Abstract
Background:
The first‐pass imaging of 64‐multidetector computed tomography (MDCT) using pharmacological stress has been used to assess myocardial perfusion. However, detection of myocardial ischemia at rest using MDCT has yet to be elucidated. We studied the incidence of myocardial perfusion defect (MPD) by 64‐MDCT at rest and the effect of coronary revascularization therapy on MPD in patients with coronary artery disease.
Hypothesis:
MPD by 64‐MDCT at rest indicates myocardial ischemia.
Methods:
We studied 76 patients with coronary artery disease who underwent 64‐MDCT before and after revascularization therapy and 55 patients who did not undergo revascularization therapy. According to percent diameter stenosis, we defined group A, B, C, and D to have stenosis between 70% and 90%, 50% and 69%, 30% and 49%, and 10% and 29%, respectively. We evaluated regional myocardial contrast enhancement by long and short axis planes. MPD was defined as hypoenhancement area of some extent with CT value <50 HU during diastole.
Results:
MPD was found in 60.0% and 32.4% of group A and B patients, respectively (P = 0.0176). The incidence was 4.8% and 0% in group C and D patients, respectively (P<0.0001 compared with group A and B). All patients in group A and B and 2 patients with MPD in group C underwent coronary revascularization therapy. MPD disappeared after revascularization therapy in all but 3 group A patients. No patients showed new MPD after revascularization therapy.
Conclusions:
Our results demonstrate that a significant percentage of patients with significant coronary artery stenosis show MPD by 64‐MDCT at rest, and these MPDs may represent myocardial ischemia. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
A promising modality to evaluate coronary artery disease called 64‐multidetector computed tomography (64‐MDCT) has emerged. 64‐MDCT has been shown to have sensitivity and specificity in the range of 95% for the detection of hemodynamically relevant stenosis, which is higher than that associated with stress myocardial perfusion imaging (MPI).1, 2, 3, 4 Particularly, its ability to detect coronary plaque noninvasively is increasingly recognized.5, 6, 7, 8
Recently, 64‐MDCT has been increasingly referred to as a potential tool to assess myocardial perfusion. The ability of 64‐MDCT to visualize areas of hypoenhancement is demonstrated in patients with acute and chronic myocardial infarction.9, 10, 11 The first‐pass imaging of 64‐MDCT using pharmacological stress has been used to assess myocardial perfusion.12, 13
There have been some reports that studied the detection of myocardial ischemia at rest using 64‐MDCT. Kachenoura et al, who compared computed tomography (CT) perfusion analysis with invasive coronary angiography, showed that a majority of areas of myocardial hypoenhancement could be explained by underlying coronary stenosis.14 In another study, the same author reported that CT detected multiple perfusion defects at rest that were not detected on resting MPI but became apparent on stress MPI.15 Nagao et al compared resting CT perfusion analysis with MPI and showed that CT myocardial imaging at rest demonstrated a characteristic enhancement pattern for ischemia.16 We studied the incidence of MPD and the effect of coronary revascularization therapy on perfusion defect in patients with coronary artery disease.
Methods
Patients
From December 2006 through November 2008, 1183 patients underwent 64‐MDCT. We selected patients who underwent diagnostic coronary angiography following 64‐MDCT. We tried to isolate patients without prior coronary artery disease to make sure that the observed MPD was likely ischemic and not a scar. Patients were excluded who had the following: 1) history of myocardial infarction or heart failure, 2) acute coronary syndrome, 3) electrocardiographic evidence of Q‐wave myocardial infarction, 4) chest pain within 24 hours of 64‐MDCT, 5) wall motion abnormality on echocardiography, and 6) multivessel disease by coronary angiography. Patients were also excluded who had myocardial bridge and coronary collateral flow findings by coronary angiography. We also excluded patients with coronary artery calcium scores >1000.
About one half of these 1183 patients were asymptomatic and referred for 64‐MDCT from primary care physicians for the purpose of diagnosis of coronary artery disease. These patients were not included in this study.
