pectoris, and myocardial infarction; (5) low blood pressure (<90/50) or uncontrolled hypertension (>170/110 mm Hg); (6) positive pregnancy test or breastfeeding practice, and (7) history or suspicion of inability to cooperate. This study complied with the Declaration of Helsinki and was approved by the institutional review board of Fu Wai Hospital. A written informed consent was obtained from all patients.
Study Design and Procedures
The study of oral sildenafil therapy on PAH associated with the different types of CHD was a 12‐week, prospective, open label, multicenter trial. Primary endpoint was defined as the change in 6MWD from baseline to week 12. Secondary endpoints were defined as changes in World Health Organization (WHO) functional class, Borg dyspnea index, arterial oxygen saturation, and hemodynamic parameters from baseline to week 12.
There were 55 patients with PAH related to CHD enrolled. Diagnosis of PAH was confirmed using right heart catheterization. In this study, 3 groups were identified based on the type of congenital heart defects, including the ASD group (n = 15), the VSD group (n = 24), and the PDA group (n = 16). In addition to their conventional therapy (cardiac glycosides, diuretics, anticoagulants) for PAH, patients received oral sildenafil (Viagra; Pfizer Ltd., New York, NY), 25 mg, 3 times daily for 12 weeks. Treatment with calcium channel blocker during the study period was prohibited. Exercise capacity, Borg dyspnea index (with 0 representing no dyspnea and 10 maximal dyspnea), WHO functional classification of PAH, hemodynamics parameters derived from right heart catheterization, and arterial oxygen saturation were assessed at baseline and after 12 weeks of sildenafil. The 6‐minute walk test was performed on all patients according to American Thoracic Society guidelines.11 Both pulmonary blood flow and systemic blood flow were calculated using the Fick equation. Pulmonary vascular resistance and systemic vascular resistance were calculated from standard equations. Adverse events were monitored throughout the study.
Statistical Methods
Continuous variables were presented as mean ± standard deviation or median (Q1, Q3). Categorical variables were presented as frequencies and percentages. Statistical comparisons among the 3 groups were performed using 1‐way analysis of variance followed by a least significant difference test or nonparametric test (Kruskal‐Wallis H test). Categorical variables were analyzed using χ 2 or Fisher exact test. Comparisons of the parameters before and after chronic sildenafil treatment were performed using the paired t test. Statistical analysis was performed using the SPSS 13.0 (SPSS Inc., Chicago, IL) statistical package. P < 0.05 was considered significant.
Results
Baseline Characteristics of Patients
Baseline characteristics of patients in the 3 groups are summarized in Tables 1, 2, 3. There was no significant difference in the sex ratio and weight among the 3 groups. The predominant WHO functional class at baseline was class II. Concomitant medications did not differ significantly among the 3 groups. Patients in the ASD group were older than those in the VSD and PDA groups, and those in the ASD group had lower mean pulmonary arterial pressure than those in the VSD and PDA groups. Pulmonary vascular resistance and systemic blood flow index were significantly higher in the PDA group than those in the ASD group. There was no significant difference in the 6MWD, WHO functional class, Borg dyspnea index, mean systemic arterial pressure, and pulmonary blood flow index among the 3 groups at baseline.
Table 1.
Baseline Characteristics of the 3 Groups of Patients With CHD
| ASD (n = 15) | VSD (n = 24) | PDA (n = 16) | P | |
|---|---|---|---|---|
| Age, y | 32.20 ± 6.32 | 26.58 ± 9.61a | 23.75 ± 7.47b | 0.020 |
| Male/ female ratio | 0/15 | 8/16 | 4/12 | 0.093 |
| Weight, kg | 50.17 ± 10.26 | 52.78 ± 12.85 | 48.81 ± 11.71 | 0.565 |
| WHO functional class (mean ± SD) | 2.3 ± 0.5 | 2.3 ± 0.4 | 2.3 ± 0.6 | 0.994 |
| II, n (%) | 11 (73.3) | 18 (75.00) | 13 (81.3) | |
| III, n (%) | 4 (26.7) | 6 (25.00) | 2 (12.5) | |
| IV, n (%) | 0 (0) | 0(0) | 1 (6.2) | |
| Shunt patency, n (%) | ||||
| Open | 14 (93.3) | 22 (91. 7) | 15 (93.7) | 1.000 |
| Closed | 1 (6.7) | 2 (8.3) | 1 (6.3) | |
| Concomitant medication, n (%) | ||||
| Diuretic | 13 (86.7) | 17 (70.8) | 14 (87.5) | 0.432 |
| Digoxin | 10 (66.7) | 16 (66.7) | 12 (75.0) | 0.869 |
| Warfarin | 7 (46.7) | 5 (20.8) | 7 (43.8) | 0.168 |
Abbreviations: ASD, atrial septal defects; CHD, congenital heart disease; PDA, patent ductus arteriosus; SD, standard deviation; VSD, ventricular septal defects; WHO, World Health Organization.
