Abstract
Background:
An experimental study showed that nebivolol is an effective agent in contrast‐induced nephropathy (CIN) prophylaxis.
Hypothesis:
We hypothesized that prophylactic nebivolol use had protective effects on renal function in human beings subjected to iodinated contrast agent since it has vasodilatory effect and antioxidant properties.
Methods:
The present study enrolled 120 patients scheduled for coronary angiography and ventriculography. All patients were hydrated with intravenous isotonic saline. The patients in group I received 600 mg N‐acetylcysteine every 12 hours for 4 days. The patients in group II received 5 mg nebivolol every 24 hours for 4 days. The patients in group III were only hydrated. The primary endpoint was the occurrence of CIN. The secondary endpoint was the change in serum creatinine (Cr) levels at 2 days and 5 days after the contrast exposure.
Results:
Nine (22.5%) patients in group I developed CIN, as did 8 patients (20.0%) in group II and 11 patients (27.5%) in group III (P = 0.72). Changes in mean Cr level from baseline to day 2 were not statistically significant in all groups. However, we detected a statistically significant increase in mean Cr levels at day 5 compared with baseline levels in group I and group III (from 1.42 ± 0.13 to 1.52 ± 0.26, p2 = 0.02; and from 1.43 ± 0.14 to 1.55 ± 0.30, p2 = 0.01, respectively). Although an increase was detected in mean Cr level from baseline to the 5‐day Cr level in group II, this did not reach statistical significance (from 1.40 ± 0.12 to 1.48 ± 0.23, P = 0.06).
Conclusions:
Pretreatment with nebivolol is protective against nephrotoxic effects of contrast media. © 2012 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Contrast‐induced nephropathy (CIN), which many clinicians (especially cardiologists) encounter in current practice, is a common complication following iodinated contrast media administration. It usually occurs in patients at risk of acute renal injury. The reported incidence of CIN varies across a large scale because of different risk factors, renal impairment at baseline, definition of CIN, and contrast type and volume. However, it can be stated that the general incidence is about 7%.1 Many studies reported that CIN has been associated with high in‐hospital morbidity and mortality.2, 3 This high incidence and morbidity and mortality rate has made clinicians more aware of this catastrophic complication and increased the need for new strategies and new drugs to avoid it.
Because CIN is a complex syndrome in which multiple factors play role, to date a variety of preventive measures and agents have been evaluated to reduce the risk of this complication. The primary supposed mechanisms for the development of CIN are vasoconstriction, oxidative stress, and direct tubular toxicity.4 Therefore, in the literature, all studies to prevent negative effects of radiocontrast on renal function were conducted based on these mechanisms. N‐acetylcysteine (NAC), saline, sodium bicarbonate, statins, aminophylline, ascorbic acid, and dopamine are the most commonly evaluated agents for this purpose. Although some promising results have been achieved by many agents, because of discrepant results and too‐small patient populations, no pharmacologic therapy yet has been suggested in the prevention of CIN, except for volume expansion.
Nebivolol, a third‐generation β‐blocker, has nitric oxide (NO)‐induced vasodilation and antioxidant properties. An experimental study5 has previously evaluated the benefit of this drug for prevention of CIN in rats and concluded that prescription of nebivolol to avoid CIN may be reasonable, on condition that similar results are obtained by clinical studies. Therefore, we attempted to demonstrate the effect of prophylactic nebivolol use on renal function in human beings subjected to iodinated contrast agent.
Methods
Study Population
Between January 2008 and March 2009, 120 patients scheduled for coronary angiography and ventriculography were enrolled into the present study. All patients in this prospective, randomized study had baseline serum creatinine (SCr) levels ≥1.2 mg/dL. Exclusion criteria included dialysis patients, recent exposure to contrast media or a nephrotoxic agent within 7 days before the study, urgent percutaneous coronary intervention (PCI), requiring loop diuretics, theophylline/aminophylline, dopamine throughout the study, hemodynamically unstable patients, and contraindications for β‐blocker prescription.
