Abstract
Neoplastic pericardial effusion is a serious and common clinical disorder encountered by cardiologists, cardiothoracic surgeons, oncologists, and radiation oncologists. It may develop from direct extension or metastatic spread of the underlying malignancy, from an opportunistic infection, or from a complication of radiation therapy or chemotherapeutic toxicity. The clinical presentation varies, and the patient may be hemodynamically unstable in the setting of constrictive pericarditis and cardiac tamponade. The management depends on the patient's prognosis and varies from pericardiocentesis, sclerotherapy, and balloon pericardiotomy to cardiothoracic surgery. Patients with neoplastic pericardial effusion face a grave prognosis, as their malignancy is usually more advanced. This review article discusses the epidemiology and etiology, pathophysiology, clinical presentation, diagnosis, management, and prognosis of neoplastic pericardial effusion. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Neoplastic pericardial effusion is a clinical disorder encountered by cardiologists, cardiothoracic surgeons, oncologists, and radiation oncologists. The immediate control of neoplastic pericardial effusions is mandatory for both the survival and quality of life of patients. This review article discusses the epidemiology and etiology, pathophysiology, clinical presentation, diagnosis, management, and prognosis of neoplastic pericardial effusion.
Epidemiology and Etiology
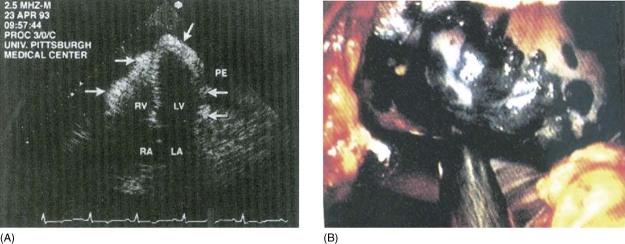
Malignancy has been noted to be the most common cause of pericardial effusion in the Western world.1, 2 The pericardial effusion that occurs in patients with malignancy could be secondary to malignancy itself or could be an inflammatory process. In a review of 31 patients with cancer and pericardial disease, the diagnoses included malignant pericardial disease (58%), benign idiopathic pericarditis (32%), and radiation‐induced pericarditis (10%).3 In autopsy series, the prevalence of pericardial involvement varies from 4% in general autopsies to 15%–30% in autopsies of cancer patients.4 In some studies, in up to two‐thirds of patients with malignancy, the pericardial effusion was caused by nonmalignant disease processes such as radiation pericarditis or opportunistic infections.5, 6 Autopsy series have shown that pericardial metastases are found particularly in lung cancer (35%), breast cancer (25%), lymphoma and leukemia (15%), and other malignancies such as esophageal cancer, Kaposi sarcoma, and melanoma.7, 8, 9, 10 A transthoracic echocardiogram with large neoplastic pericardial effusion and epicardial studding with metastatic melanoma is shown in Figure 1A, as well as epicardial melanoma (black) observed during placement of a pericardial window in Figure 1B.11
Figure 1.
Transthoracic echocardiogram (A) of a large neoplastic PE is shown in the apical 4‐chamber view. Arrows show epicardial studding with metastatic melanoma. (B) Epicardial melanoma (black) observed during placement of a pericardial window. Abbreviations: LA, left atrium; LV, left ventricle; PE, pericardial effusion; RA, right atrium; RV, right ventricle. Used with permission from Katz WE, Ferson PF, Lee RF, et al.11
Primary neoplasms of the pericardium are 40× less common than the metastatic ones.12 The malignant pericardial effusion is mainly a secondary process due to metastasis. Primary neoplasms of the pericardium are exceedingly rare, and these include malignant mesotheliomas, fibrosarcomas, lymphangiomas, hemangiomas, teratomas, neurofibromas, and lipomas. Only 12%–25% of patients who have metastasis to the pericardium have pericardial effusion, of which only a small percentage develop tamponade.13 Approximately 20% of large, symptomatic effusions without an obvious etiology based on routine diagnostic examination constitute the initial presentation of a previously unrecognized cancer.14 Symptoms and signs suggestive of pericardial involvement may be the presenting clinical feature of either primary or secondary malignant cardiac disease, but they may also be seen in patients under treatment for advanced malignancy.15, 16 With the advent of human immunodeficiency virus disease, the incidence of Kaposi sarcoma and lymphomatous involvement of the pericardium has increased markedly.