Significant compared with ASD group.
P = 0.043.
P = 0.006.
Table 2.
Exercise Capacity, Functional Status, Arterial Oxygen Saturation, and Mean Systemic Arterial Pressure in the 3 Groups at Baseline and 12 Weeks
| ASD | VSD | PDA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | P | Baseline | 12 Weeks | P | Baseline | 12 Weeks | P | |
| 6MWD, m | 377.2 ± 68.7 | 436.0 ± 70.4 | <0.001 | 371.2 ± 66.0 | 413.7 ± 83.1 | 0.006 | 384.3 ± 90.2 | 440.9 ± 71.8 | 0.006 |
| Borg dyspnea index | 2.6 ± 1.3 | 1.8 ± 1.0 | 0.041 | 2.6 ± 1.5 | 2.4 ± 1.5 | 0.496 | 2.2 ± 1.4 | 1.9 ± 1.5 | 0.647 |
| WHO functional class | 2.3 ± 0.5 | 2.1 ± 0.6 | 0.189 | 2.3 ± 0.4 | 1.8 ± 0.6 | 0.002 | 2.3 ± 0.6 | 1.8 ± 0.5 | 0.029 |
| SaO2, %a | 90.98 ± 5.04 | 93.26 ± 2.95 | 0.019 | 88.93 ± 4.83 | 89.31 ± 5.09 | 0.748 | 86.49 ± 3.88b | 87.31 ± 4.60 | 0.598 |
| MAP, mm Hg | 87.82 ± 10.74 | 86.60 ± 7.31 | 0.631 | 84.54 ± 7.14 | 85.03 ± 10.03 | 0.809 | 86.48 ± 10.47 | 82.42 ± 11.79 | 0.082 |
Abbreviations: 6MWD, 6‐minute walk distance; ASD, atrial septal defects; MAP, mean systemic arterial pressure; PDA, patent ductus arteriosus; VSD, ventricular septal defects; SaO2, arterial oxygen saturation; WHO, World Health Organization.
ASD group, n = 13; VSD group, n = 16; and PDA group, n = 11.
Significant compared with ASD group at baseline, P = 0.024.
Table 3.
Change From Baseline to Week 12: Hemodynamic Parameters
| ASD | VSD | PDA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | P | Baseline | 12 Weeks | P | Baseline | 12 Weeks | P | |
| HR, beats/min | 88.00 ± 11.88 | 81.00 ± 6.26 | 0.021 | 77.54 ± 9.22a | 75.50 ± 9.59 | 0.238 | 82.25 ± 13.06 | 80.06 ± 13.16 | 0.279 |
| mRAP,b mm Hg | 7.85 ± 3.08 | 6.33 ± 3.57 | 0.177 | 8.04 ± 4.40 | 7.49 ± 5.97 | 0.607 | 10.38 ± 9.48 | 7.69 ± 6.16 | 0.279 |
| mPAP, mm Hg | 65.58 ± 11.12 | 60.85 ± 8.96 | 0.054 | 86.32 ± 19.93c | 76.58 ± 16.81 | <0.001 | 91.71 ± 17.34c | 86.04 ± 16.83 | 0.245 |
| Qpi, L·min−1·m−2 | 2.8 ± 1.0 | 4.1 ± 1.8 | 0.008 | 2.8 ± 1.0 | 3.4 ± 1.5 | 0.013 | 2.6 ± 0.9 | 3.4 ± 1.0 | 0.004 |
| Qsid, L·min−1·m−2 | 2.5 ± 0.6 | 3.3 ± 0.7 | 0.05 | 2.7 ± 0.7 | 2.7 ± 0.6 | 0.881 | 3.4 ± 1.0e | 3.3 ± 0.7 | 0.842 |
| PVR, dyn·s·cm−5 | 1333.7 ± 427.7 | 921.7 ± 384.0 | <0.001 | 1830.8 ± 977.3 | 1377.5 ± 682.9 | <0.001 | 2190.4 ± 1348.3f | 1495.5 ± 759.9 | 0.046 |
| SVRg, dyn·s·cm−5 | 1849.2 ± 520.5 | 1479.0 ± 404.1 | 0.166 | 1569.4 ± 381.4 | 1558.9 ± 373.2 | 0.942 | 1456.4 ± 811.2 | 1339.7 ± 621.1 | 0.499 |
| PVR/SVR ratiog | 0.79 ± 0.30 | 0.66 ± 0.24 | 0.089 | 1.48 ± 0.96 | 0.91 ± 0.40 | 0.006 | 2.15 ± 1.42f | 1.46 ± 0.60 | 0.225 |
Abbreviations: ASD, atrial septal defects; HR, heart rates; MAP, mean systemic arterial pressure; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; PDA, patent ductus arteriosus; PVR, pulmonary vascular resistance; Qpi, pulmonary blood flow index; Qsi, systemic blood flow index; SVR, systemic vascular resistance; VSD, ventricular septal defects.
