Abstract
Background:
To investigate the clinical features of cardiac involvement in polymyositis (PM) or dermatomyositis (DM).
Hypothesis:
More attention will be focused on the heart in PM/DM as we would have wished, which contribute to improve the prognosis.
Methods:
All articles published in English were retrieved by searching MEDLINE via PubMed (1975–2011). After selecting eligible articles according to the predefined inclusion and exclusion criteria, a systemic review was carried out.
Results:
A total of 26 articles were included in this study, which included 1530 patients. The incidence of cardiac involvement was 9% to 72%. Heart failure was the most frequent (32% to 77%) clinical symptom. Among the abnormal electrocardiogram and ultrasonic cardiogram, the incidence of conduction abnormalities, left ventricular diastolic dysfunction, and hyperkinetic left ventricular contraction were 25% to 38.5%, 42%, and 6% to 12%, respectively. The pathologic findings revealed myocardial inflammation, degenerative changes and necrosis similar to that in skeletal muscles. Cardiac manifestations of some patients improved after glucocorticoid and immunosuppressant treatment. Thirty‐seven patients (46.3%) died as a direct result of heart disease.
Conclusions:
Heart abnormalities are frequent in patients with PM/DM, most of which were subclinical. The efficacy of glucocorticoids and immunosuppressants is uncertain. Cardiac involvement is a common cause of death. Clin. Cardiol. 2012 doi: 10.1002/clc.22026
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Polymyositis (PM) and dermatomyosits (DM) are autoimmune systemic diseases characterized by chronic muscle weakness and inflammatory cell infiltrates in skeletal muscle. Cardiac involvement in PM or DM was first reported in 1899 by Oppenheim.1 A number of studies showed that cardiac involvements were frequent and have been recognized as the main prognostic factor for death.2, 3, 4 But the nature and extent of these abnormalities varied according to different patient selection, definition of heart involvement, and methods of detection used. Although several reviews have been published that were updated in 2006,5 the issue of heart involvement in adult PM/DM has not been systemically evaluated. We conducted a systematic review of the current literature to investigate the clinical features of cardiac complications in PM/DM.
Methods
Search Strategy and Data Sources
We identified potentially relevant articles from the literature using the strategies shown in the Figure 1. We searched for articles (1975–2011) in PubMed with limits that they are “English, humans, all adults 19+ years.” Medical subject headings terms were as follows: (polymyositis OR dermatomyositis) AND (heart OR heart diseases OR myocardium OR myocarditis OR cardiomyopathy OR heart failure). We also performed a manual search of major journals and reviewed the reference lists from retrieved articles.
Figure 1.
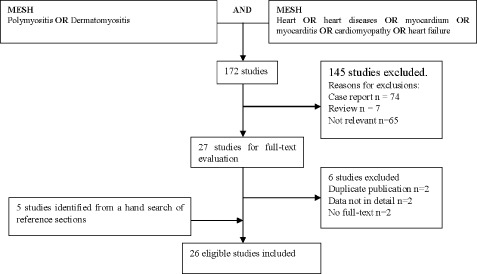
Results of the search.
Inclusion Criteria
Inclusion criteria were (1) PM/DM diagnosis based on the definitions described by Bohan and Peter in 1975, (2) articles that contained clinical characteristics as well as the results of necessary auxiliary testsm, and (3) cardiac involvement defined as being if 1 of the following rules are met: myocardial ischemia: the characteristic ECG change is horizontal or downsloping ST segment depression more than 0.5 mV in 3 consecutive leads, with wave changes or not; arrhythmias: frequent atrial or ventricular premature beats, atrial tachycardia, ventricular tachycardia, atrial fibrillation, except for physiogenic arrhythmias; conduction defects: atrioventricular and intraventricular conduction defects, incomplete right bundle branch block is excluded; cardiomyopathies: atrial and/or ventricular enlargement, cardiac hypertrophy, ventricular wall dyskinesia, systolic and/or diastolic dysfunction by ultrasonic cardiogram (UCG), left ventricular high voltage by electrocardiogram (ECG); diseases of pericardium: pericardial effusion or thickening in echocardiography; pulmonary hypertension: the mean pulmonary arterial pressure is >25 mm Hg at rest or >30 mm Hg during exercise by echocardiograph; valvular heart disease: abnormalities of valve morphology and function, physiological changes were excluded; other: any abnormality such as myocardial infarction or aortic enlargement.