Pathophysiology
Patients with malignancies may develop pericardial disease by 4 mechanisms: direct extension or metastatic spread via lymphatics or blood into the pericardium, chemotherapeutic toxicity, radiation toxicity, and opportunistic infections in the setting of immunosuppressive therapy.
Pericardial effusions due to malignancies typically arise either by direct local extension to the parietal pericardium, although visceral involvement with or without frank myocardial invasion may also be seen (for example, advanced lung carcinoma, breast carcinoma, mesothelioma, and esophageal carcinoma), or by metastatic spread via lymphatics or blood (for example, melanoma, Kaposi sarcoma, leukemia and lymphoma). Pericardial effusions may also arise because of obstruction to the lymphatic drainage, which might occur secondary to enlarged mediastinal lymph nodes.11, 17
Chemotherapeutic agents such as anthracyclines or cyclophosphamide are associated with acute pericarditis or myocarditis. These syndromes occur with high‐dose chemotherapy regimens, and the risk is dose‐dependent.
Mediastinal irradiation can produce an effusive‐constrictive pericarditis, similar to primary malignant effusion. This process is caused by pericardial effusion and by constriction of the heart by the fibrosed visceral pericardium.
Pericardial disease can also be due to opportunistic infections in the setting of immunosuppressive therapy of cancer patients. Viral pericarditis (eg, cytomegalovirus), tuberculous pericarditis, and fungal pericarditis (from opportunistic fungi such as Candida and Aspergillus) occur in immunocompromised patients.11
Clinical Presentation
The normal pericardium is a fibroelastic sac composed of visceral and parietal layers separated by the pericardial cavity and containing a thin layer (50–100 mL) of straw‐colored fluid surrounding the heart. It can accommodate >1000 mL without compromising cardiac function if the fluid is accumulated slowly. On the other hand, an extra 100 mL can cause hemodynamic compromise if accumulated rapidly.
The clinical presentation of patients with neoplastic pericardial effusion is variable. Some patients are completely asymptomatic, some might develop new‐onset atrial fibrillation with controlled or rapid ventricular rate, whereas others develop pericardial tamponade and cardiovascular collapse.18 The pericardial disease can present as 4 overlapping clinical syndromes: pericarditis, pericardial effusion, cardiac tamponade, or pericardial constriction. Of these pericardial syndromes, 2 are oncologic emergencies, cardiac tamponade and pericardial constriction, which can impair cardiac function and compromise hemodynamics.
Cardiac Tamponade
As effusion increases in the pericardial space, it compresses the cardiac chambers.19, 20, 21, 22 Patients with pericardial tamponade can present with symptoms such as syncope, chest pain, or palpitations. The symptoms could also be subtle, such as dyspnea, nonspecific chest discomfort, and simple fatigue. Beck's triad of hypotension, tachycardia, and muffled heart tones is usually present.23 Patients with more gradual onset of tamponade might have large hearts with preserved blood pressure and heart sounds.24 Patients may also have distended neck veins and a pulsus paradoxus (an exaggerated fall in systolic blood pressure of ≥10 mm Hg during inspiration). Pulsus paradoxus is a reliable physical cardiovascular sign of tamponade that might be absent in patients with increased pulmonary arterial wedge pressure elevation before the onset of tamponade.25, 26
Pericardial Constriction
Pericarditis, due to neoplastic infiltration, chemotherapy, radiation, or opportunistic infection, can lead to scarring with loss of pericardial elasticity. The volume of the pericardial sac becomes fixed, and ventricular filling is impaired. Patients with pericardial constriction may present with fatigue and dyspnea on exertion. On exam, sinus tachycardia, hypotension, elevated jugular venous pressure, and pericardial friction rub are present. Kussmaul sign (the lack of an inspiratory decline in jugular pressure) is rarely present, and pulsus paradoxus may be present.27
Diagnosis
The Role of Imaging
Although cardiac tamponade and constrictive pericarditis are clinical diagnoses, imaging studies play an important role in assessment and possible therapeutic intervention.