For patients who underwent coronary revascularization therapy we selected only patients who underwent 64‐MDCT before and after the revascularization procedure. For most patients who underwent revascularization, a second MDCT was performed for the evaluation of restenosis in the follow‐up period. Other patients underwent a second MDCT because of chest symptoms, but no patients showed abnormal findings in the treated lesions. All patients were selected after their first MDCT.
We selected 78 patients with coronary artery disease who underwent 64‐MDCT before and after revascularization therapy and 57 patients who underwent 64‐MDCT but did not undergo revascularization therapy. Four patients with myocardial contrast enhancement of poor image quality were excluded.
We divided these 131 patients into 4 groups according to the severity of coronary artery stenosis measured by quantitative coronary angiography. Patients in group A are defined as having coronary artery stenosis with percent diameter stenosis between 70% and 90%. Patients in group B, C, and D are defined as having coronary artery stenosis with percent diameter stenosis between 50% and 69%, 30% and 49%, and 10% and 29%, respectively.17 All patients in group A and B had symptoms suggestive of coronary artery disease, and 64‐MDCT showed significant coronary artery disease. Patients in group D had symptoms suggestive of vasospastic angina, and these patients underwent coronary catheterization for the purpose of provocation of coronary artery spasm.
Thus this study is a retrospective cohort analysis.
64‐MDCT
All patients were scanned with a 64‐MDCT scanner (SOMATOM Sensation 64 Cardiac; Siemens Medical Solutions, Erlangen, Germany).
Patients with a heart rate >70 bpm received 20 mg of oral metoprolol before the 64‐MDCT scan. To achieve coronary vasodilation we administered 0.8 mg of sublingual nitroglycerin before the scan.
A native scan without contrast dye was performed to determine the total calcium burden of the coronary tree (sequential scan with 32 × 0.6‐mm collimation, tube current 60 mAs at 120 kV). Contrast‐enhanced CT angiography data were acquired with the use of a spiral scan with 32 × 0.6‐mm collimation, 330‐ms gantry rotation, pitch of 0.2, and tube voltage at 120 kV. A total of 64 overlapping 0.6‐mm slices per rotation were acquired with the use of a focal spot periodically moving in the longitudinal direction (z‐flying focal spot).
Tube current was modulated according to the electrocardiogram (ECG), with a maximum current of 850 to 950 mA during a time period of approximately 330 ms centered at 375 ms before the next R‐wave, and reduction by 80% during the remaining cardiac cycle. The same contrast agent (60–70 mL; 370 mg/mL iodine) was injected intravenously (4.0 mL/s) followed by a 30‐mL saline chaser. Transaxial images were reconstructed using an ECG‐gated half‐scan reconstruction algorithm (temporal resolution 164 ms) and kernel B30f.
64‐MDCT Image Interpretation
CT data sets were transferred to an offline workstation (Aquarius NetStation; TeraRecon Inc., San Meteo, CA) for image analysis. Total calcium score of all patients was calculated using dedicated software and expressed as Agatston scores.18 An independent reviewer evaluated the contrast‐enhanced 64‐MDCT scans with maximum intensity and curved multiplanar reconstruction techniques along multiple longitudinal axes and transversely (Figure 1A and Figure 2A). Standard display settings were used for the evaluation of the contrast‐enhanced 64‐MDCT scans (window width, 800 HU; window center, 250 HU).
Figure 1.
