Abstract
As major prescribers of oral anticoagulants, cardiologists must be familiar with strategies to manage bleeding, the principal complication associated with all anticoagulants, and to reverse anticoagulant effects in acute‐care settings. The purpose of this manuscript is to review currently available information regarding dabigatran and rivaroxaban, the 2 novel oral anticoagulants approved to date in the United States. Further, we suggest reasonable interventions for the clinician faced with a patient who suffers a major bleeding event while receiving one of these agents. Data sources were peer‐reviewed publications, US Food and Drug Administration documents in the public domain, and approved US prescribing information for dabigatran (Pradaxa) and rivaroxaban (Xarelto). Strategies for management of bleeding and reversal of anticoagulant effects from warfarin include vitamin K, fresh frozen plasma, and prothrombin complex concentrates. For rivaroxaban and dabigatran, appropriate therapies include support and observation, which are likely to be effective for the majority of patients because of the short half‐lives of these agents. In severe life‐threatening hemorrhage, clotting‐factor substitutes may be appropriate in certain situations. Validated protocols specific to each agent remain to be developed. Clin. Cardiol. 2012 doi: 10.1002/clc.22037
Editorial support for this paper was provided by Janssen Scientific Affairs, LLC. W.F.P. has received research grants (>$10 000) from Abbott, Alere, Baxter, Brahms, Novartis, and The Medicines Company. He has been a consultant (<$10 000) for Abbott, Alere, Eli Lilly, and The Medicines Company; served on the speaker's bureau (<$10 000) for Abbott and Alere; and has ownership interest (<$10 000) in Comprehensive Research Associates LLC, Vital Sensors, and Emergencies in Medicine LLC. M.M.G and R.M.M. are full‐time employees of Janssen Scientific Affairs, LLC. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Anticoagulation is a common intervention in the prevention and treatment of thrombosis in multiple clinical settings.1., 2. In the United States, the oral anticoagulant most commonly prescribed in the outpatient setting is the vitamin K antagonist (VKA) warfarin.3 In 2004, 25 00 000 US patients received warfarin,4., 5. and nearly 31 million warfarin prescriptions were dispensed.6 Although efficacious, VKAs are limited by a narrow therapeutic window, slow onset/offset, variable response, a requirement for coagulation monitoring, and food and drug interactions that make their use challenging.7., 8., 9. Recently, novel oral anticoagulants that overcome many of these limitations were introduced. These include the direct thrombin inhibitor dabigatran and the direct Factor Xa inhibitor rivaroxaban. Based on their clinical advantages, these agents are likely to achieve wide acceptance.
With all anticoagulants, bleeding is the principal complication. Although relatively uncommon, severe or even life‐threatening bleeding is a major concern in emergency care. Among patients who suffer anticoagulation‐related major bleeding, the mortality rate is as high as 13.4%,10 rising to 66% in patients with spontaneous intracerebral hemorrhage and an international normalized ratio (INR) >3.0.11 Thus, when bleeding occurs in a patient receiving anticoagulation, rapid reversal may be needed either to control acute bleeding or because invasive surgical procedures are immediately required.
Although strategies to reverse warfarin are generally accepted, the literature provides little guidance as to the appropriate reversal of the newest anticoagulants, which have no specific reversal agents. The purpose of this manuscript is to review currently available information regarding dabigatran and rivaroxaban, the 2 novel oral anticoagulants approved to date in the United States, and to suggest reasonable interventions for the clinician faced with a patient who suffers a major bleeding event while receiving one of these agents.
Mechanisms of Anticoagulant Action
Vitamin K Antagonists
Warfarin inhibits vitamin K‐dependent γ‐carboxylation of coagulation factors II, VII, IX, and X and the anticoagulation factor proteins C and S12 (Figure 1). By blocking the synthesis of functional vitamin K‐dependent coagulation factors, chronic administration of warfarin leads to a deficiency of these functional proteins.13 In the setting of VKA‐related hemorrhage, the principal objective is to increase the concentration of vitamin K‐dependent clotting factors.
Figure 1.
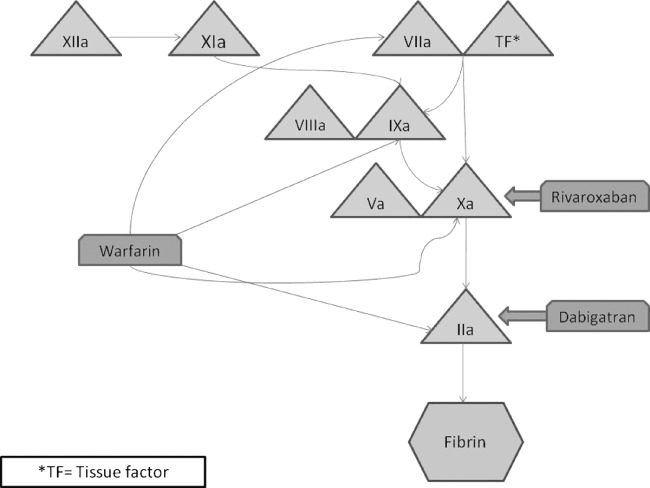
The coagulation cascade. Abbreviations: TF, tissue factor.