If pericarditis is present, the electrocardiographic findings may show widely distributed ST‐segment elevations with J‐point elevations. Sinus tachycardia and low voltage (defined as maximum QRS amplitude <0.5 mV in the limb leads) are typical findings on electrocardiograms in patients with pericardial effusion. Electrical alternans is characterized by beat‐to‐beat alterations in the QRS complex (as shown in Figure 2) that reflect the swinging of the heart in the pericardial fluid. It is relatively specific but not sensitive for tamponade. Cardiac tamponade can present as pulseless electrical activity.
Figure 2.
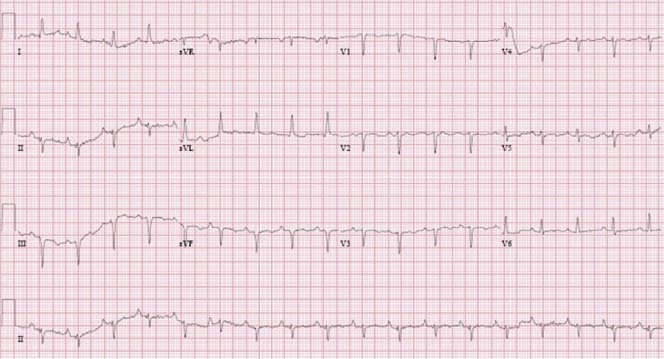
Electrocardiogram of a patient with breast cancer with a large pericardial effusion showing low voltage and electrical alternans, particularly seen in lead II rhythm strip.
Chest radiographs provide nonspecific supporting evidence for pericardial effusions. Acute tamponade may not show the classic enlarged cardiac silhouette, which requires at least 200 mL of pericardial fluid. On the other hand, a slowly developing tamponade on chest x‐ray may present as an enlarged cardiac silhouette with clear lung fields and the characteristic “water bottle” appearance, if there is adequate compensation.
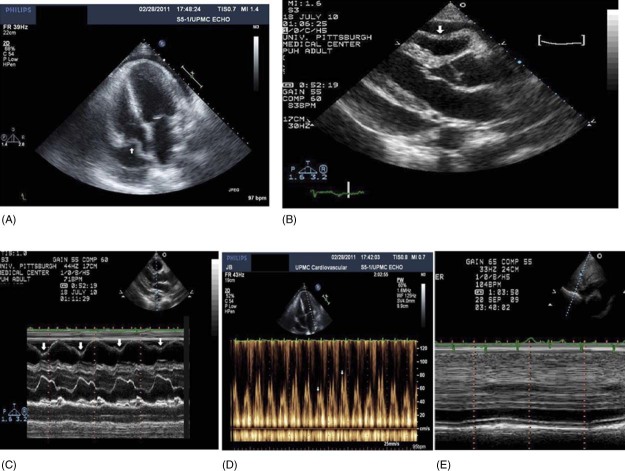
Echocardiography is considered the primary imaging modality for the evaluation of pericardial effusion because of its easy availability, lack of ionizing radiation, and low cost. Examples of transthoracic echocardiograms of a large neoplastic pericardial effusion with cardiac tamponade are in Figure 3. There are 10 echocardiographic findings described in cardiac tamponade due to neoplastic pericardial effusion, and they are summarized here and in the Table 1, 18, 28:
-
1.
Presence of pericardial effusion.
-
2.
Ventricular interdependence by volume and paradoxical motion of the interventricular septum with respiration. This pattern of motion corresponds to the physical finding of pulsus paradoxus.
-
3.
Right atrial (RA) systolic collapse has a 90% sensitivity and 68%–82% specificity for cardiac tamponade. If the RA collapse lasts up to one‐third of the cardiac cycle, this finding will have a 94% sensitivity and 100% specificity for cardiac tamponade (as shown in Figure 3A).