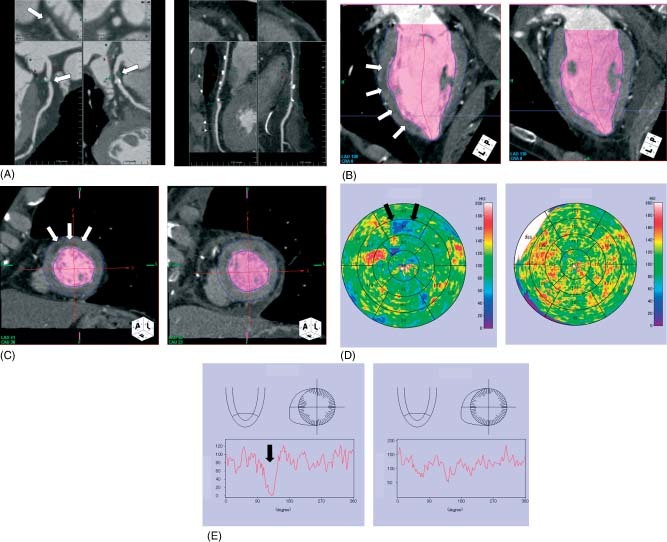
Representative case of myocardial perfusion defect. (A) A 78‐year‐old man presented with effort angina. 64‐multidetector computed tomography showed severe coronary artery stenosis in the proximal portion of the left anterior descending coronary artery (arrow, left panel). (B) and (C) Myocardial perfusion imaging demonstrated subendocardial hypoenhancement in the anterior wall of the left ventricle (arrow, left panel). (D) Myocardial perfusion map showed the clear hypoenhancement area in the anterior region (arrow, left panel). (E) Histograms of myocardial perfusion showed decreased perfusion in the anterior wall (arrow, left panel). After coronary artery bypass surgery this myocardial hypoenhancement improved (B–D, right panel).
Figure 2.
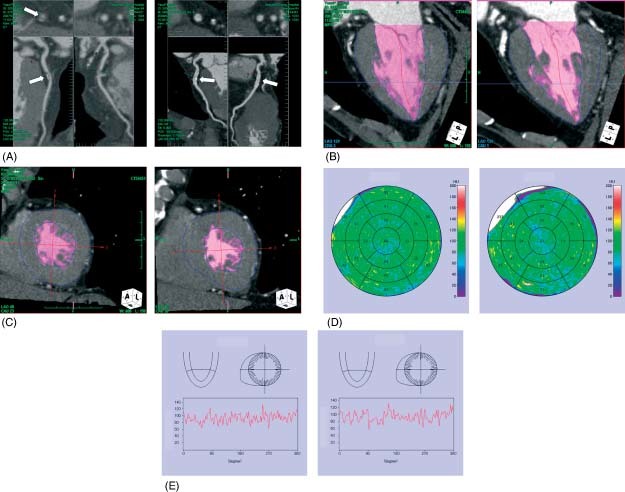
Representative case of normal myocardial perfusion. (A) A 74‐year‐old woman with chest pain underwent MDCT, which showed significant coronary artery stenosis in the proximal portion of the left anterior descending coronary artery (arrow, left panel). (B) and (C) Myocardial perfusion imaging demonstrated no hypoenhancement area in the left ventricle (left panel). (D) and (E) Myocardial perfusion map and histograms of myocardial perfusion showed no hypoenhancement area in the left ventricle (left panel). After coronary stent implantation (A; arrow, right panel) there is no hypoenhancement area in the left ventricle (B–D; right panel).
An independent observer identified coronary segments using a modified American Heart Association classification.19 Segments were classified as normal (smooth parallel or tapering borders), as having nonsignificant stenosis (luminal irregularities or <50% stenosis), or as having significant stenosis.
Assessment of Myocardial Perfusion
Time volume analysis was used to evaluate the regional myocardial contrast enhancement on the same phase of the cardiac cycle used for coronary CT angiography. Analysis was performed by an independent investigator blinded to results of all previous tests. The long axis planes and short axis planes were analyzed (Figure 1B, 1C and Figure 2B, 2C). The short axis was evaluated from a basal slice just below the leaflet tips of the open mitral valve, through a midventricular slice, to an apical slice. Myocardial perfusion map was also used (Figure 1D and Figure 2D). Histograms of myocardial perfusion were plotted separately for every 3 degrees (Figure 1E and Figure 2E).