Rivaroxaban
Rivaroxaban is the first oral, direct factor Xa inhibitor approved in the United States for the prophylaxis of deep‐vein thrombosis in patients undergoing total knee or hip replacement surgery and to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF).14 Rivaroxaban inhibits both free factor Xa activity and factor Xa that has been incorporated into the prothrombinase complex.15 Because factor Xa represents the junction of the intrinsic and extrinsic pathways of the blood‐coagulation cascade, its inhibition prevents the generation of the final common coagulation enzyme catalyst, thrombin (Figure 1). Rivaroxaban exhibits no inhibitory effects on thrombin (activated factor II) and has no direct effects on platelet function.14
The maximum concentrations (Cmax) of rivaroxaban appear 2 to 4 hours after tablet intake. The half‐life of rivaroxaban is 5 to 9 hours in healthy subjects, with little accumulation after repeated daily doses.14 Approximately one‐third of a rivaroxaban dose is excreted unchanged in urine, and about two‐thirds undergoes hepatic metabolism to inactive metabolites.16 Of these metabolites, half are cleared by the hepatobiliary route and half are renally excreted. Pharmacodynamic factor Xa inhibition persists through 24 hours, allowing for once‐daily dosing.
In drug‐interaction studies evaluating the concomitant use with drugs that are combined P‐glycoprotein and cytochrome P450 3A4 isoenzyme inhibitors, increases in rivaroxaban exposure and pharmacodynamic effects (ie, factor Xa inhibition and prothrombin time [PT] prolongation) were observed. Significant increases in rivaroxaban exposure may increase bleeding risk.14
Phase III trials have demonstrated that rivaroxaban provides predictable anticoagulation with efficacy superior to the low‐molecular‐weight heparin enoxaparin in the orthopaedic setting17., 18., 19., 20. and noninferior to warfarin for stroke prophylaxis in AF,21 but without the need for dose adjustments, routine coagulation monitoring, or dietary restrictions. Major and clinically relevant nonmajor bleeding occurred in 14.9% patients in the rivaroxaban group and in 14.5% patients in the warfarin group (P = 0.44). Rates of major bleeding were similar in the rivaroxaban and warfarin groups (3.6% and 3.4%, respectively; P = 0.58).21
Dabigatran
Dabigatran etexilate is an oral direct thrombin inhibitor that has been approved (150 mg twice daily) for the reduction of stroke risk in patients with nonvalvular AF. Dabigatran inhibits free and clot‐bound thrombin and thrombin‐induced platelet aggregation. Because thrombin enables the conversion of fibrinogen into fibrin, its inhibition prevents thrombus development (Figure 1).
Dabigatran etexilate mesylate is absorbed and hydrolyzed to form the active moiety.22 The dabigatran moiety is metabolized into 4 different acyl glucuronides with similar pharmacologic activity to the original. The pharmacokinetics described in the US labeling refers to the sum of dabigatran and its glucuronides.
The absolute bioavailability of dabigatran etexilate following oral administration is 3% to 7%, and time to Cmax in the fasted state is 1 hour postadministration in healthy volunteers. This is delayed by ∼2 hours when the drug is coadministered with a high‐fat meal. Most of the bioavailable dabigatran etexilate is cleared renally. Given twice daily, dabigatran's accumulation factor is ∼2. Dabigatran is given in fixed doses and has no requirement for routine coagulation monitoring or dietary restrictions. In healthy subjects, the half‐life of dabigatran is 12 to 17 hours.22
Clinical trials of dabigatran etexilate have demonstrated predictable anticoagulation and comparable or better efficacy to warfarin for stroke prevention in AF, depending on the dose and clinical setting. In the Randomized Evaluation of Long‐term Anticoagulant Therapy (RE‐LY) study, a life‐threatening bleed occurred at an annualized rate of 1.5% and 1.8% for dabigatran 150 mg and warfarin, respectively.22
Strategies for Anticoagulation Reversal
Because the use of any anticoagulant may be complicated by the potential for bleeding, clinicians must understand the mechanism of action, the clearance mechanisms, and the half‐life of each of these agents. When a patient presents with anticoagulant‐associated bleeding, a drug‐specific emergency‐department strategy that includes indications and agents for rapid reversal is necessary. The choice of anticoagulation‐reversal method depends upon the pharmacology of the agent, clinical urgency, and the severity of the bleeding.12
The options for reversing anticoagulation include: (1) withholding anticoagulation therapy (observation); (2) administering a specific reversal agent, such as oral or intravenous (IV) vitamin K if the bleed‐related anticoagulant agent is a VKA; and (3) administering supplemental clotting‐factor substitutes, such as fresh frozen plasma (FFP) or prothrombin complex concentrates (PCCs).12
In addition to these options, the clinician should institute appropriate supportive and symptomatic treatment, such as mechanical compression or surgical intervention. Patients should be evaluated to determine their needs for fluid replacement or hemodynamic support. In cases of suspected overdose, if the patient is alert, oriented, and able to communicate, and the anticoagulant was ingested within the previous 2 hours, liquid activated charcoal with sorbitol can be considered.14., 23.