Study Design
The study population was prospectively divided into 3 groups. All patients were hydrated with intravenous isotonic saline at a rate of 1 mL/kg/hour, for 6 hours before and 12 hours after the procedure. The patients in the first group (NAC + 0.9% saline) received 600 mg NAC every 12 hours for 4 days: 4 doses before the procedure day, 2 doses on the day of the procedure, and 2 doses on the day after the procedure. The patients in the second group (nebivolol + 0.9% saline) received 5 mg nebivolol every 24 hours for 4 days: 2 doses before the procedure day, 1 dose on the day of the procedure, and 1 dose on the day after the procedure. The patients in the third group (0.9% saline) were only hydrated as mentioned above.
Iopromide, a low‐osmolar, nonionic contrast agent, was used in all procedures. Before the coronary procedures, left ventricular function was evaluated in all patients using the Vingmed System 7 echocardiography machine and a 2.5‐MHz probe (GE Vingmed Ultrasound, Horten, Norway). Left ventricular ejection fraction was measured by 2‐dimensional echocardiography via the modified Simpson method.6 Creatinine clearance (CrCl) was estimated by the Cockcroft‐Gault formula.7 We also estimated Mehran risk score8 in all patients before the procedure. This scoring system is the most frequently used method in the prediction of CIN.
Follow‐Up
Serum Cr concentration was measured in venous blood at baseline (before initiating preprocedure hydration), and at 2 days and 5 days after the procedure. The primary endpoint was the occurrence of CIN. We defined CIN as an increase ≥0.5 mg/dL and/or ≥25% in SCr concentration at day 2 and/or day 5 of the procedure. The secondary endpoint was the change in SCr levels at 2 days and 5 days after the contrast exposure.
The study was approved by local ethics committee, and written, informed consent was obtained from all patients enrolled in the study.
Statistical Analysis
The comparison of groups for the incidence of CIN and other categorical variables was performed by the χ 2 test. Categorical variables were presented as numbers and percentages. One‐way analysis of variance was used to compare the continuous variables among the 3 groups. Continuous variables were expressed as mean and SD. A paired t test was used to investigate the time‐dependent variables. A 2‐sided P value <0.05 was considered statistically significant. All statistical analyses were performed using the SPSS statistical package for Windows, version 13.0 (SPSS Inc, Chicago, IL).
Results
Baseline demographic and clinical characteristics and medications were similar among the randomized groups, with no significant differences. There was also no significant difference with regard to Mehran risk score, which predicts CIN risk (Table 1).
Table 1.