MPD was defined as a hypoenhancement area of some extent with CT value <50 HU during diastolic phase. A spotty hypoenhancement, including papillary muscle region, and fatty infiltration were excluded. Informed consent for clinical procedure and research protocol was received from all studied patients. This study was approved by an institutional review board.
Statistics
Data are expressed as mean ± standard deviation. Continuous variables in the laboratory data were compared by 2‐group t test. Discrete variables were expressed as counts or percentages and compared with χ 2 or the Fisher exact test. Because the data for the percent diameter stenosis did not show a normal distribution, the Mann‐Whitney test was used to determine the differences between the 2 groups. A P value <0.05 was considered to be statistically significant.
Results
Clinical characteristics of studied patients are shown in the Table 1. The mean duration from the first 64‐MDCT to coronary angiography was 8.9 ± 6.1 days (range 2–45 days). And the mean duration from coronary revascularization therapy to second 64‐MDCT was 177.2 ± 42.0 days (range 30–224 days).
Table 1.
Clinical Characteristics of Studied Patients
| Group A | Group B | Group C | Group D | P | |
|---|---|---|---|---|---|
| No. | 40 | 34 | 42 | 15 | |
| Age, y | 70.4 ± 8.1 | 67.4 ± 10.6 | 70.1 ± 9.9 | 74.5 ± 8.6 | 0.1222 |
| Sex (male) | 25 (62.5%) | 23 (67.6%) | 27 (64.3%) | 11 (73.3%) | 0.8818 |
| Risk factor | |||||
| HT | 24 (60.0%) | 25 (73.5%) | 32 (76.2%) | 14 (93.3%) | 0.0586 |
| HL | 32 (80.0%) | 26 (76.5%) | 17 (40.5%) | 7 (46.7%) | 0.0003 |
| DM | 16 (40.0%) | 15 (44.1%) | 11 (26.2%) | 5 (33.3%) | 0.3752 |
| Laboratory data | |||||
| TG | 171.0 ± 121.8 | 175.0 ± 141.5 | 151.9 ± 73.0 | 143.4 ± 78.1 | 0.7044 |
| HDL‐C | 49.9 ± 13.8 | 47.4 ± 12.7 | 48.9 ± 11.6 | 51.3 ± 12.8 | 0.7713 |
| LDL‐C | 112.9 ± 33.1 | 132.2 ± 41.1 | 107.0 ± 27.6 | 121.4 ± 24.8 | 0.1593 |
| TC | 189.5 ± 39.0 | 196.6 ± 39.4 | 182.5 ± 21.6 | 189.8 ± 22.8 | 0.4948 |
| BS | 121.2 ± 33.8 | 143.8 ± 52.9 | 125.4 ± 41.1 | 132.9 ± 37.8 | 0.1427 |
| HbA1c | 6.2 ± 1.5 | 6.1 ± 1.2 | 5.7 ± 0.9 | 5.8 ± 1.0 | 0.3767 |
| Medication | |||||
| Aspirin | 35 (87.5%) | 31 (91.2%) | 19 (45.2%) | 3 (20.0%) | <0.0001 |
| Statin | 25 (62.5%) | 19 (55.9%) | 12 (28.6%) | 2 (13.3%) | 0.0008 |
| ACEI/ARB | 10 (25.0%) | 18 (52.9%) | 15 (35.7%) | 10 (66.7%) | 0.0082 |
| CCB | 13 (32.5%) | 12 (35.3%) | 14 (33.3%) | 7 (46.7%) | 0.7323 |
| BB | 11 (27.5%) | 8 (23.5%) | 7 (16.7%) | 1 (6.7%) | 0.3711 |
| Lesion location | |||||
| LAD | 22 (55.0%) | 22 (64.7%) | 22 (52.4%) | 10 (66.7%) | |
| RCA | 11 (27.5%) | 8 (23.5%) | 14 (33.3%) | 4 (26.7%) | |
| LCX | 7 (17.5%) | 4 (11.8%) | 6 (14.3%) | 1 (6.7%) | 0.8701 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β‐blocker; BS, blood sugar; CCB, calcium channel blocker; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HL, hyperlipidemia; HT, hypertension; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LDL‐C, low‐density lipoprotein cholesterol; RCA, right coronary artery; TC, total cholesterol; TG; triglyceride.