Exclusion Criteria
Exclusion criteria were (1) duplicate publications (only the most recent and/or complete one was analyzed to avoid overlap between cohorts); (2) case reports or the number of cases is <4; (3) juvenile dermatomyositis, polymyositis with malignancy, or overlap syndromes that were unique study objects; (4) publication type is a review or letter.
Results
Results of Search
The search yielded 172 abstracts that were independently reviewed by 2 authors, with disagreements resolved by consensus. Twenty‐eight papers appearing to meet inclusion criteria based on abstract alone were subject to full‐text evaluation, resulting in 26 articles that included 1530 patients meeting the eligibility criteria. More details are shown in Table 1.
Table 1.
Summary of 26 included articles
| Author | Year | Study design | No. of case | Category | Conclusion |
|---|---|---|---|---|---|
| Plazak et al20 | 2011 | prospective | 15 | NA | Pathologic changes on valve (46.7%) and/or pericardium (66.7%) are main abnormalities in PM/DM. |
| Corinne et al33 | 2010 | prospective | 8 | PM3/DM3 | Serum cTnT levels did not appear to correlate with CK level. It may identify a subgroup with subclinical myocardial involvement. |
| Vidya et al23 | 2010 | Retrospective | 227 | PM184/DM43 | The prevalence of ischemic heart disease in PM/DM was 26%, which were more likely to be present prior to the diagnosis of PM/DM rather than following it. |
| Na et al34 | 2009 | retrospective | 32 | DM | 35% adult DM showed ECG abnormalities. |
| Tisseverastinghe et al24 | 2009 | retrospective | 607 | NA | The incidence of acute myocardial infarction in PM/DM was 5.6%. |
| Allanore et al16 | 2006 | prospective | 3 | PM2/DM1 | A therapy of glucorticoid and immunosuppressant improved the cardiac clinical symptoms and markedly reduced myocardial MRI contrast enhancement in patients with PM/DM Cardiac MRI could be used for the diagnosis and monitoring of myocarditis related to PM/DM. |
| Danko et al6 | 2004 | retrospective | 117 | PM75/DM42 | Unfavorable prognostic signs were respiratory muscle and cardiac involvement. (Pulmonary complications: 4/13, cardiovascular reasons: 8/13) |
| Buchpiguel et al17 | 1996 | prospective | 22 | PM8/DM14 | 99mTc‐PYP heart scan seems to permit early detection of cardiac involvement in PM/DM. Patients with marked myocardial uptake had a poor outcome. |
| Gonzalez‐Lopez et al2 | 1996 | prospective | 32 | PM6/DM26 | Subclinical cardiac abnormalities are frequent (72%) is patients with PM/DM, Left ventricular diastolic dysfunction (42%) appears to be common. |
| Taylor et al3 | 1993 | prospective | 26 | PM22/DM4 | Cardiac symptoms and signs were common (62 and 81%, respectively), Conduction system abnormalities and left ventricular dysfunction are more likely to be related to underlying myocarditis. |
| Byrnes et al14 | 1991 | prospective | 12 | PM7/DM5 | Cardiomyopathy and valve disease were unusual. The incidence of PAH and pencardial effusion was 75% and 15.3% respectively. |
| Hebert et al21 | 1990 | prospective | 11 | PM | PAH hypertension is frequent in PM (63%), most of them are asymptomatic. |
| Agrawal et al18 | 1989 | prospective | 14 | NA | Cardiac involvement is infrequent (14%) in PM/DM. |
| Wilhelmina et al13 | 1987 | prospective | 36 | PM13/DM23 | Cardiac involvement is frequent in PM/DM (70%). Antibody (anti‐Ro) is a marker for cardiac injury in myositis. |
| Hochberg et al7 | 1986 | retrospective | 52 | PM31/DM21 | Cardiac involvement was present in 37% of patient. 15.4% (2/13) of patients died of congestive heart failure. |
| Benenbassat et al9 | 1985 | retrospective | 47 | PM18/DM29 | 31.5% of patients had ECG abnormalities, 26.7% (4/15) of patients died of heart disease. |
| Stern et al11 | 1984 | retrospective | 77 | PM | ECG abnormalities were found in 32.5% of patients with PM. Left anterior hemi‐block (13.0%) and right bundle‐branch block (9.1%) were the most common abnormalities |