Baseline Patient Characteristics
| Baseline Characteristics | Group I, NAC + 0.9% Saline, n = 40 | Group II, Nebivolol + 0.9% Saline, n = 40 | Group III, 0.9% Saline, n = 40 | P Value |
|---|---|---|---|---|
| Age, y | 64.7 ± 11.9 | 64.1 ± 9.0 | 66.4 ± 10.7 | 0.59 |
| Sex, M/F | 29/11 | 29/11 | 25/15 | 0.53 |
| Weight, kg | 74.5 ± 12.4 | 71.9 ± 12.9 | 76.1 ± 10.7 | 0.28 |
| BMI, kg/m2 | 27.7 ± 4.7 | 26.4 ± 4.2 | 27.8 ± 3.7 | 0.29 |
| LVEF, % | 50.0 | 51.5 | 49.2 | |
| DM, n (%) | 12 (30) | 14 (35) | 11 (27.5) | 0.76 |
| Hypertension, n (%) | 26 (65) | 31 (77.5) | 26 (65) | 0.37 |
| Current smoker, n (%) | 15 (37.5) | 19 (47.5) | 13 (32.5) | 0.37 |
| Baseline Cr | 1.42 ± 0.13 | 1.40 ± 0.12 | 1.43 ± 0.14 | 0.58 |
| Baseline CrCl, mL/min | 49.8 | 51.6 | 47.6 | 0.08 |
| Total cholesterol, mg/dL | 168.5 | 177.6 | 186.3 | 0.21 |
| LDL‐C, mg/dL | 108.1 | 112.6 | 116.5 | 0.61 |
| Contrast agent dose, mL | 63.4 | 61.8 | 64.2 | 0.83 |
| Contrast agent dose/BMI, mL/BMI | 2.34 | 2.38 | 2.33 | 0.93 |
| Mehran risk score | 5.7 ± 2.7 | 5.5 ± 2.5 | 6.5 ± 3.1 | 0.23 |
| Medications, n (%) | ||||
| ACEI | 21 (52.5) | 29 (72.5) | 29 (72.5) | 0.09 |
| ARB | 2 (5) | 2 (5) | 2 (5) | 1.0 |
| Thiazide | 7 (17.5) | 9 (22.5) | 9 (22.5) | 0.81 |
| Statin | 15 (37.5) | 15 (37.5) | 22 (55) | 0.19 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; Cr, creatinine; CrCl, creatinine clearance; DM, diabetes mellitus; F, female; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; M, male; NAC, N‐acetylcysteine. Data are presented as mean ± SD or number and percentage of patients. P < 0.05 is considered statistically significant.
Group I was the NAC + 0.9% saline group, group II was the nebivolol + 0.9% saline group, and group III was the 0.9% saline group.
Baseline SCr levels were similar in the 3 groups (1.42 ± 0.13 mg/dL, 1.40 ± 0.12 mg/dL, and 1.43 ± 0.14 mg/dL, respectively, for groups I, II, and III; P = 0.58) (Table 1).
Baseline CrCl, estimated by the Cockcroft‐Gault formula, was also similar between the groups (49.8, 51.6, and 47.6, respectively; P = 0.08) (Table 1).
According to our definition for CIN, there was no significant difference between the 3 groups (P = 0.72). Contrast‐induced nephropathy occurred in 9 patients (22.5%) in group I, 8 patients (20%) in group II, and 11 patients (27.5%) in group III (Figure 1).
Figure 1.
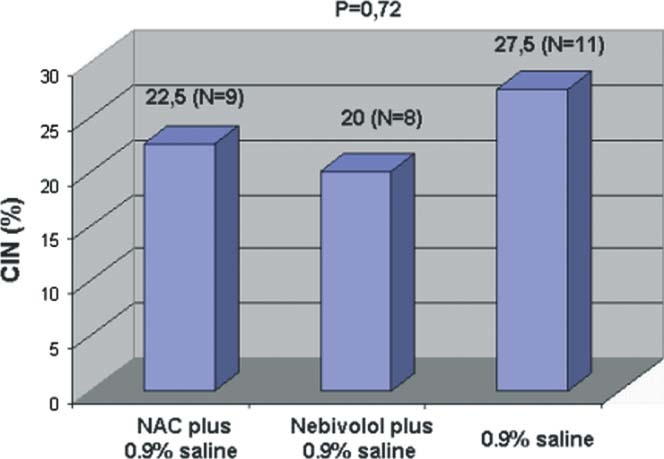
The comparison of CIN incidence between 3 treatment groups. Abbreviations: CIN, contrast‐induced nephropathy; NAC, N‐acetylcysteine.
Changes in mean Cr levels from baseline to 2‐day Cr levels were not statistically significant in all groups (from 1.42 ± 0.13 to 1.47 ± 0.2 in group I, from 1.40 ± 0.12 to 1.44 ± 0.24 in group II, and from 1.43 ± 0.14 to 1.50 ± 0.27 in group III; P 1 = 0.27, 0.33, and 0.06, respectively) (Table 2).
Table 2.