Figures 1 and 2 show the representative case of MPD and normal myocardial perfusion. Figure 3 shows the incidence of MPD in each group. MPD was found in 60.0% of group A patients and 32.4% of group B patients (P = 0.0176). The incidence of MPD was 4.8% and 0% in group C and D patients, respectively (P<0.0001 compared with group A and B). All patients in group A and B underwent coronary revascularization therapy, and 2 patients with MPD in group C also underwent coronary revascularization therapy.
Figure 3.
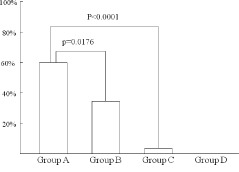
The incidence of myocardial perfusion defect among the 4 groups.
We performed coronary pressure measurement in 2 patients in group C. The first patient had 47% diameter stenosis, and the fractional flow reserve (FFR) of this patient was 0.72. After coronary stent implantation FFR improved to 0.90. The second patient had 40% diameter stenosis and his FFR was 0.75. FFR improved to 0.93 after stent implantation.
Figure 4 shows the time course of these patients. In group A, MPD disappeared after coronary revascularization therapy in 21 of 24 patients (87.5%), and none of the 16 patients with normal myocardial perfusion showed new MPD after coronary revascularization therapy. In group B, MPD disappeared in all 11 patients and no patients showed new MPD after coronary revascularization therapy. In group C, 2 patients with MPD showed a disappearance of MPD after revascularization therapy. In all patients, areas of MPD were in agreement with the territory of the diseased artery by a standard cardiac segmentation model.
Figure 4.
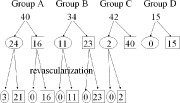
The time course of myocardial perfusion defects among the 4 groups. The number in the circle shows the number of patients with myocardial perfusion defect, and the number in the square shows the number of patients without myocardial perfusion defect.
All patients who underwent coronary revascularization therapy, except 1 patient in group A, showed improvement of symptoms suggestive of angina. When clinical characteristics of patients with and without MPD in group A were compared, percent diameter stenosis was significantly higher in patients with MPD than those without MPD (81.9 ± 7.9% vs 75.6 ± 5.6%, P = 0.0074, respectively). Other clinical characteristics were not significantly different between the 2 groups.
When clinical characteristics of patients with and without MPD in group B were compared, no clinical characteristics other than the sex (male 90.9% vs 56.5%, P = 0.0318, respectively) and β‐blocker use (54.5% vs 8.7%, P = 0.0027, respectively) were significantly different between the 2 groups.
Discussion
Our results demonstrate that a significant percentage of patients with significant coronary artery stenosis show MPD by 64‐MDCT at rest, and most of these perfusion defects improved after coronary revascularization therapy. Also, areas of MPD were in the territory of the diseased vessel in all patients. Thus, our results suggest that these MPDs represent myocardial ischemia.
MPD was found in 47.3% of patients with significant coronary artery stenosis defined as percent diameter stenosis more than 50% by quantitative coronary angiography. And this defect was more prevalent in patients with severe coronary artery stenosis compared with those with less severe coronary artery stenosis (60.0% vs 32.4%, respectively, P<0.0176). MPD was found only in 3.5% of patients without significant coronary artery stenosis.
All but 3 patients in group A with MPD showed improvement of perfusion defect after coronary revascularization therapy. For these 3 patients we found that 2 patients showed side branch occlusion after percutaneous coronary intervention, which seems to be the cause of persistent MPD. No patients with normal myocardial perfusion showed new MPD after coronary revascularization therapy. In group B, MPD disappeared in all patients and no patients showed new MPD after coronary revascularization therapy. In group C, 2 patients with MPD showed disappearance of MPD after revascularization therapy. In all patients, areas of MPD were in agreement with the territory of the diseased artery. As for symptoms of suggestive of angina, almost all patients showed improvement of their symptoms.