| Askari et al22 | 1984 | retrospective | 8 | PM2/DM6 | Cardiac involvement is usual in PM/DM. ECG. Holter and UCG are helpful to detect subclinical diseases. |
| Strongwater et al19 | 1983 | retrospective | 12 | PM | The presence of elevated CK‐MB isoenzyme activity describes a patient population at risk for developing cardiac disease. |
| Haupt and Hutchins12 | 1982 | retrospective | 7 | DM | Cardiac involvement may be common in PM. Evidence of active myocarditis was present in 25% of patients, all had congestive failure. |
| Kehoe et al10 | 1981 | prospective | 4 | PM | The severity of skeletal muscle involvement maybe related to the extent of conduction system disease. |
| Denbow et al8 | 1979 | retrospective | 18 | PM18 | Electrocardiographic abnormalities (72%) and clinicallyevident heart failure (45%) in PM are associated with active myocarditis. |
| Oka et al25 | 1978 | prospective | 12 | PM8/DM4 | Features of cardiac involvement were observed in 11 cases (69%). In 4 patients congestive heart failure progressed to death. |
| Gottdiener et al15 | 1978 | prospective | 21 | PM21 | Abnormalities on noninvasive cardiac testing are prevalent (76%) in patients with PM. |
| Bohan et al4 | 1977 | retrospectively | 97 | PM52/DM45 | Cardiac abnormalities (35%) were common in PM and DM, which were more frequent in patients with PM (50%) than DM (178%). |
| Sharratt et al35 | 1977 | prospective | 13 | PM | The prevalence of cardiac involvement in PM was 38.5%. |
Abbreviations: PM,polymyositis;DM,dermatomyositis;PAH,pulmonary artery hypertension; NA,not available.
Incidence and Risk Factor of Cardiac Involvement
The frequency of heart involvement in patients with PM/DM varies between 9% and 72%.6, 7, 8, 9 depending on patient selection, and methods used to detect cardiac involvement. Although Kehoe et alargued the extent of conduction system disease may be related to the severity of skeletal muscle involvement,10 several other studies showed that there was no correlation of overall severity of the disease with the presence of pathologic cardiac involvement.11, 12 Bohan A et al found that cardiac abnormalities were more frequent in patients with PM than in patients with DM.4 In addition, Wilhelmina et al demonstrated antibody (anti‐Ro) is a marker for cardiac injury in PM/DM.13
Clinically Manifest Heart Involvement
Clinically manifest heart problems are relatively infrequent in patients with PM/DM. The statistics show that the prevalence of asymptomatic cardiac involvement is 3% to 6%.2, 4, 7 The most frequently reported clinically manifest cardiac involvement is heart failure (dyspnea on exertion, orthopnea, nocturnal dyspnea), observed in between 32% and 77% of PM/DM patients.3, 7, 8, 14 Other clinical symptoms such as palpitation, shortness of breath, nonproductive cough, angina pectoris, chest pain, dizziness, and syncope were also described. Physical examination signs included increased jugular venous distention, muffled heart sounds, rales, wheezing, and rhonchi heard at the lung bases and peripheral edema. Heart diseases may occur at any phase of PM/DM, even when PM/DM is in remission. In a longitudinal study, conduction abnormalities progressed and new disturbances developed despite corticosteroid treatment and remission of the polymyositis.11
Subclinical Heart Involvement
The conjunctive use of noninvasive diagnostic testing such as ECG, Holter, UCG, and radionuclide ventriculography detected 13% to 72% of patients with subclinical heart complications.4, 8, 9, 13, 15 The incidence of ECG abnormalities from selected articles is from 25% to 85%.2, 3, 7, 13, 16, 17 ECG and Holter abnormalities observed in PM/DM patients include: frequent atrial or ventricular premature beats, atrial tachycardia, ventricular tachycardia, atrial fibrillation, atrioventricular conduction block, bundle branch block, abnormal Q‐waves, as well as nonspecific ST‐T–wave changes. ST‐T changes and conduction defects are the most frequent, and the occurrences are 12.5% to 56.7% and 25% to 38.5%, respectively.