-
4.
Right ventricular (RV) early diastolic collapse. This finding has 60%–90% sensitivity and 85%–100% specificity for cardiac tamponade (as shown in Figure 3B and 3C).
-
5.
Left atrial (LA) compression (rarely seen).
-
6.
Left ventricular (LV) diastolic compression (rarely seen).
-
7.
Respiratory variation in RV and LV diastolic filling. Doppler interrogation of mitral valve inflow in cardiac tamponade shows ≥25% respiratory variation in the E wave (as shown in Figure 3D). The respiratory diastolic tricuspid valve inflow E variation is ≥40% in cardiac tamponade. Doppler flow velocity paradoxus has a good correlation with clinical features of tamponade, with a higher sensitivity (75%) than RV collapse and a much higher specificity (91%) than RA collapse.29, 30
-
8.
Diastolic reversal of hepatic vein flow on expiration.
-
9.
Plethora of the inferior vena cava (IVC) is an indicator of an elevated RA pressure. Dilated IVC with <50% inspiratory reduction in the diameter near the RA/IVC junction reflects increased RA pressure. Figure 3E shows plethora of the IVC. This finding has 97% sensitivity but only 40% specificity.
-
10.
Swinging motion of the heart in the pericardial sac.
Figure 3.
Transthoracic echocardiogram (A) of a patient with lung cancer with a large neoplastic pericardial effusion in the apical 4‐chamber view causing RA collapse (arrow). Parasternal long axis (B) showing RV diastolic collapse (arrow). M‐mode echocardiogram (C) of a large neoplastic pericardial effusion showing RV diastolic collapse (arrows). Pulse Doppler interrogation (D) of mitral valve inflow showing >25% respiratory variation in early diastolic velocity of the mitral inflow with respiration in a patient with cardiac tamponade. Dilated IVC (E) with no significant change with respiration (plethora). Abbreviations: IVC, inferior vena cava; RA, right atrial; RV, right ventricular.
Table 1.
Echocardiographic Findings in Cardiac Tamponade
| 1. Pericardial effusion. |
| 2. Ventricular interdependence: abnormal inspiratory increase of RV dimensions and abnormal inspiratory decrease of LV dimensions. |
| 3. RA systolic collapse (>one‐third of the cardiac cycle). |
| 4. RV early diastolic collapse. |
| 5. LA compression. |
| 6. LV diastolic compression. |
| 7. Abnormal inspiratory increase in blood flow velocity through the tricuspid valve and pulmonic valves and abnormal inspiratory decrease of mitral and aortic valves' flow velocity. |
| 8. Respiratory variations of pulmonary and hepatic venous flow. |
| 9. Plethora of the IVC: dilated IVC with lack of inspiratory collapse. |
| 10. Swinging heart. |
Abbreviations: IVC, inferior vena cava; LA, left atrial; LV, left ventricular; RA, right atrial; RV, right ventricular.
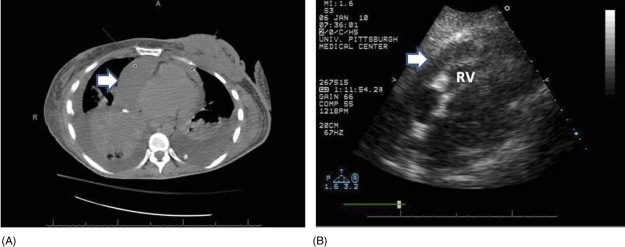
Constrictive pericarditis is characterized by pericardial thickening from chronic fibrosis, resulting in severe diastolic dysfunction. Echocardiography allows visualization of a thick pericardium and Doppler echocardiography is useful to evaluate hemodynamic changes in constrictive pericarditis. In constrictive pericarditis, the early diastolic mitral annular velocity (E′) cutoff value ≥8 cm/second is 95% sensitive and 96% specific for constrictive pericarditis rather than restrictive cardiomyopathy.31 Figure 4 shows a computed tomography (CT) scan and subcostal 4‐chamber echocardiogram showing a loculated effusion adjacent to the RV of a patient with breast carcinoma and effusive constrictive pericarditis.