These results suggest that MPD demonstrated by 64‐MDCT at rest in patients with significant coronary artery disease may represent myocardial ischemia.
Why is MPD detected by 64‐MDCT at rest? There are several suggested mechanisms. First, Linder et al, Jayaweera et al, and Le et alinvestigated the role of capillaries with graded coronary stenosis.20, 21, 22 Jayaweera et al studied the role of capillaries in determining coronary blood flow reserve using myocardial contrast echocardiography.21 They found that in the absence of stenosis, the contribution of capillaries to total myocardial vascular resistance was only 25 ± 5% at rest, which increased to 75 ± 14% during hyperemia, whereas total myocardial vascular resistance decreased by 51 ± 13%. In the presence of a noncritical stenosis, total myocardial vascular resistance decreased by 22 ± 10% at rest without change in capillary resistance. During hyperemia, total myocardial vascular resistance increased by 58 ± 50%, and the contribution of capillaries to total myocardial vascular resistance increased to 84 ± 8% because arteriolar and venular resistance were already minimal. In the presence of a flow‐limiting stenosis at rest, capillary blood volume decreased, which suggested that decrease in coronary blood flow was associated with an increase in capillary resistance. Therefore, we speculate that MPD represents the myocardial perfusion disturbance due to decreased capillary blood volume.
Second, vasodilatory effects of contrast media used during MDCT imaging would induce some degree of coronary hyperemia, which may to some extent mimic that of vasodilator stress agents.23, 24, 25 Interestingly, in group A patients, the percent diameter stenosis was significantly higher in those with MPD compared with those without perfusion defect. However, to achieve coronary vasodilation we administered 0.8 mg sublingual nitroglycerin before the scan. Nitroglycerin causes redistribution of blood flow from normally perfused to ischemic areas, particularly in the subendocardium, which improves myocardial ischemia.26 Therefore, this mechanism is unlikely. Third, although we excluded patients with chest pain within 24 hours of MDCT, prolonged silent myocardial ischemia may cause MPD.27, 28
Why did some patients with presumably obstructive coronary artery disease not have MPD and also why would some patients with lesions only 30%–49% stenosis have MPD? Interestingly, we had a chance to perform coronary pressure measurement in 2 group C patients. These patients had functionally significant coronary stenosis. One possible explanation is that coronary angiography underestimated or overestimated the coronary stenosis severity. It is well recognized that for intermediate stenosis (percent diameter stenosis 40%–70%), the evaluation of stenosis severity by coronary angiography is often inaccurate.29 The other possibility is that improvement of spatial resolution by 64‐MDCT may not sufficiently detect myocardial perfusion disturbance in all patients with significant coronary artery stenosis.
There are some reports that studied the myocardial perfusion abnormality by 64‐MDCT at rest. Kachenoura et al studied 84 patients and found myocardial hypoenhancement in 29 of 64 patients in 47 vascular territories, of which 36 were abnormal by the reference technique.14 Of these 36 abnormalities, 28% were associated with previous myocardial infarction, whereas 72% corresponded to severely stenotic coronary arteries. In another study, Kachenoura et al developed a technique for quantification of myocardial perfusion from MDCT images and studied its diagnostic value against MPI.15 Resting MPI detected mild or worse abnormalities in 20/78 patients. MDCT detected abnormalities in 15/20 patients. Additional abnormalities found in 16/78 patients were not confirmed on resting MPI. However, 8 of these 16 apparently false‐positive MDCT perfusion tests had abnormal stress MPI. Nagao et al studied 75 patients with suspected coronary artery disease using 64‐MDCT and stress myocardial perfusion scintigraphy.16 They defined the ischemic pattern on MDCT as a pattern of transient endocardial hypoenhancement at systole and normal enhancement at diastole. They found that this ischemic pattern identified patients with myocardial ischemia demonstrated by stress myocardial perfusion scintigraphy with a sensitivity of 90%, specificity of 83%, positive predictive value of 86%, and negative predictive value of 88%.
