Abstract
Background:
Potential benefits of off‐label cardiac resynchronization therapy (CRT) in end‐stage heart failure (EHF) patients have not been fully investigated.
Hypothesis:
Some EHF patients who are dependent on intravenous inotropes can benefit from CRT.
Methods:
We retrospectively enrolled 14 EHF patients who were dependent on intravenous inotropes at the time of CRT implantation. Mean duration of inotropic support was 51 ± 47 days before CRT device implantation. To identify the efficacy of CRT, we assessed the successful withdrawal rate from inotropic support and survival estimates after device implantation. We also tried to identify possible predictors for withdrawal by comparing patient demographics between successful withdrawal (SW) and nonwithdrawal (NW) groups.
Results:
Successful withdrawal was achieved in 9 (64%) of 14 patients 46 ± 33 days after CRT device implantation. Event‐free survival was longer in the SW group than in the NW group (810 ± 169 days vs 114 ± 34 days; P = 0.007). In addition, patients in the SW group showed a higher previous surgery rate (89% vs 20%; P = 0.010) and a lower grade of mitral regurgitation (median, 0 vs 2; P = 0.010) than those in the NW group.
Conclusions:
Our retrospective data showed potential benefits of CRT among EHFpatients. Treatment of mitral regurgitation might be an essential qualification for managing EHF patients with CRT. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Heart transplantation is a promising treatment choice and has provided the greatest survival benefit in end‐stage heart failure (EHF) patients.1, 2 Because of the critical shortage of donor hearts, however, heart transplantation programs have increasingly tended to emphasize the importance of aggressive medical management, including devices, prior to accepting a patient as a heart transplantation candidate.1
On the other hand, the beneficial effects of cardiac resynchronization therapy (CRT) have been proven by a body of evidence in heart failure patients with left ventricular (LV) ejection fraction ≤35 percent, a QRS duration ≥120 msec, and New York Heart Association (NYHA) functional class III or ambulatory class IV symptoms with optimal medical therapy.3, 4, 5, 6 However, it has not been fully investigated whether and to what extent EHF patients, especially those corresponding to United Network of Organ Sharing (UNOS) status 1A patients, benefit from CRT.7, 8, 9, 10, 11 In the present study, we retrospectively evaluated the clinical impact of off‐label CRT among EHF patients who are dependent on in‐hospital intravenous (IV) inotropic support. The efficacy of CRT was evaluated by assessing the successful withdrawal (SW) rate from inotropic support and survival estimates after CRT device implantation.
Methods
Study Population
Among 62 consecutive patients with CRT registered in our database between April 2003 and August 2010, we enrolled 14 NYHA functional class IV EHF patients who were dependent on continuous in‐hospital IV inotropic support despite maximum tolerated medical therapy. Patients with significant aortic valve disease or mitral stenosis were excluded from the study. Dependence on IV inotropic therapy was defined as the inability to wean or withdraw from these supports without occurrence of symptomatic hypotension, oligo‐ or anuria, hypoxemia, and/or worsening of heart failure symptoms despite gradual dose reduction of inotropes under careful clinical monitoring. For example, some patients could not tolerate even a 0.5 µg/kg/minute reduction of inotropic support. We used dobutamine, milrinone, or dopamine as IV inotropic agents. Oral use of digoxin, pimobendan, and docarpamine were considered as oral inotropic support. All data were acquired preprocedurally unless otherwise indicated during hemodynamic supports with IV inotropes, mechanical ventilation, and/or intra‐aortic balloon pumping.