The prevalence of echocardiographic abnormalities in patients with PM/DM varied from 14% to 62% depending on differences in patient selection and echocardiography techniques.18 Abnormalities obtained by echocardiography include the following (Table 2): (1) Changes of heart structure: a number of articles described the major changes of structure as being left atrial and/or left ventricular enlargement (8%–12%), left ventricular hypertrophy (8%–15%), and some case showed septal hypertrophy (8%). (2) Disorder of heart function: there were 42% patients with left ventricular diastolic dysfunction2 and 6% to 12% of patients with hyperdynamic left ventricular contraction.2, 12, 16, 19, In addition, segmental or global hypokinetics appeared in some cases.16 (3) Heart valve disease: the incidence of clinically significant valve diseases was relatively low (7%–23%),2, 14, 20 which included thickened valve leaflet, valve prolapses, and valve stenosis or regurgitation in mitral valve and aortic valve most frequently. (4) Diseases of pericardium: all pericardial effusions (8% to 66.7%) were small and hemodynamically insignificant.2, 3, 12, 14 (5) Pulmonary hypertension: limited studies showed that the incidence of pulmonary hypertension detected by echocardiography parameters was 63% to 75%, which merits further evaluation.14, 21
Table 2.
Echocardiographic Abnormalities
| Echocardiographic Abnormalities | Frequency (%) | |
|---|---|---|
| Heart Structure | Left artrial/ventricular enlargement | 8∼12 |
| Left ventricular hypertrophy | 8∼15 | |
| Septal hypertrophy | 8 | |
| Heart function | Left ventricular diastolic dusfuction | 42 |
| Hyperdynamic left ventricular contraction | 6∼12 | |
| Segmental/global hypokinetics | Not available | |
| Heart valve | Thickened valve leaflet, valve prolepses, valve stenosis or regurgitation | 7∼23 |
| Pericardium | Pericardial effusion | 8∼66.7 |
| Pulmonary artery | Pulmonary hypertension | 63∼75 |
Technetium99m‐pyrophosphate (99mTc‐PYP) scintigraphy permits detection of left ventricular global and regional wall abnormalities. Both decreased function and hyperkinetic left ventricular contraction systolic function can be detected. Taylor found 15% patients had wall motion abnormalities (mild global hypokinesis or regional hypokinesis) with normal ejection fractions.3 The conclusion of Buchpiguel et al's study is that 99mTc‐PYP heart scan seems to permit early detection of cardiac involvement in PM/DM. Patients with marked myocardial uptake had a poor outcome.
Besides 99mTc‐PYP scintigraphy, Gadolinium diethylenetriaminepantaacetic enhanced magnetic resonance imaging (Gd‐DTPA‐MRI) has been used to detect early myocarditis in 4 cases with PM/DM.16 Cardiac magnetic resonance imaging (MRI) showed an area of early and delayed enhancement consistent with the diagnosis of myocarditis. After 6 months of treatment, cardiac MRI showed sensitively that the kinetic abnormalities had normalized and that contrast enhancement with scarring tissue was clearly reduced. Results indicate that Gd‐DTPA‐MRI may be a prospective technique to detect myocardial inflammation.
As biochemical markers of myocardial injury, isoenzyme of creatinine kinase (CK‐MB) and cardiac troponin T(cTnT) had been investigated. Studies by Askari22 and Strongwater et al19 indicated that elevated CK‐MB was correlated to cardiac involvement. Cullen et al21 concluded that raised cTnT may identify a subgroup with subclinical myocardial involvement. However, the results have been questioned by some excluded research.