Our results demonstrate that 64‐MDCT has potential as a noninvasive method for the detection of myocardial ischemia at rest. In patients with severe coronary artery disease 64‐MDCT often overestimates or cannot estimate the severity of coronary artery stenosis because of severe coronary artery calcification. In these patients the MPD suggests the presence of significant coronary artery stenosis. Thus, 64‐MDCT has a significant advantage in assessing patients with coronary artery disease by noninvasive evaluation of both coronary artery stenosis and myocardial perfusion using the same raw data of the 1 modality.
There are some limitations in our study. First, this is a retrospective study and only patients who underwent 64‐MDCT both before and after coronary angiography and coronary revascularization therapy were selected. Therefore, the true incidence of this MPD may differ from our results. Second, analysis of MPD is qualitative and there is a subjective nature to the visual assessment. However, there is only 1 report that studied the quantitative assessment of MPD. Recently, Kachenoura et al developed a new technique for quantitative assessment of myocardial enhancement based on analysis of MDCT images acquired for computed tomographic coronary angiography.14 The development of a quantitative assessment method would elucidate the significance of MPD at rest further.
Conclusion
Our results demonstrate that a significant percentage of patients with significant coronary artery stenosis show MPD by 64‐MDCT at rest, and these MPDs may represent myocardial ischemia.
Acknowledgements
The authors thank Dr. Yasushi Koyama for his valuable advice.
References
- 1. Mollet NR, Cademartiri F, van Mieghem C, et al. High‐resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–2323. [DOI] [PubMed] [Google Scholar]
- 2. Ropers D, Rixe J, Anders K, et al. Usefulness of multidetector row spiral computed tomography with 64‐× 0.6‐mm collimation and 330‐ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97:343–348. [DOI] [PubMed] [Google Scholar]
- 3. Leber AW, Knez A, von Ziegler F, et al. Quantitation of obstructive and nonobstructive coronary lesions by 64‐slice computed tomography. J Am Coll Cardiol. 2005;46:147–154. [DOI] [PubMed] [Google Scholar]
- 4. Raff GL, Gallagher MJ, O'Neill WW, et al. Diagnostic accuracy of noninvasive coronary angiography using 64‐slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–557. [DOI] [PubMed] [Google Scholar]
- 5. Leber AW, Becker A, Knez A, et al. Accuracy of 64‐slice computed tomography to classify and quantify plaque volumes in the proximal coronary system. J Am Coll Cardiol. 2006;47: 672–677. [DOI] [PubMed] [Google Scholar]
- 6. Ferencik M, Nieman K, Achenbach S. Noncalcified and calcified coronary plaque detection by contrast‐enhanced multi‐detector computed tomography: a study of interobserver agreement. J Am Coll Cardiol. 2006;47:207–209. [DOI] [PubMed] [Google Scholar]
- 7. Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast‐enhanced, submillimeter multidetector spiral computed tomography. A segment‐based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. [DOI] [PubMed] [Google Scholar]
- 8. Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques. A comparative study with intravascular ultrasound. J Am Coll Cardiol. 2004;43:1241–1247. [DOI] [PubMed] [Google Scholar]
- 9. Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast‐enhanced magnetic resonance. Circulation. 2006;113:823–833. [DOI] [PubMed] [Google Scholar]
- 10. Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16‐slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042–2047. [DOI] [PubMed] [Google Scholar]