Observation
Because of the short half‐lives of the newer agents, observation and supportive care is the preferred strategy in patients with minor or mechanically controlled bleeding. In patients with adequate hepatic and renal function, any of the novel oral agents will be largely cleared within a few hours after the most recent dose. Dabigatran and rivaroxaban do not require routine monitoring; however, in the event of a bleed or the need to take a patient emergently to surgery, there are pharmacodynamic parameters that can be measured to determine the approximate level of anticoagulation (Table 1).
Table 1.
Summary of Anticoagulation Reversal Agents
| Reversal Agents | Monitoring | Comments | |
|---|---|---|---|
| Warfarin | Vitamin K, FFP, rFVIIa, PCC, aPCC | PT/INR | Vitamin K has a delayed reversal effect. To significantly increase coagulation proteins, 2 to 10 L of FFP may be required. PCCs and rFVIIa are powerful procoagulant agents that can significantly increase the risk of thrombotic events. |
| Rivaroxaban | rFVIIa, PCC, aPCC | PT, TT, anti‐factor Xa | |
| Dabigatran | rFVIIa | ECT, aPTT, TT |
Abbreviations: aPCC, activated prothrombin complex concentrate; aPTT, activated partial thromboplastin time; ECT, ecarin clotting time; FFP, fresh frozen plasma; INR, international normalized ratio; PCC, prothrombin complex concentrate; PT, prothrombin time; rFVIIa, recombinant factor VIIIa; TT, thrombin time.
Vitamin K Antagonist Reversal
Although in minor bleeding discontinuation of VKA therapy may be adequate for anticoagulation reversal, vitamin K administration may speed reversal.12 With IV vitamin K, INR normalization has occurred at 6 hours in 45% of patients.24 It is critical to emphasize that vitamin K will have no effect on reversing non‐VKA antagonists.
With an isolated, significantly elevated INR, in the absence of bleeding or when bleeding is minor, vitamin K may be the only therapy indicated. However, because of its slow onset of action, vitamin K alone is inadequate if emergency correction of warfarin‐related bleeding is indicated.12
Factor Replacement
Table 2 shows the mechanisms of action of the reversal agents discussed below.11., 25., 26.
Table 2.
Mechanisms of Action of Anticoagulation Reversal Agents
| Reversal Agent | Mechanism of Action | Role in Reversal | |
|---|---|---|---|
| Rivaroxaban | Dabigatran | ||
| PCC | Replaces deficient clotting factors. Factor IX is activated by factor XIa in the intrinsic coagulation pathway. Activated factor IX (IXa) in combination with factor VII:C activates factor X to Xa, resulting in the conversion of prothrombin to thrombin and the formation of a fibrin clot. The infusion of exogenous factor IX to replace the deficiency restores hemostasis.25 | Data in healthy volunteers suggest a dose of 50 U/kg | Data in healthy volunteers do not demonstrate efficacy based on coagulation parameters |
| aPCC (FEIBAa) | aPCCs contain prothrombin, FVII, FIX, FX, and protein C; the zymogen forms of important hemostatic enzymes and very small amounts of their activation products, except for FVIIa, which is contained in greater amounts. The mechanism of action comprises multiple biochemical interactions, which induce or facilitate thrombin generation even in the absence of FVIII.11 | Animal data available for the use of FEIBA for the reversal of rivaroxaban | No data exist |
| rVIIa | aFVII binds to the TF that is found on the surface of subendothelial cells. TF‐FVIIa complex then activates FIX and FX, which leads to the formation of a small initial amount of thrombin. Thrombin activates blood platelets and other coagulation factors (FV and FVIII), resulting in the “thrombin burst.” This large amount of thrombin then enables the conversion of fibrinogen to fibrin and the formation of a fibrin clot.26 | Not recommended | No data in human patients or volunteers, but reversed bleeding in rat tail model |
| FFP | FFP is the plasma separated from a unit of whole blood. Each bag has a volume of 175 to 250 mL. FFP is used to replace coagulation factors. FFP contains an average of 1 IU/mL of all the coagulation proteins, including the labile factors V and VIII, and 400 to 800 mg of fibrinogen. It also contains fibrinolytic and complement factors.11 | Not recommended | Not recommended |
Abbreviations: aFVII, activated factor VII; aPCC, activated prothrombin complex concentrate; FEIBA, Factor VIII Inhibitor Bypassing Activity; FFP, fresh frozen plasma; FIX, factor IX; FVII, factor VII; FVIIa, factor VIIa; FVIII, factor VIII; FX, factor X; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa. a Baxter Healthcare Corp., Westlake Village, CA.