Ischemic Heart Disease
Myocardial ischemia is important in heart diseases, and the prevalence was 26% in study by Vidya et al.23 The incidence of angina symptoms and acute myocardial ischemia were 4% to 18% and 5.6%, respectively.3, 24, 25 There were 3.3% to 5.6% of patients who were diagnosed with old myocardial infarction by ECG.8, 17 Another 2 articles8, 25 reported myocardial infarction and remote infarction also detected in autopsied patients, and some of them were clearly related to severe coronary atherosclerosis.
Pathophysiology
The histopathology of the myocarditis resembles inflammation in the skeletal muscle including active myocarditis, focal fibrosis, vasculitis, intimal proliferation, and medial sclerosis of vessels.12 All patients in the study by Haupt and Hutchins12 and 66% of patients reported by Denbow et al8 with active myocarditis had heart failure. Similar pathophysiology in the myocardium were observed in the conducting system including lymphocytic infiltration, fibrosis of the sinoatrial node, and contraction‐band necrosis in autopsies of PM/DM patients. Some of these cases developed complete heart blockage. In addition, significant coronary artery disease (more than 75% luminal narrowing of at least 1 coronary artery) was present in some patients. Meanwhile, myocardial infarction and remote infarction were also reported in autopsied patients, and some of them were clearly related to severe coronary atherosclerosis. Vascular changes was a prominent pathology that included medial sclerosis and intimal proliferation.
Treatment
In some cases, heart failure, frequent atrial or ventricular premature beats, and pericardial effusions improved during corticosteroid treatment, but progressed in other individuals.11, 16, 25 Besides immunosuppressive therapy, traditional heart medications such as nitrates, β‐blockers, and calcium antagonists diuretics are also of benefit to patients. There are a few reports of patients with AV block who have been successfully treated with pacemakers.25
Prognosis
One hundred thirty‐two deaths occurred among the 1530 patients. Cause of death could be determined through physician and/or autopsy record in 80 of the fatal cases. Thirty‐seven patients (46.3%) died as a direct result of heart disease. Additional causes included respiratory failure in 22 and sepsis in 11. Among those deaths, arrhythmia, heart failure, cardiac arrest, and myocardial infarction were most commonly referred to. Moreover, the study by Danko et al6 showed that cardiovascular complications caused death mainly after a 5‐year disease duration.
In addition, 11 deaths were recorded in prospective studies that included 78 patients. The detailed information is shown in Table 3. As can be clearly seen from Table 3, nine fatal cases (81.8%) had cardiac complications.
Table 3.
Death cases in prospective studies
| Author | Year | No. of cases | No. of death | Cause of death/Autopsy |
|---|---|---|---|---|
| Corinne et al33 | 2010 | 8 | 3 | Aspiration pneumonia n = 2; respiratory failure/myocarditis n = 1 |
| Buchpiguel et al17 | 1996 | 22 | 2 | Autopsy: fibrosis in the myocardium n = 2 |
| Wilhelmina MHB et al13 | 1987 | 36 | 3 | Adams‐Stokes attacks n = 3/complete fibrosis of the atrioventricular node with focal fibrosis of the bundle of His and the bundle branches |
| Oka et al25 | 1978 | 12 | 3 | Congestive heart failure n = 3 |
Discussion
Cardiac involvement is a usual complication of PM/DM in present studies, which was demonstrated to be a poor prognostic factor.4, 6, 9 Because clinical manifestations of heart diseases are relatively infrequent, clinicians have not paid much attention to it.
This review suggests that heart failure is the most common clinical symptom. Any abnormalities of heart construction or function can lead to systolic or diastolic dysfunction. Because cardiac complications lack symptoms in early stages, this factor was ignored by patients and doctors. Acute or chronic heart failure is the manifestation of heart deterioration and damage that was reported by physicans.