Change in Creatinine During Follow‐Up Period for N‐Acetylcysteine + 0.9% Saline, Nebivolol + 0.9% Saline, and 0.9% Saline Groups
| Basal Cr | 2‐Day Cr | 5‐Day Cr | P Value (P 1) | P Value (P 2) | |
|---|---|---|---|---|---|
| NAC + 0.9% saline | 1.42 ± 0.13 | 1.47 ± 0.29 | 1.52 ± 0.26 | 0.27 | 0.02 |
| Nebivolol + 0.9% saline | 1.40 ± 0.12 | 1.44 ± 0.24 | 1.48 ± 0.23 | 0.33 | 0.06 |
| 0.9% saline | 1.43 ± 0.14 | 1.50 ± 0.27 | 1.55 ± 0.30 | 0.06 | 0.01 |
Abbreviations: Cr, creatinine; NAC, N‐acetylcysteine; P 1, difference between baseline Cr and 2‐day Cr; P 2, difference between baseline Cr and 5‐day Cr. P < 0.05 is considered statistically significant.
However, we detected a statistically significant increase in mean Cr levels at day 5 compared with baseline Cr levels in group I and group III (from 1.42 ± 0.13 to 1.52 ± 0.26, P 2 = 0.02; and from 1.43 ± 0.14 to 1.55 ± 0.30, P 2 = 0.01, respectively) (Table 2). Although an increase was detected in mean Cr level from baseline to day 5 in Group II, this did not reach statistical significance (from 1.40 ± 0.12 to 1.48 ± 0.23, P = 0.06) (Table 2, Figure 2).
Figure 2.
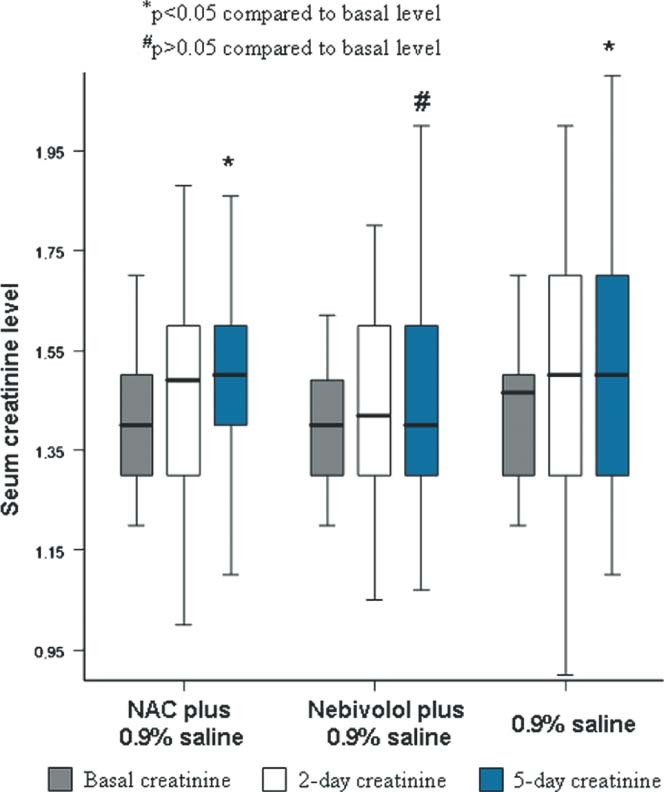
Change in serum creatinine levels over time in every group, showing that only the nebivolol + 0.9% saline group had a nonsignificant increase in serum creatinine at 5 days compared with baseline level. Abbreviations: NAC, N‐acetylcysteine.