Fresh Frozen Plasma:
Fresh frozen plasma is a blood product manufactured by isolating the liquid portion of whole blood. It contains all soluble coagulation factors, including the vitamin K‐dependent factors: II, VII, IX, and X.27 Because it is widely available and relatively inexpensive, FFP is the standard of care in the United States for emergency VKA anticoagulation reversal, despite the fact that only a small number of randomized controlled trials have studied its use for this purpose.28 Fresh frozen plasma is not without limitations; as a blood product, it presents a risk of transfusion‐transmitted infections, allergic reactions, and transfusion‐related lung injury. Moreover, FFP can require substantial time to obtain from the local blood bank and to warm, and full anticoagulation reversal may not be realized until several hours after administration.29., 30. In addition, the total dose of FFP needed to reverse VKA anticoagulation is quite large. Up to 2 L or 10 U are frequently required to increase the level of coagulation proteins in the circulatory system by 0.4 to 0.5 U/mL. As a result, underdosing is common, and the potential for volume overload must be considered and monitored.31
Recombinant Factor VIIa:
Recombinant factor VIIa (rFVIIa) is a non–plasma‐derived activated factor VII approved by the US Food and Drug Administration (FDA). It was originally developed for the treatment of spontaneous or surgical bleeding in hemophiliacs with inhibitors to factors VIII and IX. Although it may be administered for anticoagulation reversal, it does not contain Factors II, IX, or X, and VKA reversal will not occur without concurrent administration of FFP. There are no clinical data to support the use of rFVIIa to reverse bleeding associated with the novel oral agents.
In the last decade, the off‐label use of rFVIIa has increased more than 140‐fold, raising concerns about its use in unsupported indications.32 Administration of rFVIIa has not reduced mortality in intracerebral hemorrhage; however, it does increase the risk for thromboembolism.33 In a randomized, placebo‐controlled, double‐blind trial of 114 healthy subjects receiving warfarin, rFVIIa reduced INR values, but not to levels seen at baseline, and it did not restore hemostasis.34
Prothrombin Complex Concentrates:
Prothrombin complex concentrates (PCCs) is a generic term describing a number of virus‐reduced, concentrated, pooled plasma products typically containing a combination of either 3 or 4 vitamin K‐dependent clotting factors.13., 31. Prothrombin complex concentrates may be used for the rapid replacement of vitamin K‐dependent clotting factors when excessive anticoagulation is related to a VKA. Second‐generation PCCs contain both procoagulation and anticoagulation factors. These “balanced PCCs” are thought to maintain hemostatic balance and minimize the risk of thrombosis.35
The currently accepted model of the clotting process emphasizes the interaction between coagulation factors and platelets.36 It includes 4 consecutive overlapping stages: initiation, amplification, propagation, and stabilization. Prothrombin complex concentrates act at both the initiation and amplification stages of the cascade. Most data on the safety and efficacy of PCCs in warfarin reversal have included non–FDA‐approved products. Prothrombin complex concentrates currently available in the United States include Bebulin (factor IX complex, vapor heated; Baxter Healthcare Corp., Westlake Village, CA) and Profilnine SD (factor IX complex, solvent detergent treated; BDI Pharma, Columbia, SC), both of which are 3‐factor PCCs. Neither one has received FDA approval for reversing anticoagulants.37 Table 3 lists the available PCCs and the factor levels in each.29., 38.
Table 3.
| FII | FVII | FIX | FX | Availability in United States | |
|---|---|---|---|---|---|
| 3‐factor PCCs | |||||
| Prothombinex HT | 100 U | ‐ | 100 U | 100 U | No (only in Austria) |
| Bebulin (Baxter) | 120 U | 0.05–0.20 U1 | 100 U | 139 U | Yes |
| Profilnine SD (Grifols) | 148 U | <35 U | 100 U | 64 U | Yes |
| 4‐factor PCCs | |||||
| Cofact (Sanquin) | 50–75 U | ∼ 20 U | 100 U | ∼ 75 U | No (only in Europe) |
| Beriplex (CSL Behring) | 128 U | 68 U | 100 U | 152 U | No (only in Europe and Canada) |
| Prothromplex T (Baxter) | 100 U | 85 U | 100 U | 100 U | No (only in Austria) |
| Octaplex (Octapharma) | 38–152 U | 36–96 U | 100 U | 72–120 U | No (only in Europe and Canada) |
| PPSB‐HT | 100 U | 100 U | 100 U | 100 U | No (only in Japan) |
Abbreviations: FII, factor II; FIX, factor IX; FVII, factor VII; FX, factor X; PCC, prothrombin complex concentrate.