ECG and UCG are the most often used auxiliary tests to evaluate cardiac abnormalities. In the polymorphism of the cardiac manifestations in PM/DM, conduction defects, left ventricular diastolic dysfunction, and hyperdynamic left ventricular contraction manifest relatively higher incidence. Lymphocytic infiltration, fibrosis of conduction system lesions, and active myocarditis are the pathologic foundation of the diseases listed above.8, 12 The presumption of enhanced left ventricular function by Gottdiener et al15 was indicated by a reduction of afterload by arteriovenous shunting across involved skeletal muscle beds and high output state secondary, due either to increased metabolic demand of diseased skeletal muscle or to the effects of vasoactive amines released by involved muscle. It is possible that myocarditis may predispose the patient to development of congestive heart failure over a period of time or under certain circumstances. Systolic left ventricular function decrease is the concurrent outcome of persistent hyperkinetic cardiovascular state and necrosis of myocardium.
Most heart abnormalities are subclinical, and traditional techniques such as ECG, Holter, and UCG are limited by their poor sensitivity and specificity. Cardiac MRI is a noninvasive technique that has been efficiently used in early diagnosis of viral myocarditis. Allanore et al16 proposed that cardiac MRI could be used to diagnosis and monitor myocarditis sensitively in PM/DM. Although its evaluation on large scale is warranted, cardiac MRI may be of considerable potential value in this field.
Pathologic findings of myocardium with myocardial inflammatory, degenerative changes and necrosis are similar to that of skeletal muscles, which indicate that the heart is a target organ of PM/DM. Recent research demonstrated that overexpression of major histocompatibility complex in muscle fibers is related to the mechanism of PM/DM.26, 27 Therefore, more detailed characterization of the cellular phenotype or localization of inflammation in myocardium should be helpful to understand the possible mechanism of cardiac involvement.
Many excluded studies demonstrated that CK‐MB and cTnT may be expressed in regenerating skeletal muscles of patients with muscle disease. A study of 3 patients by Kiely et al28 suggested a noncardiac origin for CK‐MB in DM. On the other hand, due to the multiple manifestations of heart damage, sometimes the level of CK‐MB is normal when cardiac involvement is present.29 CK‐MB was increased in 51% and cTnT in 41% of patients without clinical evidence for myocardial damage.30 Given the limited state of knowledge about cardiac troponin I(cTnI) in PM/DM at present, cTnI was only elevated in a few patients (2.5%). The correlation between cTnI and myocardial cell injury is unclear right now.
The therapy for heart complications is empirical. The effects of corticosteroids and immunosuppressants on cardiac manifestations in patients with PM/DM are conflicting. In addition, there are case reports about successful treatment with rituximab31and heart transplantation.32 Because of the pathophysiological analogy of myocardial tissue and skeletal muscle, corticosteroids should be effective for myocarditis as well as inflammation in the skeletal muscles. Therefore, carefully designed controlled studies are needed.
In the past decades, earlier diagnosis and adequate treatment regimes have become the standard for care, so survival of patients with PM/DM has improved progressively worldwide.6 Cancer, lung, and cardiovascular complications are generally cited as the most common causes of death in PM/DM patients. In the articles included, 37 patients (46.3%) died as a direct result of heart disease. Cancer‐associated myositis were excluded in this review. The top 2 causes of death are heart disease and respiratory failure. Because cardiac complications were paid close attention to in this review, there may be a selection of literature bias. Therefore, the ratio of heart disease as a direct cause was as high as 46.3%, but the data also illustrated that the presence of cardiac involvement was associated with a significantly worse survival.
As stated in many articles, most cardiac involvement in PM/DM was subclinical. Also, noninvasive technologies for detection lack sensitivity. The most frequently reported clinical manifest is heart failure with poor prognosis. Therefore, we prefer ECG and UCG as routine examinations to detect subclinical heart disease. See Figure 2 for the flowchart illustrating the evaluation of heart in PM/DM patient. It is beneficial to prevent heart disease from progressing.
Figure 2.
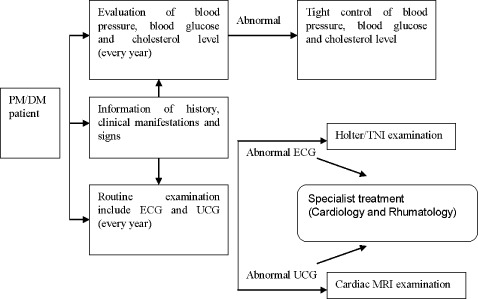
Flowchart to evaluate involvement of heart in PM/DM.