Echocardiographic Evaluation
All echocardiographic data were acquired retrospectively and were completely available. The following indexes were evaluated: LV end‐systolic, LV end‐diastolic, interventricular septum, posterior wall, and left atrial diameters, evaluated in the parasternal long‐axis view. The LV end‐systolic and end‐diastolic volumes and ejection fraction were calculated by biplane Simpson's method using apical 2‐ and 4‐chamber views.12 The severity of mitral regurgitation was classified into 5 grades (0–4) using percent regurgitation jet area of the left atrium with color Doppler images in apical 2‐, 3‐, or 4‐chamber views, with the most severe result being used for analysis.13 The interventricular and atrioventricular delay were optimized by echocardiography after CRT device implantation.4
Cardiac Resynchronization Therapy Device Implantation
A CRT device with or without an implantable cardioverter‐defibrillator could be used in the study. Left ventricular leads were placed in the lateral or posterolateral coronary veins according to previously published implantation techniques.3, 4 Four patients already had an epicardial LV lead that had been placed at the LV lateral wall during a previous cardiac surgery. The clinical indication for CRT in this patient cohort was limited to using CRT to manage EHF. Although all patients corresponded to UNOS 1A status at the time of CRT device implantation, none of the patients were able to undergo heart transplantation or LV assist device (LVAD) implantation because of the donor heart shortage, contraindications, or patient refusal to undergo LVAD implantation. As a consequence, 3 narrow QRS patients underwent CRT implantation in this study.
Efficacy of Cardiac Resynchronization Therapy
The clinical impact of CRT among EHF patients was evaluated by assessing the SW rate. Successful withdrawal was defined as withdrawal from all inotropic, mechanical ventilation, and intra‐aortic balloon pumping supports if applied for managing EHF before CRT device implantation. Weaning off from inotropic supports was performed gradually, for example by 0.5 µg/kg/minute every 3 days, with careful clinical monitoring during hospitalization because some patients could not tolerate this before CRT device implantation. We divided our patients into SW and nonwithdrawal (NW) groups and compared patient demographics between the 2 groups to identify possible predictors for withdrawal. We also evaluated event‐free or vital survival rate after CRT device implantation in the 2 groups. The endpoint of the present study was set as composite of death from any cause, worsening of heart failure, any‐cause hospitalization (other than generator exchange for the CRT device), and additional mechanical or surgical interventions such as LVAD implantation or heart transplantation. Worsening of heart failure was indicated by escalation of the inotropic dose for managing heart failure symptoms after CRT device implantation, or restart of IV inotropes after successful weaning during hospitalization. Readmission or unscheduled hospital visit for heart failure symptoms were also defined as worsening of heart failure for outpatients. Event‐free survival was analyzed from the day of CRT implantation to the time of the first event. We also analyzed vital survival estimates, in which the endpoint was set as composite of all‐cause mortality, LVAD implantation, or heart transplantation. All patients were followed up until the vital composite endpoint or November 2010.
Statistical Analysis
Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, IL). Continuous variables were presented as mean ± SD. If data were normally distributed due to Shapiro‐Wilk test, the Student t or Welch t test was applied following Levene's test for equality of variance; if data were not normally distributed, the Mann‐Whitney test was employed for the analysis. Ordinal variables were presented as median and analyzed with the Mann‐Whitney test. Categorical variables were presented as frequencies, and differences between groups were examined with the χ 2 statistics. Event‐free and vital survival durations were calculated from the day of CRT device implantation to the first and vital composite endpoints, respectively. Survival estimates were based on the Kaplan‐Meier method and compared by log‐rank test. A P value <0.05 was considered statistically significant.
The study complies with the Declaration of Helsinki and was approved by the institutional review board. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written.
Results
Baseline Characteristics
Patient and device demographics are shown in Tables 1, 2, 3. All 9 (100%) patients in the SW group were male, as compared with 2 (40%) of 5 in the NW group. All 3 patients with left bundle branch block showed a beneficial effect of CRT in the present study, although it did not meet statistical significance. The SW group showed a higher rate of previous cardiac surgery, especially mitral surgery, and a lower mitral regurgitation grade than the NW group. Total bilirubin value was higher in the NW group than in the SW group. In addition, patients in the SW group showed larger LV dimensions than those in the NW group, although LV volumes were not significantly different (Table 3). There were 4 procedure‐related complications (Table 3). Two patients in the NW group required prolonged antibiotic therapy for occurrences of fever and suspected infections, 1 in the NW group showed worsening of heart failure, and 1 in the SW group underwent CRT device removal and reimplantation due to device infection. However, these complications were successfully overcome with medications or reimplantation.