- 11. Henneman MM, Schuijf JD, Jukema JW, et al. Comprehensive cardiac assessment with multislice computed tomography: evaluation of left ventricular function and perfusion in addition to coronary anatomy in patients with previous myocardial infarction. Heart. 2006;92:1779–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurata A, Mochizuki T, Koyama Y, et al. Myocardial perfusion imaging using adenosine triphosphate stress multi‐slice computed tomography—alternative to stress myocardial perfusion scintigraphy. Circ J. 2005;69:550–557. [DOI] [PubMed] [Google Scholar]
- 13. George RT, Silva C, Cordeiro MA, et al. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48:153–160. [DOI] [PubMed] [Google Scholar]
- 14. Kachenoura N, Gasper T, Lodato JA, et al. Combined assessment of coronary anatomy and myocardial perfusion using multidetector computed tomography for the evaluation of coronary artery disease. Am J Cardiol. 2009;103:1487–1494. [DOI] [PubMed] [Google Scholar]
- 15. Kachenoura N, Lodato JA, Gasper T, et al. Value of multidetector computed tomography evaluation of myocardial perfusion in the assessment of ischemic heart disease: comparison with nuclear perfusion imaging. Eur Radiol. 2009;19:1897–1905. [DOI] [PubMed] [Google Scholar]
- 16. Nagao M, Matsuoka H, Kawakami H, et al. Detection of myocardial ischemia using 64‐slice MDCT—comparison with stress/rest myocardial scintigraphy. Circ J. 2009;73:905–911. [DOI] [PubMed] [Google Scholar]
- 17. Stadius ML, Alderman EL. Coronary artery revascularization. Critical need for, and consequences of, objective angiographic assessment of lesion severity. Circulation. 1990;82: 2231–2234. [DOI] [PubMed] [Google Scholar]
- 18. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 19. Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. [DOI] [PubMed] [Google Scholar]
- 20. Linder JR, Skyba DM, Goodman NC, et al. Changes in myocardial blood volume with graded coronary stenosis. Am J Physiol Heart Circ Physiol. 1997;272:H567–H575. [DOI] [PubMed] [Google Scholar]
- 21. Jayaweera AR, Wei K, Coggins MP, et al. Role of capillaries in determining CBF reserve: new insights using myocardial contrast echocardiography. Am J Physiol Heart Circ Physiol 1999;277:H2363–H2372. [DOI] [PubMed] [Google Scholar]
- 22. Le DE, Jayaweera AR, Wei K, et al. Changes in myocardial blood volume over a wide range pf coronary driving pressures: role of capillaries beyond the autoregulatory range. Heart. 2004;90:1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tatinesi S, Kern MJ, Deligonul U, et al. The effects of ionic and nonionic contrast media on coronary hyperemia in patients during coronary angiography. Am Heart J. 1992;123:621–627. [DOI] [PubMed] [Google Scholar]
- 24. Crystal GJ, Salem MR. Investigations into the mechanisms of coronary vasodilation by contrast media in dogs. Invest Radiol. 1996;31:556–562. [DOI] [PubMed] [Google Scholar]
- 25. Baile EM, Pare PD, D'yachkova Y, et al. Effect of contrast media on coronary vascular resistance: contrast‐induced coronary vasodilation. Chest. 1999;116:1039–1045. [DOI] [PubMed] [Google Scholar]
- 26. Boottcher M, Madsen MM, Randsbaek F, et al. Effect of oral nitroglycerin and cold stress on myocardial perfusion in areas subtended by stenosed and nonstenosed coronary arteries. Am J Cardiol. 2002;89:1019–1024. [DOI] [PubMed] [Google Scholar]
- 27. Stern S. Symptoms other than chest pain may be important in the diagnosis of ‘silent ischemia’, or ‘the sounds of silence’. Circulation. 2005;111:e435–e437. [DOI] [PubMed] [Google Scholar]
- 28. Sajadieh A, Nielsen OW, Rasmussen V, et al. Prevalence and prognostic significance of daily‐life silent myocardial ischemia in middle‐aged and elderly subjects with no apparent heart disease. Eur Heart J. 2005;14:1402–1409. [DOI] [PubMed] [Google Scholar]
- 29. Bech GJW, de Bruyne B, Pijls NHJ, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis. A randomized trial. Circulation. 2001;103:2928–2934. [DOI] [PubMed] [Google Scholar]