Some limitations of this systematic review should be acknowledged. First, due to the differences of patient selection, heart disease definition and detection methods used analyzed sources with relatively heterogeneous and cannot be systematically quantitatively evaluated. Second, case reports were excluded from this analysis.
Conclusion
Heart abnormalities are common and so to is polymorphism in PM/DM, most of which are subclinical. Cardiac involvement is a common cause of death. Through qualitative evaluation, we narratively reviewed the incidences and clinical characteristics of cardiac involvement in adult PM/DM, which was not been investigated previously. Although auxiliary examinations can evaluate heart complications, there is no method that is excellent both in sensitivity and specificity. It is worthwhile to pay more attention to the heart in PM/DM.
Acknowledgements
The authors thank those who kindly provided the data necessary for our systematic evaluations.
References
- 1. Oppenheim HZ. Dermatomyostitis. Berl klin Wochenschrift. 1899: 805–807. [Google Scholar]
- 2. Gonzalez‐Lopez L, Gamez‐Nava JI, Sanchez L, et al. Cardiac manifestations in dermato‐polymyositis. Clin Exp Rheumatol. 1996;14:373–379. [PubMed] [Google Scholar]
- 3. Taylor AJ, Wortham DC, Burge JR, et al. The heart in polymyositis: a prospective evaluation of 26 patients. Clin Cardiol. 1993;16: 802–808. [DOI] [PubMed] [Google Scholar]
- 4. Bohan A, Peter JB, Bowman RL, et al. Computer‐assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore). 1977;56:255–286. [DOI] [PubMed] [Google Scholar]
- 5. Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology (Oxford). 2006;45(suppl 4):iv18–iv21. [DOI] [PubMed] [Google Scholar]
- 6. Danko K, Ponyi A, Constantin T. Long‐term survival of patients with idiopathic inflammatory myopathies according to clinical features. Medicine (Baltimore). 2004;83:35–42. [DOI] [PubMed] [Google Scholar]
- 7. Hochberg MC, Feldman D, Stevens MB. Adult onset polymyositis/dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Semin Arthritis Rheum. 1986;15:168–178. [DOI] [PubMed] [Google Scholar]
- 8. Denbow CE, Lie JT, Tancredi RG, et al. Cardiac involvement in polymyositis: a clinicopathologic study of 20 autopsied patients. Arthritis Rheum. 1979;22:1088–1092. [DOI] [PubMed] [Google Scholar]
- 9. Benenbassat J, Gefel D, Larholt K. Progognostic factors in polymyositis/dermatomyosits. Arthritis Rheum. 1985;28:249–255. [DOI] [PubMed] [Google Scholar]
- 10. Kehoe RF, Robert B, Carl T. Cardiac conduction defects in polymyositis. Ann Intern Med. 1981;94:41–43. [DOI] [PubMed] [Google Scholar]
- 11. Stern R, Godbold JH, Chess Q, et al. ECG abnormalities in polymyositis. Arch Intern Med. 1984;144:2185–2189. [PubMed] [Google Scholar]
- 12. Haupt HM, Hutchins GM. The heart and cardiac conduction system in polymyositis‐dermatomyositis: a clinicopathologic study of 16 autopsied patients. Am J Cardiol. 1982;50:998–1006. [DOI] [PubMed] [Google Scholar]
- 13. Wilhelmina MHB, Behan Po, Gairns IJ. Cardiac damage in polymyositis associated with antibodies to tissue ribonucleoproteins. Br Heart J. 1987;57:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byrnes TJ, Baethge BA, Wolf RE. Noninvasive cardiovascular studies in patients with inflammatory myopathy. Angiology. 1991; 42:843–848. [DOI] [PubMed] [Google Scholar]
- 15. Gottdiener JS, Sherber HS, Hawley RJ, et al. Cardiac manifestations in polymyositis. Am J Cardiol. 1978,41:1141–1149. [DOI] [PubMed] [Google Scholar]