Table 1.
Patient Characteristics
| All (N = 14) | SW (n = 9) | NW (n = 5) | P Value (SW vs NW) | |
|---|---|---|---|---|
| Age, y | 58 ± 14 | 61 ± 11 | 52 ± 17 | 0.241 |
| Male sex | 11 (79) | 9 (100) | 2 (40) | 0.009 |
| Height, cm | 166 ± 8 | 167 ± 7 | 164 ± 11 | 0.604 |
| Weight, kg | 52.2 ± 10.0 | 52.9 ± 4.1 | 50.8 ± 17.0 | 0.798 |
| Body surface area, m2 | 1.56 ± 0.17 | 1.58 ± 0.07 | 1.53 ± 0.28 | 0.694 |
| Etiology | 0.122 | |||
| Ischemic | 3 (21) | 2 (22) | 1 (20) | |
| Nonischemic | 11 (79) | 7 (78) | 4 (80) | |
| iDCM | 8 (57) | 7 (78) | 1 (20) | |
| dHCM | 1 (7) | 0 (0) | 1 (20) | |
| Myocarditis | 1 (7) | 0 (0) | 1 (20) | |
| Sarcoidosis | 1 (7) | 0 (0) | 1 (20) | |
| Coronary risk factors | ||||
| Diabetes | 4 (29) | 3 (33) | 1 (20) | 0.597 |
| Hypertension | 1 (7) | 1 (11) | 0 (0) | 0.439 |
| Dyslipidemia | 5 (36) | 4 (44) | 1 (20) | 0.360 |
| Past smoker | 8 (57) | 5 (56) | 3 (60) | 0.872 |
| Mechanical ventilation | 2 (14) | 1 (11) | 1 (20) | 0.649 |
| IABP | 2 (14) | 2 (22) | 0 (0) | 0.255 |
| Previous cardiac surgery | 9 (64) | 8 (89) | 1 (20) | 0.010 |
| CABG | 3 (21) | 2 (22) | 1 (20) | 0.923 |
| Mitral surgery | 9 (64) | 8 (89) | 1 (20) | 0.010 |
| Surgery to CRT, d | 1801 ± 3278 | 1917 ± 3485 | 877 | NA |
| Medication | ||||
| IV inotrope | 0.326 | |||
| 1 kind | 8 (57) | 6 (67) | 2 (40) | |
| 2 kinds | 5 (36) | 3 (33) | 2 (40) | |
| 3 kinds | 1 (7) | 0 (0) | 1 (20) | |
| Duration of inotropic support prior to CRT, d | 51 ± 47 | 41 ± 23 | 69 ± 73 | 0.436 |
| Amiodarone | 8 (57) | 4 (44) | 4 (80) | 0.198 |
| ACEI/ARB | 9 (64) | 6 (67) | 3 (60) | 0.803 |
| β ‐Blocker | 10 (71) | 6 (67) | 4 (80) | 0.597 |
| Loop diuretics | 14 (100) | 9 (100) | 5 (100) | NA |
| Aldosterone blocker | 12 (86) | 7 (78) | 5 (100) | 0.255 |
| Oral inotropic agent | 5 (36) | 3 (33) | 2 (40) | 0.803 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CRT, cardiac resynchronization therapy; dHCM, dilated‐phase hypertrophic cardiomyopathy; IABP, intra‐aortic balloon pumping; iDCM, idiopathic dilated cardiomyopathy; IV, intravenous; NA, not applicable; NW, nonwithdrawal; SW, successful withdrawal. Data are presented as mean ± SD or n (%), and were acquired preprocedurally unless otherwise indicated.
Table 2.