Figure 4.
A CT scan and subcostal 4‐chamber echocardiogram showing a loculated effusion adjacent to the RV. The patient is the same patient as in Figure 2, several months later after pericardiocentesis. Abbreviations: CT, computed tomography; RV, right ventricle.
Both CT and magnetic resonance (MR) imaging are indicated when loculated or hemorrhagic effusion or pericardial thickening is suspected. Contrast‐enhanced helical CT provides valuable information about the possible nature of pericardial effusions based on the attenuation measurements of the collection.32, 33 The appearance of pericardial fluid is different on spin‐echo (SE) and gradient‐recalled echo (GRE) cine MR images. Nonhemorrhagic fluid has low signal intensity on T1‐weighted SE images and high intensity on GRE cine images.34 Conversely, hemorrhagic effusion is characterized by high signal intensity on T1‐weighted SE images and low intensity on GRE cine images.34
Despite echocardiographic signs of tamponade, right heart catheterization remains the definitive and quantifiable standard for diagnosing tamponade of any etiology. The procedure provides 2 important pieces of information: (1) elevated RA pressure, especially during inspiration; and (2) the intracardiac diastolic pressures has equalized among multiple chambers—there is equalization of the RA pressure, RV pressure, and pulmonary capillary wedge pressure. Recently, a novel criteria for diagnosis of constrictive pericarditis in the cardiac catheterization laboratory is systolic area index (ratio of RV to LV systolic area during inspiration and expiration) >1.1, with 97% sensitivity, 100% specificity, 100% positive predictive accuracy, and 95% negative predictive accuracy.35
Laboratory Investigations
Pericardiocentesis and the cytology of pericardial fluid or pericardial biopsy are essential for the definitive diagnosis of malignant pericardial disease. In almost two‐thirds of the patients with known malignancy, pericardial effusion is caused by nonmalignant inflammatory or infectious processes (eg, radiation pericarditis, pericarditis due to chemotherapy, or opportunistic infections).5, 6 Culture, cytology, adenosine deaminase (for tuberculous pericarditis), and polymerase chain reaction of the pericardial fluid and tissue can be helpful if indicated with a reasonable pretest probability. High white blood cell count with high polymorphonuclear neutrophils is suggestive of inflammation, particularly bacterial and rheumatologic. High monocytes is suggestive of malignancy and hypothyroidism.
Management
The management of neoplastic pericardial effusion involves the relief of symptoms and the prevention of recurrences. Symptomatic pericardial effusions are managed with percutaneous drainage.36 No randomized controlled studies have established the optimal initial approach to the management of symptomatic pericardial effusion. In most cases, pericardiocentesis is both therapeutic and diagnostic.35, 37, 38, 39 If the neoplastic pericardial effusion is associated with new‐onset atrial fibrillation, a rhythm‐control strategy is preferred, especially in the setting of hemorrhagic pericardial effusion secondary to metastases, to avoid anticoagulation and prevent thromboembolism.
Pericardiocentesis is the technique of catheter‐based aspiration of pericardial fluid. It serves as a diagnostic modality via fluid analysis and a therapeutic modality especially with hemodynamic compromise in patients with pericarditis with pericardial effusion, pericardial effusion with pericardial tamponade, and effusive‐constrictive pericarditis. Pericardiocentesis is also ill‐advised when the effusion is very small or loculated. Malignant effusions are usually a marker of advanced malignancy. Thus, recurrence is a major issue. Pericardiocentesis alone is inadequate for long‐term palliation, with recurrence rates as high as 90% within 90 days.39
Percutaneous balloon pericardiotomy offers a nonsurgical management of pericardial effusion.40 It is particularly useful for critically ill patients with advanced malignancy and limited to palliate malignant pericardial disease successfully for the duration of their survival.
Percutaneous balloon pericardiotomy should be considered if pericardial fluid recurs after primary pericardiocentesis.