Compared with baseline values:
VSD group vs ASD group, P < 0.01.
ASD group, n = 13; VSD group, n = 23; PDA group, n = 14.
PDA group or VSD group vs ASD group, P < 0.01.
ASD group, n = 10; VSD group, n = 16; PDA group, n = 11.
PDA group vs ASD or VSD group, P < 0.05.
PDA group vs ASD group, P < 0.05.
ASD group, n = 9; VSD group, n = 15; PDA group, n = 9.
Effect of Sildenafil on Exercise Capacity, Functional Status, Arterial Oxygen Saturation, and Mean Systemic Arterial Pressure
Results of the exercise capacity, functional status, arterial oxygen saturation and mean systemic arterial pressure in the 3 groups at baseline and at 12 weeks are shown in Table 2. The 6MWD increased significantly in all groups compared with baseline values (Figure 1). There was a significant decrease in Borg dyspnea index in the ASD group compared with baseline, but it did not reach statistical significance in the VSD and PDA groups. The WHO functional class improved significantly in both the VSD group and the PDA group. In the ASD group, arterial oxygen saturation improved significantly from 90.98% ± 5.04% to 93.26% ± 2.95% (P = 0.019). There was no significant change in the mean systemic arterial pressure compared to baseline in all groups.
Figure 1.
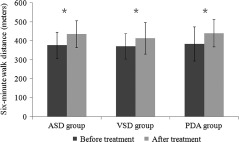
The 6‐minute walk distance increased significantly in all groups after 12‐weeks of sildenafil therapy (*P < 0.01). Abbreviations: ASD, atrial septal defects; PDA, patent ductus arteriosus; VSD, ventricular septal defects.
Effect of Sildenafil on Hemodynamic Parameters
The results of the baseline and the 12‐week postsildenafil hemodynamic data in the 3 groups are shown in Table 3. Heart rate decreased significantly in the ASD group. For mean pulmonary artery pressure, significant reduction was only observed in the VSD group. However, pulmonary blood flow index increased significantly in all groups. There was a borderline significant increase in systemic blood flow index in the ASD group. Pulmonary vascular resistance decreased by 30.89% (ASD group), 24.76% (VSD group), and 31.72% (PDA group) to 921.7 ± 384.0 dyn·s·cm−5 (ASD group), 1377.5 ± 682.9 dyn·s·cm−5 (VSD group), and 1495.5 ± 759.9 dyn·s·cm−5 (PDA group) (P<0.001, P<0.001, P = 0.046, respectively). As compared with baseline, sildenafil administration for 12 weeks did not result in a significant decrease in mean right atrial pressure or systemic vascular resistance in all groups. The pulmonary vascular resistance/systemic vascular resistance ratio decreased significantly in VSD group, whereas there was a trend toward decrease in the ASD and PDA groups.