Factor units per 1 factor IX unit.
Activated PCCs (aPCCs) are nanofiltered, vapor‐heated, freeze‐dried sterile human plasma fractions. The aPCCs have a role as hemostatic agents in VKA reversal in patients with clinically significant bleeding events.23 The FDA‐approved aPCCs available in the United States include the anti‐inhibitor coagulant complex FEIBA NF (Factor VIII Inhibitor Bypassing Activity, nonfiltered; Baxter Healthcare). In vitro, FEIBA NF shortens the activated partial thromboplastin time (aPPT) of plasma that contains a factor VIII inhibitor. Factor VIII inhibitor‐bypassing activity is expressed in arbitrary units. One unit of activity is defined as the amount of FEIBA that shortens the aPTT of a high‐titer factor VIII inhibitor reference plasma to 50% of the blank value. FEIBA contains factors II, IX, and X (mainly nonactivated) and factor VII (mainly in the activated form). The product contains approximately equal units of factor VIII inhibitor‐bypassing activity and prothrombin complex factors.39
Reversal of the Newly Approved Anticoagulants
Recommendations for the treatment of bleeding due to rivaroxaban or dabigatran are shown in Figure 2.1., 23., 40.
Figure 2.
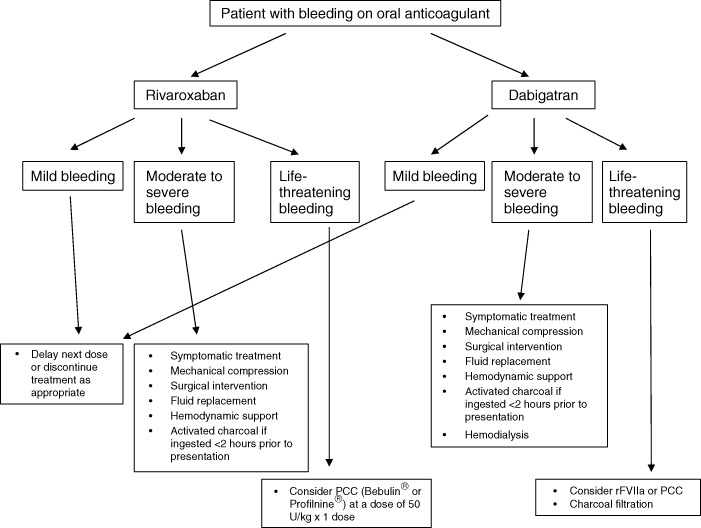
Recommendation for the treatment of bleeding due to rivaroxaban or dabigatran. Abbreviations: PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa.
Rivaroxaban
Time/Observation:
The anticoagulant effect of rivaroxaban can be assessed by following the PT, preferably as determined with the Neoplastin Plus (Roche Diagnostics) reagent. It is critical to note that the actual PT, not the INR, must be used for this assessment. In most patients who discontinue rivaroxaban after steady‐state dosing, the PT will fall rapidly to near‐normal times within 24 hours. However, a clinically relevant reduction of PT occurs within 4 to 6 hours after peak elevation.41 Figure 3 shows serial Neoplastin PT determinations on the first and third days of dosing in a series of patients who had taken rivaroxaban 10 mg daily.41 In non–life‐threatening presentations, supportive care combined with simply withholding rivaroxaban may be adequate therapy. However, when a life‐threatening hemorrhage occurs, intervention with reversal agents may be necessary.
Figure 3.
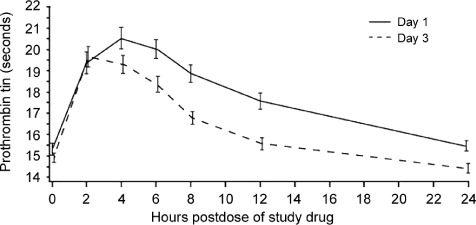
Serial neoplastin prothrombin determinations on the first and third day of dosing in patients receiving rivaroxaban 10 mg daily. From Mills et al.41
Fresh Frozen Plasma:
Not recommended.
Recombinant Factor VIIa:
Administration of rFVIIa has been evaluated for reversal of rivaroxaban in 2 animal studies.42., 43. Gruber et al dosed primates with high‐dose rivaroxaban, prolonging the bleeding time to 2.54‐fold ± 0.79× baseline and PT to 3.2× baseline.42 An infusion of 210 µg/kg rFVIIa only partially reduced bleeding time (34%) at 30 minutes postdose. These data have been published in abstract form only, not full manuscripts.
Prothrombin Complex Concentrates:
Four separate studies have evaluated the use of PCC or aPCC for reversal of rivaroxaban. Two of these were in animal models (primates and rats),42., 43. and 2 were performed in healthy human subjects.40., 44. FEIBA was the aPCC used in both animal models. In these trials the aPCCs showed promise; bleeding time and PT were significantly reduced within 5 minutes of FEIBA infusion.