Surgery has also shown to be effective in managing symptomatic neoplastic pericardial effusions. Consideration has to be made about the patient's prognosis and life expectancy before proceeding with surgery. Creating a window for fluid to drain to another reabsorptive cavity, such as the pleura, peritoneum, or preperitoneal subcutaneous space, may provide palliation.41, 42, 43, 44, 45 Subxyphoid pericardiotomy with percutaneous balloon pericardial window has shown to produce immediate relief of symptoms and prevention of local recurrences.44
There have been suggestions of obliteration of the pericardial space by maintaining pericardial drains on suction for between 3 and 28 days to effect fusion of parietal and visceral pericardium.45 Since then there have been studies with sclerosing agents, especially tetracycline.45 Other agents such as bleomycin, cisplatin, nitrogen mustard, fluorouracil, teniposide, thiotepa, mitomycin‐C, or even radionuclides also have been studied. Systemic antineoplastic treatment as baseline therapy can prevent recurrences in up to 67% of cases (European Society of Cardiology guidelines, level of evidence B, class I indication).7
Prognosis
Cardiac symptoms are mainly related to the presence of tamponade, which is present in a significant number of patients. In previously asymptomatic patients, cardiac tamponade was the immediate cause of death in about 85% of patients in a case series.32 Pericardial cytology remained an independent predictor of death. Pericardial effusions due to lung cancer or solid tumors other than breast cancer were associated with limited survival compared with effusions due to breast cancer or hematologic malignancies.
There are no randomized controlled trials regarding optimal treatment for patients with malignant and symptomatic pericardial effusion. Clinicians and investigators must continue to search for safe, flexible, and durable modes to palliate these patients while simultaneously containing costs and preserving the quality of their limited remaining life span.
Conclusion
In summary, the neoplastic pericardial effusion is a serious and common problem. It may develop from direct extension or metastatic spread of the underlying malignancy via lymphatics or blood into the pericardium, from an opportunistic infection, or from a complication of radiation therapy or chemotherapeutic toxicity. The clinical presentation varies, and the patient may be hemodynamically unstable in the setting of constrictive pericarditis and cardiac tamponade. The management depends on the patient's prognosis and varies from pericardiocentesis and balloon pericardiotomy to cardiothoracic surgery.
References
- 1. Gornik HL, Gerhard‐Herman M, Beckman JA. Abnormal cytology predicts poor prognosis in cancer patients with pericardial effusion. J Clin Oncol. 2005;23:521–526. [DOI] [PubMed] [Google Scholar]
- 2. Wilkes JD, Fidias P, Vaickus L, et al. Malignancy‐related pericardial effusion. Cancer. 1995;76:1377–1387. [DOI] [PubMed] [Google Scholar]
- 3. Posner MR, Cohen GI, Skarin AT. Pericardial disease in patients with cancer: the differentiation of malignant from idiopathic and radiation‐induced pericarditis. Am J Med. 1981;71:407–413. [DOI] [PubMed] [Google Scholar]
- 4. DeCamp MM Jr, Mentzer SJ, Swanson SJ, et al. Malignant effusive disease of the pleura and pericardium. Chest. 1997;112(4 suppl): 291S–295S. [DOI] [PubMed] [Google Scholar]
- 5. Millaire A, Wurtz A, de Groote P, et al. Malignant pericardial effusions: usefulness of pericardioscopy. Am Heart J. 1992;124: 1030–1034. [DOI] [PubMed] [Google Scholar]