Hemodynamic, Electrocardiographic, and Laboratory Data
| All (N = 14) | SW (n = 9) | NW (n = 5) | P Value (SW vs NW) | |
|---|---|---|---|---|
| Hemodynamic data | ||||
| Systolic BP, mm Hg | 87 ± 11 | 87 ± 12 | 87 ± 9 | 0.989 |
| Diastolic BP, mm Hg | 54 ± 7 | 54 ± 9 | 53 ± 5 | 0.785 |
| Heart rate, bpm | 80 ± 8 | 82 ± 5 | 75 ± 12 | 0.168 |
| Electrocardiographic data | ||||
| Basic rhythm | 0.138 | |||
| Sinus | 9 (64) | 7 (78) | 2 (40) | |
| AF | 1 (7) | 1 (11) | 0 (0) | |
| Pacemaker | 4 (29) | 1 (11) | 3 (60) | |
| Conduction disorder | 0.209 | |||
| None (QRS <120 msec) | 3 (21) | 2 (22) | 1 (20) | |
| LBBB | 3 (21) | 3 (33) | 0 (0) | |
| RBBB | 1 (7) | 0 (0) | 1 (20) | |
| Intraventricular conduction defect | 2 (14) | 2 (22) | 0 (0) | |
| Pacing | 5 (36) | 2 (22) | 3 (60) | |
| QRS interval, msec | 159 ± 48 | 159 ± 44 | 159 ± 59 | 0.988 |
| QRS after CRT, msec | 159 ± 35 | 162 ± 28 | 154 ± 48 | 0.702 |
| Laboratory data | ||||
| BNP on admission, pg/mL | 1045 ± 858 | 885 ± 824 | 1333 ± 934 | 0.371 |
| BNP before CRT, pg/mL | 742 ± 543 | 626 ± 522 | 950 ± 575 | 0.317 |
| Hemoglobin, g/dL | 11.4 ± 1.9 | 11.3 ± 1.7 | 11.6 ± 2.4 | 0.768 |
| Sodium, mEq/L | 133 ± 5 | 133 ± 4 | 134 ± 6 | 0.553 |
| Creatinine, mg/dL | 1.4 ± 0.8 | 1.4 ± 0.9 | 1.3 ± 0.8 | 0.739 |
| Total bilirubin, mg/dL | 1.0 ± 0.9 | 0.6 ± 0.2 | 1.6 ± 1.3 | 0.015 |
| Uric acid, mg/dL | 6.2 ± 2.1 | 6.7 ± 1.7 | 5.4 ± 2.7 | 0.303 |
Abbreviations: AF, atrial fibrillation; BNP, brain natriuretic peptide; BP, blood pressure; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; NW, nonwithdrawal; RBBB, right bundle branch block; SD, standard deviation; SW, successful withdrawal. There is a discrepancy between the number of pacemaker rhythm (4) and paced QRS conduction (5) because 1 AF patient received ventricular pacing. Data are presented as mean ± SD or n (%), and were acquired preprocedurally unless otherwise indicated.
Table 3.