Eerenberg et al40 conducted a randomized, double‐blind, placebo‐controlled study in which 12 healthy male subjects received rivaroxaban 20 mg twice daily for 2.5 days. One group (n = 6) was then randomized to receive a single bolus of 50 IU/kg PCC (Cofact human prothrombin complex; Sanquin, the Netherlands) and the other group (n = 6) was given a similar volume of saline. Prothrombin time was significantly prolonged by rivaroxaban (15.8 seconds ± 1.3 vs 12.3 ± 0.7 at baseline; P < 0.001). Immediately after the infusion of PCC, PT normalized almost completely (12.8 ± 1.0; P < 0.001), and the reduction was sustained for 24 hours. This study provided the first evidence that PCC reverses the anticoagulant effect of rivaroxaban in humans and may serve as a reversal agent.
The same investigators conducted a second randomized study evaluating the reversal of rivaroxaban and dabigatran etexilate by PCC in healthy subjects.40 Healthy male volunteers received rivaroxaban 20 mg (n = 6) or dabigatran etexilate 150 mg (n = 6) twice daily for 2.5 days, followed by 50 IU/kg PCC (Cofact) or saline. After a washout period, a crossover period followed with the alternate anticoagulant. Rivaroxaban significantly prolonged PT (15.8 ± 1.3 sec vs 12.3 ± 0.7 sec at baseline), and PCC caused an immediate and complete reversal (12.8 ± 1.0; P < 0.001) (figure 4).40 The endogenous thrombin potential was inhibited by rivaroxaban (51% ± 22%, baseline 92% ± 22%; P = 0.002) and was normalized with PCC (114 ± 26, P < 0.001).
Figure 4.
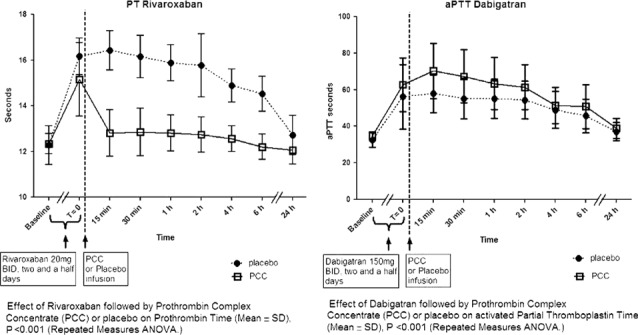
Time courses of mean PT after rivaroxaban or aPPT after dabigatran etexilate, bid dosing (±SD) aPTT. Abbreviations: ANOVA, analysis of variance; aPTT, activated partial thromboplastin time; bid, twice a day; PCC, prothrombin complex concentrate; PT, prothrombin time; SD, standard deviation. Reproduced with permission from Eerenberg et al.40
Dabigatran
Time/Observation:
Although activated clotting time could be used in some clinical situations to measure the anticoagulant effect of dabigatran etexilate, no systematic investigation of its use has been performed in patients. However, the anticoagulant effect of dabigatran may be monitored by measurement of aPPT. When dabigatran etexilate is abruptly withdrawn from patients on steady‐state dosing, aPTT returns toward baseline within 24 hours,40 with measurable improvement within 4 to 6 hours.40 As with rivaroxaban, in non–life‐threatening presentations, supportive care and withholding dabigatran etexilate may be sufficient therapy. However, in the presence of a life‐threatening hemorrhage, intervention with reversal agents may be necessary.
Hemodialysis:
Dabigatran can be dialyzed, with the removal of about 60% of the drug over 2 to 3 hours, but data supporting this approach are limited.22
Fresh Frozen Plasma:
This is not recommended, because there is no clinical evidence that FFP will reverse the anticoagulant effect of dabigatran.23
Recombinant Factor VIIa:
There is some experimental evidence that rFVIIa may antagonize the anticoagulant effect of dabigatran. In a rat tail model of template bleeding, addition of rFVIIa (0.1 or 0.5 mg/kg) significantly reduced bleeding time and prolongation of aPTT associated with high‐dose dabigatran (1 µmol/kg bolus + 0.5 µmol/kg/h infusion for 25 min) in a dose‐dependent manner. Recombinant factor VIIa at 0.5 mg/kg reduced bleeding time from 11.6‐fold (ratio to control) to 1.1‐fold and aPTT prolongation by dabigatran from 8.2‐fold to 3.8‐fold.23 However, the use of rFVIIa in clinical settings has not been firmly established.1., 22.
Prothrombin Complex Concentrates or Activated Prothrombin Complex Concentrates:
Recommendations for the use of PCC in patients receiving dabigatran therapy are largely based on limited nonclinical data. There is little experience in patients or volunteers.