Discussion
Increase in the use of imaging methods and interventional techniques that require iodinated contrast agents have made CIN the third leading cause of acute renal failure in in‐hospital patients.9 Widely different incidences have been reported in the literature as a function of the population type being studied. Patient baseline characteristics tremendously affect the frequency of CIN. Diabetic, hypertensive, anemic, and older patients are more vulnerable to contrast agents.8 In addition, chronic heart failure, chronic renal insufficiency, patient hemodynamic status at the moment of injection of contrast dye, and contrast volume and type are the other well‐known factors that have an impact on the development of CIN.8, 10, 11 Also, different definitions used in different studies have led to inconsistent incidences reported by many researchers.10 However, CIN incidence can be predicted via some risk‐scoring methods.10, 12, 13 Although many developed risk‐scoring schemes exist, Mehran et al8 have published the simplest, most effective, and easiest system to use. With this method, CIN risk score is determined according to 8 readily available patient‐ and procedure‐related characteristics. The patient‐related variables are chronic renal failure, anemia, congestive heart failure, diabetes mellitus, hypotension, and age >75 years. The procedure‐ related variables are the need for an intra‐aortic balloon pump within 24 hours periprocedurally and contrast media volume. A risk score of 1–6, 6–10, 11–16, and >16 shows a risk for CIN of 7.5%, 14%, 26%, and 57%, respectively. We determined our patients' risk stage by using this method. The CIN ratios were extremely high in all groups when compared with the expected values according to the scoring system. One of the reasons for this high CIN incidence in our study can be attributed to the different hydration protocols. In the study conducted by Mehran et al, the hydration was performed with 1 mL/kg/hour of half‐normal saline for 4 to 12 hours before PCI and 18 to 24 hours after the procedure. But, we hydrated all patients with intravenous isotonic saline at a rate of 1 mL/kg/hour for 6 hours before and 12 hours after the procedure. The other contributory factor to high CIN incidence of our study when compared with that of the Mehran et al study can be the different definitions of CIN between the 2 studies. We defined CIN as an increase of ≥0.5 mg/dL and/or ≥25% in SCr concentration at 2 days and/or 5 days after the procedure. However, Mehran et al defined CIN according to the SCr levels measured only at 48 hours after the procedure.
As well as high morbidity rates, prolonged hospitalization, and increased costs to the healthcare system, it has been confirmed by many studies that CIN is associated with high in‐hospital mortality. For instance, McCullough and colleagues noted a 7.1% in‐hospital mortality rate in patients who developed CIN, but 1.1% without CIN.10 Also, an association between CIN and late cardiovascular events was demonstrated by a registry study of 5967 patients. In that study, Lindsay et al14 found a 24% myocardial infarction rate in patients who developed CIN after PCI, compared with 11.6% in patients without CIN (P < 0.001). It can be clearly emphasized that CIN is such a detrimental complication that it directly affects not only short‐term, but also long‐term prognosis. Therefore, many CIN‐preventive strategies have been evaluated by the researchers. In determining these strategies, probable pathogenesis of CIN has been the main issue of focus.
There are many data on the role of vasoconstrictor agents in the pathogenesis of CIN. Klause et al showed rapidly elevated plasma endothelin levels after contrast exposure.15 Adenosine is another vasoconstrictor that is thought to be a factor in renal injury.16 Decrease in NO production, a potent vasodilator, has also been shown and suggested as one of the underlying mechanisms contributing to vasoconstriction and CIN pathophysiology.17, 18 Because direct renal tubular damage caused by reactive oxygen species has been confirmed to play a role in CIN pathogenesis by many studies,19, 20 many agents with antioxidative properties have been evaluated for avoidance of kidney injury.
Patti et al showed that pretreatment with atorvastatin has protective effect on CIN in patients undergoing PCI. This protective effect was mainly attributed to atorvastatin's antioxidative, anti‐inflammatory properties and its increasing NO bioavailability.4 Sodium bicarbonate is another agent that has been demonstrated to exert preventive effects on CIN development by means of disrupting some pathways leading to free‐radical formation.21 Because of vasodilatory effects, some calcium channel blockers have been tried to prevent CIN. Unfortunately, most studies using these drugs as a prophylactic agent resulted in disappointment in the prevention of CIN.22, 23 It was hypothesized that dopamine, with its vasodilatory effect on renal arteries, can reduce the vasoconstriction caused by the contrast agent, whereby it can lower the incidence of CIN. But dopamine turned out to show no consistent evidence of benefit for this purpose.24
Up to now, NAC, an antioxidant, is the most commonly used agent for CIN prophylaxis. Nevertheless, the benefit of NAC in the prevention of CIN remains unclear, because the studies of NAC have demonstrated variable efficacy. The observed benefit in some studies may be due to the Cr‐decreasing effect of NAC by increasing tubular secretion of Cr, or another mechanism.25 Considering all the studies, neither oral nor intravenous NAC should be routinely prescribed for CIN prophylaxis.26, 27 As reported in many studies, we also found that NAC did not confer any benefit over saline alone.