Preprocedural Echocardiographic Findings and Device Data
| All (N = 14) | SW (n = 9) | NW (n = 5) | P Value (SW vs NW) | |
|---|---|---|---|---|
| Echocardiographic findings | ||||
| LVEDd, mm | 68.8 ± 8.3 | 72.6 ± 7.1 | 62.0 ± 6.0 | 0.016 |
| LVESd, mm | 62.9 ± 9.1 | 66.8 ± 7.9 | 55.8 ± 6.8 | 0.023 |
| LVEDV, mL | 254 ± 82 | 266 ± 94 | 232 ± 57 | 0.739 |
| LVESV, mL | 204 ± 76 | 216 ± 85 | 181 ± 59 | 0.386 |
| LVEF, % | 20.9 ± 6.3 | 19.6 ± 4.7 | 23.2 ± 8.7 | 0.321 |
| IVS, mm | 6.2 ± 1.3 | 6.1 ± 1.5 | 6.4 ± 0.9 | 0.574 |
| PW, mm | 6.9 ± 2.3 | 7.4 ± 2.1 | 6.0 ± 2.6 | 0.285 |
| LAD, mm | 49.1 ± 11.4 | 51.9 ± 11.3 | 44.2 ± 10.7 | 0.239 |
| MR, grade | 0.5 | 0 | 2 | 0.010 |
| MR ≥2 | 5 (36) | 1 (11) | 4 (80) | 0.010 |
| TR‐PG, mm Hg | 29 ± 15 | 29 ± 14 | 29 ± 19 | 0.998 |
| IVC, mm | 15.8 ± 5.8 | 15.8 ± 5.7 | 15.8 ± 6.5 | 0.995 |
| Device data | ||||
| Device | 0.164 | |||
| CRT without defibrillator | 1 (7) | 0 (0) | 1 (20) | |
| CRT with defibrillator | 13 (93) | 9 (100) | 4 (80) | |
| Hospitalization to CRT, d | 75 ± 48 | 68 ± 24 | 88 ± 79 | 0.606 |
| Manufacturer | 0.803 | |||
| SJM | 5 (36) | 3 (33) | 2 (40) | |
| Medtronic | 9 (64) | 6 (67) | 3 (60) | |
| LV lead placement | 0.078 | |||
| Coronary sinus lead | 10 (71) | 5 (56) | 5 (100) | |
| Surgical epicardial lead | 4 (29) | 4 (44) | 0 (0) | |
| Upgrade from PM/ICD | 6 (43) | 3 (33) | 3 (60) | 0.334 |
| Procedure‐related complication | 4 (29) | 1 (11) | 3 (60) | 0.052 |
| BiV pacing rate after CRT, % | 96 ± 5 | 95 ± 6 | 98 ± 2 | 0.166 |
Abbreviations: BiV, biventricular; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter‐defibrillator; IVC, inferior vena cava; IVS, interventricular septum; LAD, left atrial diameter; LV, left ventricular; LVEDd, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESd, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; MR, mitral regurgitation; NW, nonwithdrawal; PM, pacemaker; PW, posterior wall; SJM, St. Jude Medical; SW, successful withdrawal; TR‐PG, tricuspid regurgitation pressure gradient. Data are presented as mean ± SD, median, or n (%).
Efficacy of Cardiac Resynchronization Therapy
Figure 1 depicts the cumulative SW rate. Successful withdrawal was achieved in 9 of 14 patients (64%) 46 ± 33 days after CRT device implantation. Of these 9 patients, 7 were successfully discharged and followed up as outpatients.
Figure 1.
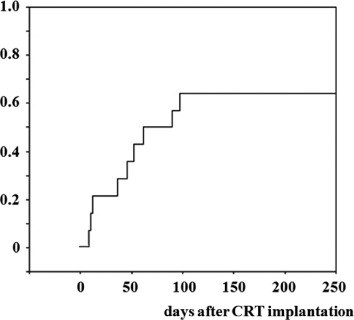
Cumulative successful withdrawal rate. Successful withdrawal was achieved in 9 (64%) of 14 patients 46 ± 33 days after CRT device implantation. Abbreviations: CRT, cardiac resynchronization therapy.
Regarding the vital composite endpoint of the SW group, 6 of 9 patients (67%) remained alive during the study period, 1 (11%) underwent LVAD implantation, and 2 (22%) died due to worsening of heart failure. On the other hand, in the NW group, 1 of 5 patients (20%) remained alive at the hospital with careful medical management, 2 (40%) underwent LVAD implantation, and 2 (40%) died due to worsening of heart failure. As a consequence, patients in the SW group showed longer event‐free survival than those in the NW group (810 ± 169 days vs 114 ± 34 days; P = 0.007) (Figure 2A). However, there was no significant difference in vital survival between the SW and NW groups (826 ± 163 days vs 337 ± 140 days, P = 0.148) (Figure 2B).
Figure 2.