In a rat tail model of template bleeding, the addition of aPCC at a dose of 50 or 100 U/kg significantly reduced prolongation of bleeding time associated with high‐dose dabigatran. At 100 U/kg, aPCC reduced bleeding time prolongation by dabigatran from 11.6‐fold (ratio to control) to 1.4‐fold.23
In the study by Eerenberg et al discussed above, dabigatran increased aPTT, ecarin clotting time, and thrombin time. Administration of PCC did not restore coagulation to the same extent in the subjects who received dabigatran as it did in those who received rivaroxaban.40
Conclusion
Novel oral anticoagulants that specifically target a single step in the coagulation cascade offer substantial clinical advantages over warfarin, including predictable pharmacokinetic and pharmacodynamic effects, fixed dosing without the need for laboratory monitoring of coagulation status, and minimal drug and dietary interactions. Two of the novel agents, rivaroxaban and dabigatran, have now been approved for use in the United States, including use for stroke prophylaxis in AF.
Bleeding is the most common adverse event associated with therapeutic anticoagulation. Cardiologists and emergency and critical‐care physicians can expect to encounter patients who are receiving these agents and who have serious bleeding. Institutional emergency protocols for the management of anticoagulant‐associated bleeding specific to each new agent must be developed. These should include relevant laboratory studies, indications for reversal, and specific appropriate agents for rapid reversal.
Because of the short duration of action of the new agents, supportive care should suffice for most patients. In the absence of clinical evidence, we believe the available data offer theoretical support for the use of PCCs if a reversal agent is required for either rivaroxaban or dabigatran. Most of the data included in this review are published in abstract form as opposed to full manuscript publications. There is a need for more data on reversal of the novel anticoagulants. Most recently, the Beriplex (4‐factor PCC) biologics license application was accepted for US review by the FDA. If approved, the United States would have available its first 4‐factor PCC.
Current guidelines addressing anticoagulation make recommendations on what patients are most appropriate for therapeutic anticoagulation.45., 46. Unfortunately, these guidelines do not address how to reverse the anticoagulant effect of these novel agents when a patient bleeds. When making the decision to use one of the agents discussed in this review, the clinician must take into consideration the procoagulant effect that can be seen with these reversal agents.25., 26., 29. Therefore, the risk/benefit ratio should be carefully weighed by the clinician.
References
- 1. Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111:4871–4879. [DOI] [PubMed] [Google Scholar]
- 2. Vigué B. Bench‐to‐bedside review: Optimising emergency reversal of vitamin K antagonists in severe haemorrhage—from theory to practice. Crit Care. 2009;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman RJ, Gallus AS, Cushner FD, et al. Physician compliance with guidelines for deep‐vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin. 2008;24:87–97. [DOI] [PubMed] [Google Scholar]
- 4. Pengo V, Pegoraro C, Cucchini U, et al. Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77. [DOI] [PubMed] [Google Scholar]
- 5. Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. [DOI] [PubMed] [Google Scholar]
- 6. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–1419. [DOI] [PubMed] [Google Scholar]
- 7. Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31:326–343. [DOI] [PubMed] [Google Scholar]
- 8. Zikria J, Ansell J. Oral anticoagulation with Factor Xa and thrombin inhibitors: is there an alternative to warfarin? Discov Med. 2009;8:196–203. [PubMed] [Google Scholar]
- 9. Kimmel SE. Warfarin therapy: in need of improvement after all these years. Expert Opin Pharmacother. 2008;9:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prasad S, Wootten MR, Kulinski N, et al. What to do when warfarin therapy goes too far. J Fam Pract. 2009;58:346–352. [PubMed] [Google Scholar]
- 11. Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin‐associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82–92. [DOI] [PubMed] [Google Scholar]
- 12. Wiedermann CJ, Stockner I. Warfarin‐induced bleeding complications—clinical presentation and therapeutic options. Thromb Res. 2008;122(suppl 2):S13–S18. [DOI] [PubMed] [Google Scholar]
- 13. Pabinger I, Tiede A, Kalina U, et al. Impact of infusion speed on the safety and effectiveness of prothrombin complex concentrate: a prospective clinical trial of emergency anticoagulation reversal. Ann Hematol. 2010;89:309–316. [DOI] [PubMed] [Google Scholar]
- 14. Xarelto (rivaroxaban) [package insert]. Titusville, NJ: Janssen Pharmaceuticals. 2011.. [Google Scholar]
- 15. Roehrig S, Straub A, Pohlmann J, et al. Discovery of the novel antithrombotic agent 5‐chloro‐N‐({(5S)‐2‐oxo‐3‐ [4‐(3‐oxomorpholin‐4‐yl)phenyl]‐1,3‐oxazolidin‐5‐yl}methyl)thiophene‐2‐carboxamide (BAY 59‐7939): an oral, direct Factor Xa inhibitor. J Med Chem. 2005;48:5900–5908. [DOI] [PubMed] [Google Scholar]
- 16. Johnson & Johnson Pharmaceutical Research & Development . Rivaroxaban for the prevention of stroke and non‐central nervous system (CNS) systemic embolism in patients with atrial fibrillation. Advisory committee briefing document; 2011..