Nebivolol, a third‐generation β‐adrenergic blocker, possesses some pharmacologic properties, affecting some pathways in CIN pathogenesis. It has a vasodilatory effect via increasing NO availability.28 Also, it has antioxidant properties that already have been proven.29 Based on these characteristics of nebivolol, Toprak et al conducted an experimental study investigating the protective effect of nebivolol on CIN in rats.5 At the end of the study, they found that nebivolol decreased medullary congestion, proteinaceous casts, and tubular necrosis caused by contrast media, and reported protective features of nebivolol against CIN. Therefore, we planned to evaluate the efficacy of nebivolol in the prevention of CIN in humans for the first time. This study of the patients subjected to a contrast agent extends the understanding of the protective effects of nebivolol on CIN beyond that reported in rats. In our study, nebivolol + saline did not reduce the CIN ratio, which is determined by the classical CIN definition. The reason why our study did not provide a benefit in the reduction of the CIN ratio is unclear. But it is clear that some discrepancies exist between our study and that of Toprak et al. First, contrast media was administered to the rats after 72 hours of dehydration; our patients were not in a dehydrated state. Second, nebivolol was given to the rats at a dose of 2 mg/kg. Hovewer, it is practically impossible to prescribe nebivolol at this dose in human beings. We were to give a dose of 5 mg/day, an extremely low dose compared with that used in the experimental study. Last, they used ionic high‐osmolar contrast medium (diatrizoate), but we administered nonionic low‐osmolar iopromide. Hovewer, in the nebivolol + saline group, contrary to the other 2 groups, we did not find a statistically significant increase in Cr. Namely, although CIN ratios are similar in all groups, it is not a mistake to say that nebivolol can be an agent in the future that will be prescribed for prevention of CIN, on the condition that our results are reached in further studies. But, of course, the number of cases here is too small to evaluate the clinical impact of nebivolol in the prevention of CIN.
Conclusion
Pretreatment with nebivolol could prevent significant increases in SCr levels in patients subjected to contrast media. Large, prospective, double‐blind, placebo‐controlled trials will be required to explore the future role of nebivolol for the prevention of CIN. It is also unknown how long nebivolol therapy should be initiated before contrast administration to achieve more benefit.
References
- 1. McCullough PA, Adam A, Becker CR, et al. CIN Consensus Working Panel. Epidemiology and prognostic implications of contrast‐induced nephropathy. Am J Cardiol. 2006;98:5–13. [DOI] [PubMed] [Google Scholar]
- 2. Gruberg L, Mintz GS, Mehran R. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with preexistent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–1548. [DOI] [PubMed] [Google Scholar]
- 3. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 4. Patti G, Nusca A, Chello M, et al. Usefulness of statin pretreatment to prevent contrast‐induced nephropathy and to improve long‐term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101:279–285. [DOI] [PubMed] [Google Scholar]
- 5. Toprak O, Cirit M, Tanrisev M, et al. Preventive effect of nebivolol on contrast‐induced nephropathy in rats. Nephrol Dial Transplant. 2008;23:853–859. [DOI] [PubMed] [Google Scholar]
- 6. Folland ED, Parisi EF, Moynihan PF, et al. Assessment of left ventricular ejection fractions by real‐time, two‐dimensional echocardiography: a comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–766. [DOI] [PubMed] [Google Scholar]