- 6. Porte HL, Janecki‐Delebecq TJ, Finzi L, et al. Pericardioscopy for primary management of pericardial effusion in cancer patients. Eur J Cardiothorac Surg. 1999;16:287–291. [DOI] [PubMed] [Google Scholar]
- 7. Buck M, Ingle JN, Guilani ER, et al. Pericardial effusion in women with breast cancer. Cancer. 1987;60:263–271. [DOI] [PubMed] [Google Scholar]
- 8. Hanfling SM. Metastatic cancer to the heart: review of the literature and report of 127 cases. Circulation. 1960;22:474–483. [DOI] [PubMed] [Google Scholar]
- 9. Adebisi D, Adenle AD, Edwards JF. Clinical and pathologic features of metastatic neoplasms of the pericardium. Chest. 1982:81:166–169. [DOI] [PubMed] [Google Scholar]
- 10. Hancock EW. Neoplastic pericardial disease. Cardiol Clin. 1990;8:673–682. [PubMed] [Google Scholar]
- 11. Katz WE, Ferson PF, Lee RF, et al. Images in cardiovascular medicine: metastatic malignant melanoma to the heart. Circulation. 1996;93:1066. [DOI] [PubMed] [Google Scholar]
- 12. Maisch B, Seferović PM, Ristić AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology . Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25:587–610. [DOI] [PubMed] [Google Scholar]
- 13. Levitan Z, Kaplan AL, Gordon A. Survival after malignant pericardial effusion and cardiac tamponade in advanced ovarian cancer. South Med J. 1990:83:241–242. [DOI] [PubMed] [Google Scholar]
- 14. LeWinter MM, Tischler MD. Pericardial diseases In: Bonow RO, Mann DL, Zipes DP, et al, eds. Braunwald's Heart Disease. 9th ed. Philadelphia, PA: Saunders; 2011:1651–1670. [Google Scholar]
- 15. Galve E, Permanyer‐Miralda G, Tornos MP, et al. Self limited acute pericarditis as initial manifestation of primary cardiac tumor. Am Heart J. 1992;123:1690–1692. [DOI] [PubMed] [Google Scholar]
- 16. Soler‐Soler J, Permanyer‐Miralda G, Sagristǎ‐Sauleda J. A systematic diagnostic approach to primary acute pericardial disease: the Barcelona experience. Cardiol Clin. 1990;8:609–620. [PubMed] [Google Scholar]
- 17. Theologides H. Neoplastic cardiac tamponade. Semin Oncol. 1978;5:181–190. [PubMed] [Google Scholar]
- 18. Jneid H, Maree AO, Palacios I. Pericardial tamponade: clinical presentation, diagnosis and catheter‐based therapies In: Parrillo JE, Dellinger RP, eds. Critical Care Medicine: Principles of Diagnosis &Management in the Adult. 3rd ed. Philadelphia, PA: Mosby; 2007. [Google Scholar]
- 19. Metcalfe J, Woodbury JW, Richards V, et al. Studies in experimental pericardial tamponade. Circulation. 1952;5:518–524. [DOI] [PubMed] [Google Scholar]
- 20. Morgan BC, Guntheroth WG, Dillard DH. Relationship of pericardial to pleural pressure during quiet respiration and cardiac tamponade. Circ Res. 1965;16:493–498. [DOI] [PubMed] [Google Scholar]
- 21. Fowler NO. Physiology of cardiac tamponade and pulsus paradoxus. II: physiological, circulatory, and pharmacological responses in cardiac tamponade. Mod Concepts Cardiovasc Dis. 1978;47:115–118. [PubMed] [Google Scholar]
- 22. Millard RW, Fowler NO, Gabel M. Hemodynamic and regional blood flow distribution responses to dextran, hydralazine, isoproterenol, and amrinone during experimental cardiac tamponade. J Am Coll Cardiol. 1983;1:461–470. [DOI] [PubMed] [Google Scholar]
- 23. Beck CS. Two cardiac compression triads. JAMA. 1935;104: 714–716. [Google Scholar]
- 24. Guberman BA, Fowler NO, Engel PJ, et al. Cardiac tamponade in medical patients. Circulation. 1981;64:633–640. [DOI] [PubMed] [Google Scholar]
- 25. Reddy PS, Curtiss EI, O'Toole JD, et al. Cardiac tamponade: hemodynamic observations in man. Circulation. 1978;58:265–272. [DOI] [PubMed] [Google Scholar]