- 17. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. [DOI] [PubMed] [Google Scholar]
- 18. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet. 2008;372: 31–39. [DOI] [PubMed] [Google Scholar]
- 19. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786. [DOI] [PubMed] [Google Scholar]
- 20. Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 21. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365: 883–891. [DOI] [PubMed] [Google Scholar]
- 22. dummy Pradaxa (dabigatran etexilate capsules) [package insert]. Ingelheim, Germany: Boehringer Ingelheim Pharma GmbH & Co. 2011.. http://bidocs.boehringer‐ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. [Google Scholar]
- 23. Van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. [DOI] [PubMed] [Google Scholar]
- 24. Lubetsky A, Yonath H, Olchovsky D, et al. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469–2473. [DOI] [PubMed] [Google Scholar]
- 25. Bershad EM, Suarez JI. Prothrombin complex concentrates for oral anticoagulant therapy‐related intracranial hemorrhage: a review of the literature. Neurocrit Care. 2010;12:403–413. [DOI] [PubMed] [Google Scholar]
- 26. Lisman T, Bijsterveld NR, Adelmeijer J, et al. Recombinant factor VIIa reverses the in vitro and ex vivo anticoagulant and profibrinolytic effects of fondaparinux. J Thromb Haemost. 2003;1: 2368–2373. [DOI] [PubMed] [Google Scholar]
- 27. Hall AB, Carson BC. Reversal of warfarin‐induced coagulopathy: review of treatment options. J Emerg Nurs. 2012;38:98–101. [DOI] [PubMed] [Google Scholar]
- 28. Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. [DOI] [PubMed] [Google Scholar]
- 29. Leissinger CA, Blatt PM, Hoots WK, et al. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83:137–143. [DOI] [PubMed] [Google Scholar]
- 30. Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin‐related intracerebral hemorrhage. Stroke. 2006;37:151–155. [DOI] [PubMed] [Google Scholar]
- 31. Makris M, van Veen JJ, Maclean R. Warfarin anticoagulation reversal: management of the asymptomatic and bleeding patient. J Thromb Thrombolysis. 2010;29:171–181. [DOI] [PubMed] [Google Scholar]
- 32. Logan AC, Yank V, Stafford RS. Off‐label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med. 2011;154:516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in‐hospital use of recombinant factor VIIa for off‐label indications. Ann Intern Med. 2011;154:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skolnick BE, Mathews DR, Khutoryansky NM, et al. Exploratory study on the reversal of warfarin with rFVIIa in healthy subjects. Blood. 2010;116:693–701. [DOI] [PubMed] [Google Scholar]
- 35. Scott LJ. Prothrombin complex concentrate (Beriplex P/N). Drugs. 2009;69:1977–1984. [DOI] [PubMed] [Google Scholar]
- 36. Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26:41–48. [DOI] [PubMed] [Google Scholar]
- 37. Morgenstern LB, Hemphill JC III, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prothrombin complex concentrates to reverse warfarin‐related bleeding. Med Lett Drugs Ther. 2011;53:78–79. [PubMed] [Google Scholar]
- 39. Anti‐Inhibitor Coagulant Complex, nanofiltered and vapor‐heated lyophilized powder for solution [package insert] . Deerfield, IL: Baxter Healthcare Corporation. 2011.
- 40. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. [DOI] [PubMed] [Google Scholar]
- 41. Mills RM, Berkowitz RD, Damaraju CV, et al. Initiation of rivaroxaban following low molecular weight heparin for thromboprophylaxis after total joint replacement: the Safe, Simple Transitions (SST) study. Poster presented at: International Society for Thrombosis and Haemostasis (ISTH); July 23–29, 2011; Kyoto, Japan. [DOI] [PubMed] [Google Scholar]
- 42. Gruber A, Marzec UM, Buetehorn U, et al. Potential of activated prothrombin complex concentrate and activated factor VII to reverse the anticoagulant effects of rivaroxaban in primates. Blood. 2008;112:3825. [Google Scholar]
- 43. Perzborn E, Harwardt M. Recombinant factor VIIA partially reverses the effects of the factor XA inhibitor rivaroxaban on thrombin generation, but not the effects of thrombin inhibitors, in vitro. J Thromb Haemost. 2007;5(suppl 2): Abstract P‐W‐640. [Google Scholar]
- 44. Eerenberg ES, Sijpkens MK, Kamphuisen PW, et al. Prothrombin complex concentrate reverses the anticoagulant effect of rivaroxaban in healthy volunteers. Blood. 2010;116:Abstract 1094. [Google Scholar]
- 45. Guyatt GH, Norris SL, Schulman S, et al. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e89S–e119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cairns JA, Connolly S, McMurtry S, et al; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol. 2011;27:74–90. [DOI] [PubMed] [Google Scholar]