- 7. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 8. Mehran R, Aymong E, Nikolsky E, et al. A simple risk score for prediction of contrast induced nephropathy after coronary intervention. J Am Coll Cardiol. 2004;44:393–399. [DOI] [PubMed] [Google Scholar]
- 9. Nash K, Hafeez A, Hou S. Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. [DOI] [PubMed] [Google Scholar]
- 10. McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. [DOI] [PubMed] [Google Scholar]
- 11. Kamdar A, Weidmann P, Makoff DL, et al. Acute renal failure following intravenous use of radiographic contrast dyes in patients with diabetes mellitus. Diabetes. 1977;26:643–649. [DOI] [PubMed] [Google Scholar]
- 12. Bartholomew BA, Harjai KJ, Dukkipati S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. [DOI] [PubMed] [Google Scholar]
- 13. Marenzi G, Lauri G, Assanelli E, et al. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 14. Lindsay J, Apple S, Pinnow EE, et al. Percutaneous coronary intervention‐associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv. 2003;59: 338–343. [DOI] [PubMed] [Google Scholar]
- 15. Klause N, Arendt T, Lins M, et al. Hypoxic renal tissue damage by endothelin‐mediated arterial vasoconstriction during radioangiography in man. Adv Exp Med Biol. 1998;454: 225–234. [DOI] [PubMed] [Google Scholar]
- 16. Arakawa K, Suzuki H, Naitoh M, et al. Role of adenosine in the renal responses to contrast medium. Kidney Int. 1996;49:1199–1206. [DOI] [PubMed] [Google Scholar]
- 17. Agmon Y, Peleg H, Greenfeld Z, et al. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ribeiro L, de Assunção e Silva F, Kurihara RS, et al. Evaluation of the nitric oxide production in rat renal artery smooth muscle cells culture exposed to radiocontrast agents. Kidney Int. 2004;65:589–596. [DOI] [PubMed] [Google Scholar]
- 19. Sandhu C, Newman DJ, Morgan R, et al. The role of oxygen free radicals in contrast induced nephrotoxicity. Acad Radiol. 2002;9:436–437. [DOI] [PubMed] [Google Scholar]
- 20. Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies [published correction appears in CMAJ. 2005;173:1210]. CMAJ. 2005;172:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masuda M, Yamada T, Mine T, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast‐induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007;100:781–786. [DOI] [PubMed] [Google Scholar]
- 22. Khoury Z, Schlicht JR, Como J, et al. The effect of prophylactic nifedipine on renal function in patients administered contrast media. Pharmacotherapy. 1995;15:59–65. [PubMed] [Google Scholar]
- 23. Madsen JK, Jensen JW, Sandermann J, et al. Effect of nitrendipine on renal function and on hormonal parameters after intravascular iopromide. Acta Radiol. 1998;39:375–380. [DOI] [PubMed] [Google Scholar]
- 24. Lameire NH. Contrast‐induced nephropathy—prevention and risk reduction. Nephrol Dial Transplant. 2006;21:i11–i23. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann U, Fischereder M, Krüger B, et al. The value of N‐acetylcysteine in the prevention of radiocontrast agent‐induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–410. [DOI] [PubMed] [Google Scholar]
- 26. Oldemeyer JB, Biddle WP, Wurdeman RL, et al. Acetylcysteine in the prevention of contrast‐induced nephropathy after coronary angiography. Am Heart J. 2003;146:E23. [DOI] [PubMed] [Google Scholar]
- 27. Carbonell N, Blasco M, Sanjuán R, et al. Intravenous N‐acetylcysteine for preventing contrast‐induced nephropathy: a randomised trial. Int J Cardiol. 2007;115:57–62. [DOI] [PubMed] [Google Scholar]
- 28. Weiss R. Nebivolol: a novel beta‐blocker with nitric oxide‐induced vasodilatation. Vasc Health Risk Manag. 2006;2: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Münzel T, Gori T. Nebivolol: the somewhat‐different beta‐adrenergic receptor blocker. J Am Coll Cardiol. 2009;54: 1491–1499. [DOI] [PubMed] [Google Scholar]