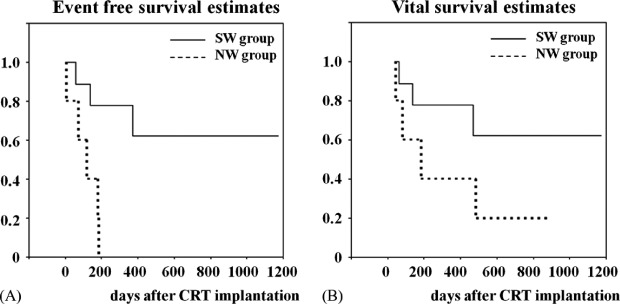
Kaplan‐Meier event‐free (A) and vital (B) survival estimates. Patients in the SW group showed statistically significant longer event‐free survival than those in the NW group (810 ± 169 days vs 114 ± 34 days, P = 0.007). However, there was no significant difference in vital survival between the SW and NW groups (826 ± 163 days vs 337 ± 140 days, P = 0.148). Abbreviations: CRT, cardiac resynchronization therapy; NW, nonwithdrawal; SW, successful withdrawal.
Discussion
Although it is an off‐label use in current guidelines,6 we treated NYHA functional class IV EHF patients who were dependent on in‐hospital IV inotropic support with CRT. The severity of heart failure corresponded to that of LVAD or heart transplantation candidates (UNOS status 1A) in all cases.1, 2, 14 Successful withdrawal was achieved in 64% of patients, even with 51 ± 47 days of inotropic support before CRT implantation, and 50% of patients were successfully discharged.
Because the SW group showed statistically significant longer event‐free survival than the NW group, SW from inotropic support can be a strong surrogate marker for EHF patients. This event‐free survival rate shown in Figure 2A may be relatively high, considering the very poor prognosis of UNOS status 1A EHF patients with medical treatment.15 Thus, identifying predictors for SW from inotropic support in these patients is important. To the best of our knowledge, there is no report that tries to predict patient response to CRT in inotrope‐dependentEHF patients. Because few data are available about the clinical impact of CRT in this patient cohort as yet,7, 8, 9, 10, 11 our data provide additional insights into CRT and are useful to further clarify the role of CRT in inotrope‐dependent EHF patients.
Our data suggest that male gender may play a role in SW from inotropic supports among EHF patients (Table 1). This is consistent with the previously reported data.16 A higher total bilirubin value in the NW group than in the SW group is also consistent with the published data, which revealed the association between high bilirubin value and poor prognosis in heart failure patients.17 In addition, we also demonstrated that LV end‐diastolic and end‐systolic diameters were larger in the SW group than those in the NW group, even though LV end‐diastolic and end‐systolic volumes were not significantly different between the 2 groups. We speculated that one potential reason for this discrepancy between LV volume and dimension is LV configuration. For example, minor axis diameter is different between a sphere and ellipsoid that have the same volume.
Of note, a greater extent of mitral regurgitation was associated with worse outcomes in this study. This is instinctively understandable, considering the very low LV function in this cohort. We speculated that higher previous mitral surgery rate and lower mitral regurgitation grade in the SW group implies that management of mitral regurgitation is crucial for managing EHF patients with CRT. Although CRT may have the potential to improve mitral regurgitation, this beneficial effect is usually gained gradually over time and limited to only some patients.18 Thus, it is important for EHF patients that mitral regurgitation has already been treated at the time of CRT implantation to gain the benefits of CRT efficiently.19 We believe our data could be applicable for a future treatment strategy of EHF patients using CRT in conjunction with surgical or percutaneous mitral intervention, although the mitral surgery in itself failed to demonstrate long‐term survival benefit among EHF patients.20
In addition, patients in the SW group showed statistically significant longer event‐free survival than those in the NW group, whereas there was no significant difference in vital survival between the groups. This may be, at least in part, due to the underpowered small sample size. In summary, we speculate that CRT may be one of the therapeutic options for managing EHF patients who refuse or who are ineligible to receive LVAD implantation or heart transplantation due to contraindications. Treatment of mitral regurgitation might be an essential qualification for managing EHF patients with CRT.