- 16. Allanore Y, Vignaux O, Arnaud L, et al. Effects of corticosteroids and immunosuppressors on idiopathic inflammatory myopathy related myocarditis evaluated by magnetic resonance imaging. Ann Rheum Dis. 2006;65:249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchpiguel CA, Roizemblatt S, Pastor EH, et al. Cardiac and skeletal muscle scintigraphy in dermato‐ and polymyositis: clinical implications. Eur J Nucl Med. 1996;23:199–203. [DOI] [PubMed] [Google Scholar]
- 18. Agrawal CS, Behari M, Shrivastava S, et al. The heart in polymyositis‐dermatomyositis. J Neurol. 1989;236:249–250. [DOI] [PubMed] [Google Scholar]
- 19. Strongwater SL, Annesley T, Schnitzer TJ. Myocardial involvement in polymyositis. J Rheumatol. 1983;10:459–463. [PubMed] [Google Scholar]
- 20. Plazak W, Kopeec G, Tomkiewica‐Pajak L. Heart structure and function in patients with generalized autoimmune diseases: echocardiography with tissue Doppler study. Acta Cardiol. 2011; 66:159–165. [DOI] [PubMed] [Google Scholar]
- 21. Hebert CA, Byrnes TJ, Baethge BA, et al. Exercise limitation in patients with polymyositis. Chest. 1990;98:352–357. [DOI] [PubMed] [Google Scholar]
- 22. Askari AD. Inflammatory disorders of muscle. Cardiac abnormalities. Clin Rheum Dis. 1984;10:131–149. [PubMed] [Google Scholar]
- 23. Vidya SL, Sue L, Peter B, et al. Idiopathic inflammatory myositis is associated with a high incidence of hypertension and diabetes mellitus. Int J Rheum Dis. 2010;13:132–137. [DOI] [PubMed] [Google Scholar]
- 24. Tisseverastinghe A, Bernatsky S, Christian AP. Arterial events in persons with dermatomyositis and polymyositis. J Rheumatol. 2009;36:1943–1946. [DOI] [PubMed] [Google Scholar]
- 25. Oka M, Raasakka T. Cardiac involvement in polymyositis. Scand J Rheumatol. 1978;7:203–208. [DOI] [PubMed] [Google Scholar]
- 26. Vander PJ, Hengstman GJ, LaaK T, et al. Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2004;75:136–139. [PMC free article] [PubMed] [Google Scholar]
- 27. Englund P, Nennesmo I, Klareskog L, et al. Interleukin‐1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum. 2002;46:1044–1055. [DOI] [PubMed] [Google Scholar]
- 28. Kiely PD, Bruckner FE, Nisbet JA, et al. Serum skeletal troponin I in inflammatory muscle disease: relation to creatione kinase, CKMB and cardiac troponin. Ann Rheum Dis. 2000;59: 750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rasmussen LH, Madsen HN, Ladefoged SD. Creatine phosphokinase MB and lactate dehydrogenase isoenzyme 1 in polymyositis. Scand J Rheumatol. 1985;14:427–430. [DOI] [PubMed] [Google Scholar]
- 30. Petra E, Angelika L, Jurgen F, et al. Cardiac troponin and b‐type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clinica Chimica Acta. 2001;306:27–33. [DOI] [PubMed] [Google Scholar]
- 31. Touma Z, Arayssi T, Kibbi L, et al. Successful treatment of cardiac involvement in dermatomyositis with rituximab. Joint Bone Spine. 2008;75:334–337. [DOI] [PubMed] [Google Scholar]
- 32. Afzal A, RSD H, Philbin EF. Heart transplant for dilated cardiomyopathy associated with polymyositis. Heart. 1999; 82:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corinne F, Sumeet A, Way MW, et al. Clinical observations on the significance of raised cardiac troponin‐T in patients with myositis of varying etiologies seen in rheumatology practice. Clin Rheumatol. 2010;29:1107–1111. [DOI] [PubMed] [Google Scholar]
- 34. Na S, Kim SM, Sunwoo N. Clinical characteristics and outcomes of juvenile and adult dermatomyositis. J Korean Med Sci. 2009;24: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharratt GP, Danta G, Carson PH. Cardiac abnormality in polymyositis. Ann Rheum Dis. 1977;36:575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]