Comparision of Changes in Clinical and Hemodynamic Parameters in Patients With the Different Types of CHD
There were no significant differences among the 3 groups in terms of changes in 6MWD, WHO functional class, arterial oxygen saturation, and hemodynamics (Table 4).
Table 4.
Comparision of Changes in Clinical and Hemodynamic Parameters in Patients With Different Types of Congenital Heart Desease
| ASD | VSD | PDA | ||
|---|---|---|---|---|
| Change From Baseline | Change From Baseline | Change From Baseline | P Value | |
| 6MWD, m | 50.00 (29.00, 85.00) | 38.00 (15.50, 86.25) | 42.50 (20.50, 70.75) | 0.679 |
| Borg dyspnea index | −1.00 (−1.50, 0) | 0 (−1.00, 1.00) | 0 (−1.63, 0.75) | 0.408 |
| Improved WHO functional class | 2 (13.3) | 9 (37.5) | 5 (31.3) | 0.288 |
| SaO2, %a | 1.90 (0.05, 3.25) | 0.80 (−3.73, 4.95) | 1.40 (−0.30, 3.20) | 0.751 |
| MAP, mm Hg | −4.67 (−5.00, 3.33) | 0.83 (−6.67, 6.67) | −3.33 (−9.17, 0.00) | 0.437 |
| HR, beats/min | −6.00 (−13.00, 2.00) | 0 (−6.75, 2.75) | 0 (−7.25, 3.75) | 0.286 |
| mRAP,b mm Hg | −2.00 (−5.00, −0.34) | −1.00 (−3.00, 1.00) | −0.50 (−9.75, 4.49) | 0.627 |
| mPAP, mm Hg | −3.00 (−15.00, 2.00) | −7.00 (−15.75, −1.50) | −5.00 (−14.00, 11.42) | 0.396 |
| Qpi, L·min−1·m−2 | 0.60 (0.07, 1.95) | 0.46 (‐0.24, 0.84) | 0.63 (0.05, 1.54) | 0.527 |
| Qsic, L·min−1·m−2 | 0.62 (−0.11, 1.37) | 0.22 (−0.28, 0.41) | 0.11 (−0.62, 0.51) | 0.244 |
| PVR, dyn·s·cm−5 | −305.88 (−673.30, −238.25) | −493.03 (−755.15, −127.30) | −327.89 (−804.23, −46.99) | 0.971 |
| SVRd, dyn·s·cm−5 | −244.20 (−536.89, 166.01) | −218.71 (−467.51, 446.91) | −18.79 (−513.48, 257.78) | 0.757 |
| PVR/SVR ratiod | −0.13 (−0.30, 0.02) | −0.36 (−1.26, −0.08) | −0.56 (−0.80, 0.29) | 0.369 |
Abbreviations: 6MWD, 6‐minute walk distance; ASD, atrial septal defects; HR, heart rates; MAP, mean systemic arterial pressure; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; PDA, patent ductus arteriosus; PVR, pulmonary vascular resistance; Qpi, pulmonary blood flow index; Qsi, systemic blood flow index; SaO2, arterial oxygen saturation; SVR, systemic vascular resistance; VSD, ventricular septal defects; WHO, World Health Organization.
Changes from baseline data are expressed as median (Q1, Q3) or n (%).
ASD group, n = 13; VSD group, n = 16; and PDA group, n = 11.
ASD group, n = 13; VSD group, n = 23; PDA group, n = 14.
ASD group, n = 10; VSD group, n = 16; PDA group, n = 11.
ASD group, n = 9; VSD group, n = 15; PDA group, n = 9.
Safety
There were 8 patients who reported 10 adverse events during the study period. Two patients reported more than 1 adverse event. Recorded adverse events included headache (1 in the ASD group and 2 in the VSD group), flushing (1 in the VSD group), nasal congestion (1 in the PDA group), tinnitus (1 in the ASD group and 1 in the VSD group), menorrhagia and irregular menstrual cycles (2 in the VSD group and 1 in the PDA group). Most adverse events were mild to moderate in degree. No patient died during the 12‐week trial period. The incidence of adverse events was similar among the 3 groups.