Study Limitations
There are several limitations in this study. First, this is a single‐center, retrospective observational study without a control arm. Second, only a small number of patients were enrolled because of the particular entry criteria, as in the previously reported study data.7, 8, 9, 10, 11 Moreover, echocardiographic data on right ventricular function, LV shape information such as spherical index, or mechanical dyssynchrony are not available due to the retrospective nature of the study, although the Predictors of Response to CRT (PROSPECT) study demonstrated that echocardiographic evaluation of mechanical dyssynchrony could not predict CRT responsiveness at present.21 These limitations remind us to interpret the data with caution.
Conclusion
Our retrospective data showed potential benefits of CRT among EHF patients. Treatment of mitral regurgitation might be an essential qualification for managing EHF patients with CRT. Studies in much larger cohorts are needed to validate our conclusions.
References
- 1. Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant. 2006;25:1024–1042. [DOI] [PubMed] [Google Scholar]
- 2. Lietz K, Miller LW. Improved survival of patients with end‐stage heart failure listed for heart transplantation: analysis of organ procurement and transplantation network/U.S. United Network of Organ Sharing data, 1990 to 2005. J Am CollCardiol. 2007;50:1282–1290. [DOI] [PubMed] [Google Scholar]
- 3. Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 4. Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 5. McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–2514. [DOI] [PubMed] [Google Scholar]
- 6. Epstein AE, DiMarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. [DOI] [PubMed] [Google Scholar]
- 7. Cazeau S, Ritter P, Lazarus A, et al. Multisite pacing for end‐stage heart failure: early experience. Pacing Clin Electrophysiol. 1996;19:1748–1757. [DOI] [PubMed] [Google Scholar]
- 8. Cowburn PJ, Patel H, Jolliffe RE, et al. Cardiac resynchronization therapy: an option for inotrope‐supported patients with end‐stage heart failure? Eur J Heart Fail. 2005;7:215–217. [DOI] [PubMed] [Google Scholar]
- 9. Herweg B, Ilercil A, Cutro R, et al. Cardiac resynchronization therapy in patients with end‐stage inotrope‐dependent class IV heart failure. Am J Cardiol. 2007;100:90–93. [DOI] [PubMed] [Google Scholar]
- 10. Konstantino Y, Iakobishvili Z, Arad O, et al. Urgent cardiac resynchronization therapy in patients with decompensated chronic heart failure receiving inotropic therapy: a case series. Cardiology. 2006;106:59–62. [DOI] [PubMed] [Google Scholar]
- 11. James KB, Militello M, Barbara G, et al. Biventricular pacing for heart failure patients on inotropic support: a review of 38 consecutive cases. Tex Heart Inst J. 2006;33:19–22. [PMC free article] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, et al; Chamber Quantification Writing Group; American Society of Echocardiographyś Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiographyś Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am SocEchocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 13. Zoghbi WA, Enriquez‐Sarano M, Foster E, et al; American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am SocEchocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 14. Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post‐REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. [DOI] [PubMed] [Google Scholar]
- 15. Birks EJ. Left ventricular assist devices. Heart. 2010;96:63–71. [DOI] [PubMed] [Google Scholar]
- 16. Bhattacharya S, Abebe K, Simon M, et al. Role of cardiac resynchronization in end‐stage heart failure patients requiring inotrope therapy. J Card Fail. 2010;16:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen LA, Felker GM, Pocock S, et al; CHARM Investigators . Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sitges M, Vidal B, Delgado V, et al. Long‐term effect of cardiac resynchronization therapy on functional mitral valve regurgitation. Am J Cardiol. 2009;104:383–388. [DOI] [PubMed] [Google Scholar]
- 19. Muratsu J, Hara M, Mizote I, et al. The impact of cardiac resynchronization therapy in an end‐stage heart failure patient with a left ventricular assist device as a bridge to recovery. Int Heart J. 2011;52:246–247. [DOI] [PubMed] [Google Scholar]
- 20. Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am CollCardiol. 2005;45:381–387. [DOI] [PubMed] [Google Scholar]
- 21. Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]