- 26. Reddy PS, Curtiss EI. Cardiac tamponade. Cardiol Clin. 1990;8:627–637. [PubMed] [Google Scholar]
- 27. Curtiss EI, Reddy PS, Uretsky BF, et al. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J. 1988;115:391–398. [DOI] [PubMed] [Google Scholar]
- 28. D'Cruz I, Rehman AU, Hancock HL. Quantitative echocardiographic assessment in pericardial disease. Echocardiography. 1997;14:207–214. [DOI] [PubMed] [Google Scholar]
- 29. Mercé J, Sagristǎ‐Sauleda J, Permanyer‐Miralda G, et al. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Heart J. 1999;138:759–764. [DOI] [PubMed] [Google Scholar]
- 30. Chong HH, Plotnick GD. Pericardial effusion and tamponade: evaluation, imaging modalities, and management. Compr Ther. 1995;21:378–385. [PubMed] [Google Scholar]
- 31. Ha JW, Ommen SR, Tajik J, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocity by tissue Doppler echocardiography. Am J Cardiol. 2004;94:316–319. [DOI] [PubMed] [Google Scholar]
- 32. Nunziata A, Catalano O, Cusati B, et al; for European Society of Radiology . Two signs of hemodynamic disturbance: contrast material reflux within the azygos vein (AV) and within the inferior vena cava (IVC) [abstract]. Presented at: Proceedings of the European Congress of Radiology; March 5–10, 2000; Vienna, Austria. Presentation 15‐028.
- 33. Harries SR, Fox BM, Roobottom CA. Azygos reflux: a CT sign of cardiac tamponade. Clin Radiol. 1998;53:702–704. [DOI] [PubMed] [Google Scholar]
- 34. Mulvagh SL, Rokey R, Vick GW III, et al. Usefulness of nuclear magnetic resonance imaging for evaluation of pericardial effusions, and comparison with two‐dimensional echocardiography. Am J Cardiol. 1989;64:1002–1009. [DOI] [PubMed] [Google Scholar]
- 35. Talreja DR, Nishimura RA, Oh JK, et al. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol. 2008;51:315–319. [DOI] [PubMed] [Google Scholar]
- 36. Soler‐Soler J, Sagristǎ‐Sauleda J, Permanyer‐Miralda G. Management of pericardial effusion. Heart. 2001;86:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsang TS, Enriquez‐Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–436. [DOI] [PubMed] [Google Scholar]
- 38. Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111:1213–1221. [DOI] [PubMed] [Google Scholar]
- 39. Celermajer D, Boyer M, Bailey B, et al. Pericardiocentesis of symptomatic malignant pericardial effusion: a study of 36 patients. Med J Aust. 1991;154:19–22. [DOI] [PubMed] [Google Scholar]
- 40. Jneid H, Ziskind A, Palacios IF. Pericardial interventions In: Topol EJ, Teirstein, eds. Textbook of Interventional Cardiology. 6th ed. Philadelphia, PA: Saunders; 2011, In press. [Google Scholar]
- 41. Hazelrigg SR, Mack MJ, Landreneau RJ, et al. Thoracoscopic pericardiectomy for effusive pericardial disease. Ann Thorac Surg. 1993;56:792–795. [DOI] [PubMed] [Google Scholar]
- 42. Piehler JM, Pluth JR, Schaff HV, et al. Surgical management of effusive pericardial disease: influence of extent of pericardial resection on clinical course. J Thorac Cardiovasc Surg. 1985;90: 506–516. [PubMed] [Google Scholar]
- 43. Olson JE, Ryan MB, Blumenstock DA. Eleven years experience with pericardial‐peritoneal window in the management of malignant and benign pericardial effusions. Ann Surg Oncol. 1995;2:165–169. [DOI] [PubMed] [Google Scholar]
- 44. Maher EA, Shepherd FA, Todd TJ. Pericardial sclerosis as the primary management of malignant pericardial effusion and cardiac tamponade. J Thorac Cardiovasc Surg. 1996;112: 637–643. [DOI] [PubMed] [Google Scholar]
- 45. Martinoni A, Cipolla CM, Cardinale D, et al. Long‐term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest. 2004;126:1412–1416. [DOI] [PubMed] [Google Scholar]