Discussion
There was no data available regarding the effect of sildenafil on pulmonary arterial hypertension in the different types of CHD. Our study, for the first time, demonstrated that the use of sildenafil could improve exercise capacity and pulmonary hemodynamics without deterioration in systemic hemodynamics and systemic arterial oxygen saturation in patients with PAH secondary to ASD, VSD, or PDA.
Significant improvement in exercise capacity, decrease in Borg dyspnea index, and improvement in WHO functional class were observed in the 3 groups, indicating that patients were able to increase their exercise capacity with a similar or even lower level of perceived dyspnea after use of sildenafil. The mean increase in 6MWD observed in our study was in the range of 42 to 59 m, which is similar to the finding in the sildenafil use in the PAH study in which the mean placebo‐corrected treatment effect was about 45 m after sildenafil therapy (20 mg, 3 times daily).12 As poor exercise capacity is associated with increased risk of hospitalization or death in patients with CHD,13 and it has been widely used as the primary endpoint in most pivotal PAH clinical trials,12, 14, 15 the use of sildenafil, which can improve exercise capacity, may also result in reduction in hospitalization and death as shown in the Dimopoulos et al study.16
In our study, mean pulmonary arterial pressure and pulmonary vascular resistance in the VSD or PDA groups were higher than those in the ASD group. However, pulmonary blood flow index and functional status were similar among the 3 groups. This phenomenon may be attributed to the adaptation of the right ventricle to high pressure with time.7
Treatment with sildenafil caused significant improvement in pulmonary hemodynamics in terms of pulmonary vascular resistance and pulmonary blood flow index in all groups. Sildenafil did not adversely affect systemic hemodynamics. This is consistent with previous studies and reflects the pulmonary selectivity of sildenafil.17, 18 The fact that heart rates did not increase in this study suggests that sympathetic activation was probably not the mechanism for the observed increase in the pulmonary blood flow index.9, 19 The increase in pulmonary blood flow index accompanied by a significant reduction in pulmonary vascular resistance in our study suggested that reduction in right ventricular afterload might be 1 of the reasons leading to increase in pulmonary blood flow index. These results are consistent with previous studies.9, 19 Sildenafil is able to relax pulmonary arterial smooth muscle by enhancing nitric oxide‐mediated pathway and has an antiproliferative effect on human pulmonary vascular smooth muscle cells.20, 21 In our study, patients with VSD or PDA suffered from more severe vascular remodeling and therefore might be less responsive to vasodilator stimuli. However, the observed reduction in pulmonary vascular resistance was similar among the 3 groups. This may imply that reversal of the pulmonary vascular remodeling plays important part in the decrease in pulmonary vascular resistance.
Our study demonstrated that the changes from baseline in exercise capacity and hemodynamics were not significantly dissimilar among the 3 groups. These results are in agreement with the results of Breathe‐5 subgroup analysis.4 These data suggest that the use of sildenafil in the treatment of patients with PAH related to simple CHD may be considered irrespective of the type of defect.
Instead of causing reduction of arterial oxygen saturation due to aggravation of the right‐to‐left shunt, sildenafil actually improved arterial oxygen saturation in patients with ASD significantly as well as in patients with VSD and PDA, although not significantly. The increase in arterial oxygen saturation may be the result of improvement in pulmonary blood flow, better ventilation/perfusion matching, and reduction in right‐to‐left shunt secondary to decrease in pulmonary vascular resistance.9, 18
Interestingly, the baseline arterial oxygen saturation in the ASD group was found to be higher than that in VSD and PDA group, and the improvement in arterial oxygen saturation was also the greatest among the 3 groups. One possible reason is that PAH secondary to ASD is usually less severe compared to that in VSD and PDA. It is demonstrated that pulmonary vascular abnormalities correlated with the overall index of ventilation‐perfusion mismatch.22 The less severe remodeling of the vascular bed in the ASD group may be associated with better improvement in ventilation/perfusion matching and therefore the increase in arterial oxygen saturation.
There were several limitations in our study such as uncontrolled and nonblinded design, relatively small sample size, and short‐term observation periods. The long‐term effects of sildenafil on patients with CHD need to be investigated.
Conclusion
In our present study, the data, for the first time, demonstrated that sildenafil therapy seems to be effective and safe for PAH secondary to ASD, VSD, and PDA, although some clinical and hemodynamic parameters were changed in a different manner between the 3 groups.
Acknowledgements
The authors would like to acknowledge Dr. Wong Ka Lam for his editorial assistance in the preparation of this manuscript.
Contributor Information
Jian‐Jun Li, Email: lijnjn@yahoo.com.cn.
Jian‐Guo He, Email: hejianguofw@gmail.com.
References
- 1. Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–2537. [DOI] [PubMed] [Google Scholar]
- 2. Beghetti M, Tissot C. Pulmonary arterial hypertension in congenital heart diseases. Semin Respir Crit Care Med. 2009;30:421–428. [DOI] [PubMed] [Google Scholar]
- 3. van Albada ME, Berger RM. Pulmonary arterial hypertension in congenital cardiac disease—the need for refinement of the Evian‐Venice classification. Cardiol Young. 2008;18:10–17. [DOI] [PubMed] [Google Scholar]
- 4. Berger RM, Beghetti M, Galie N, et al. Atrial septal defects versus ventricular septal defects in BREATHE‐5, a placebo‐controlled study of pulmonary arterial hypertension related to Eisenmenger's syndrome: a subgroup analysis. Int J Cardiol. 2010;144:373–378. [DOI] [PubMed] [Google Scholar]
- 5. Wort SJ. Sildenafil in Eisenmenger syndrome: safety first. Int J Cardiol. 2007;120:314–316. [DOI] [PubMed] [Google Scholar]
- 6. Lim ZS, Salmon AP, Vettukattil JJ, et al. Sildenafil therapy for pulmonary arterial hypertension associated with atrial septal defects. Int J Cardiol. 2007;118:178–182. [DOI] [PubMed] [Google Scholar]
- 7. Chau EM, Fan KY, Chow WH. Effects of chronic sildenafil in patients with Eisenmenger syndrome versus idiopathic pulmonary arterial hypertension. Int J Cardiol. 2007;120:301–305. [DOI] [PubMed] [Google Scholar]
- 8. Singh TP, Rohit M, Grover A, et al. A randomized, placebo‐controlled, double‐blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J. 2006;151:e851–e855. [DOI] [PubMed] [Google Scholar]
- 9. Garg N, Sharma MK, Sinha N. Role of oral sildenafil in severe pulmonary arterial hypertension: clinical efficacy and dose response relationship. Int J Cardiol. 2007;120:306–313. [DOI] [PubMed] [Google Scholar]
- 10. Lu XL, Xiong CM, Shan GL, et al. Impact of sildenafil therapy on pulmonary arterial hypertension in adults with congenital heart disease. Cardiovasc Ther. 2010;28:350–355. [DOI] [PubMed] [Google Scholar]
- 11. Brooks D, Solway S, Gibbons WJ. ATS statement on six‐minute walk test. Am J Respir Crit Care Med. 2003;167:1287. [DOI] [PubMed] [Google Scholar]
- 12. Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 13. Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. [DOI] [PubMed] [Google Scholar]
- 14. Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. [DOI] [PubMed] [Google Scholar]
- 15. Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 16. Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation. 2010;121:20–25. [DOI] [PubMed] [Google Scholar]
- 17. Gupta A, Kerkar P. Sildenafil for pulmonary hypertension secondary to congenital heart diseases. Indian Heart J. 2007;59:342–345. [PubMed] [Google Scholar]
- 18. Ghofrani HA, Voswinckel R, Reichenberger F, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase‐5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004;44:1488–1496. [DOI] [PubMed] [Google Scholar]
- 19. Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. [DOI] [PubMed] [Google Scholar]
- 20. Corbin JD, Francis SH. Cyclic GMP phosphodiesterase‐5: target of sildenafil. J Biol Chem. 1999;274:13729–13732. [DOI] [PubMed] [Google Scholar]
- 21. Tantini B, Manes A, Fiumana E, et al. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol. 2005;100:131–138. [DOI] [PubMed] [Google Scholar]
- 22. Barbera JA, Riverola A, Roca J, et al. Pulmonary vascular abnormalities and ventilation‐perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149:423–429. [DOI] [PubMed] [Google Scholar]


