Abstract
Background
Testis-specific gene antigen 10 (TSGA10) is a tumor suppressor in several types of human malignancy. However, there have been few studies that have investigated the role of TSGA10 in bladder cancer. This study aimed to investigate the expression of TSGA10 in human bladder cancer cell lines and bladder cancer tissues and its effects on patient prognosis.
Material/Methods
The expression of TSGA10 in 40 tissue samples of bladder cancer and matched normal adjacent bladder tissue, and five human bladder cancer cell lines was assessed by immunohistochemistry, Western blot, quantitative reverse transcription polymerase chain reaction (qRT-PCR), and flow cytometry. The correlation between the expression level of TSGA10 and the clinicopathological features of patients with bladder cancer was analyzed and overall survival (OS) in patients with bladder cancer was determined by Kaplan-Meier curves.
Results
Upregulation of TSGA10 expression in tissues from patients with bladder cancer was compared with normal adjacent bladder tissue and was significantly correlated with gender, metastasis, lymphovascular invasion, and tumor stage in bladder cancer. In bladder cancer cell lines, down-regulation of TSGA10 reduced cell apoptosis and increased cell migration, and resulted in the formation of an epithelial-mesenchymal transition (EMT) phenotype. Overexpression of TSGA10 resulted in an increased apoptosis rate of tumor cells, reduced cell migration, and contributed to the reversal of the EMT phenotype.
Conclusions
These findings support that TSGA10 deserves further study as a potential novel prognostic biomarker in bladder cancer.
MeSH Keywords: Apoptosis, Gallbladder Neoplasms, Neoplasm Metastasis
Background
Worldwide, bladder cancer, or transitional cell carcinoma of the bladder, ranks fourth among all malignancies in men in terms of incidence and is the eighth most common cause of cancer-related mortality in the USA [1]. Due to the high fatality rate and low 5-year survival rate, it is important to identify novel and diagnostic and prognostic biomarkers for bladder cancer [2].
Cancer-testis antigens (CTAs) have been shown to be expressed in several human tumor types [3]. For example, Shida et al. reported that the expression of Kita-Kyushu lung cancer antigen-1 (KK-LC-1) was increased in gastric cancer when compared with other CTAs and that this biomarker might have potential in the diagnosis of gastric cancer [4]. Hanagiri et al. detected reduced expression of HLA class I antigen and identified this as a poor prognostic indicator in patients with expression of CTAs, that represented a hurdle to antigen-based tumor immunotherapy [5]. Testis-specific gene antigen 10 (TSGA10) was initially isolated by differential mRNA display and was initially found to be expressed only in the adult human testis [6]. Tanaka et al. showed that TSGA10 over-expression induced an IgG response in patients with hepatocellular carcinoma and melanoma [7]. The TSGA10 gene is located on chromosome 2q11.2 and is 157,701 base pairs in length. Also, the putative TSGA10 protein is cleaved into two fragments, consisting of an N-terminal fragment of 27 kDa, located in the fibrous sheath of the sperm tail, and a C-terminal fragment of 55 kDa [7]. There is an accumulation of the large fragment in the midpiece of the sperm that co-localizes with hypoxia-inducible factor 1α (HIF-1α) and subsequently prevents the nuclear localization of HIF-1α in the process of spermatogenesis [8].
Although TSGA10 has been shown to be a tumor suppressor in several types of human malignancy, there have been few studies that have investigated the role of TSGA10 in bladder cancer. Therefore, this study aimed to investigate the expression of TSGA10 in human bladder cancer cell lines and bladder cancer tissues and its effects on patient prognosis.
Material and Methods
Ethical approval and patient consent
This study was approved by the Ethics Committee of Qinghai University Affiliated Hospital, Peoples’ Republic of China and was conducted according to the criteria of the Declaration of Helsinki. Informed consent was obtained from each patient who participated in the study.
Tissues and cells
The study included 41 samples of bladder cancer tissue and matched adjacent normal tissue from patients who were diagnosed and treated in the Department of Urological Surgery, Qinghai University Affiliated Hospital between April 1st, 2016 and April 1st, 2017 (Table 1). All patients provided written informed consent. None of the subjects enrolled in the study underwent antitumor therapy before surgery. Tissue samples of bladder tumor and adjacent normal bladder were immediately stored in liquid nitrogen for RNA extraction or were fixed in formalin for histology.
Table 1.
Correlation between testis-specific gene antigen 10 (TSGA10) protein expression and clinical characteristics of patients with bladder cancer.
| Characteristic | Number (%) | Expression | χ2 | P-value | |
|---|---|---|---|---|---|
| Low## | High# | ||||
| Gender | |||||
| Male | 22/41 (53.7) | 2 | 20 | 7.609 | 0.006** |
| Female | 19/41 (46.3) | 9 | 10 | ||
| Age (years) | |||||
| <60 | 8/41 (20.0) | 3 | 5 | 0.577 | 0.448 |
| ≥61 | 33/41 (80.0) | 8 | 25 | ||
| Smoking | |||||
| Yes | 24/41 (58.5) | 5 | 19 | 1.060 | 0.303 |
| No | 17/41 (41.5) | 6 | 11 | ||
| Tumor stage | |||||
| <IV | 20/41 (48.8) | 4 | 16 | 0.928 | 0.335 |
| ≥IV | 21/41 (51.2) | 6 | 11 | ||
| Lymphovascular invasion | |||||
| <70 | 18/41 (56.1) | 18 | 9 | 8.775 | 0.003** |
| ≥70 | 23/41 (44.0) | 2 | 21 | ||
| Tumor stage | |||||
| T1–T3 | 31/41 (75.6) | 6 | 25 | 6.034 | 0.014* |
| T4 | 10/41 (24.4) | 10 | 6 | ||
TSGA10 protein expression, (++/+++);
TSGA10 protein expression, (−/+);
statistically significant (P<0.05);
statistically significant (P<0.01).
Four human bladder cancer cell lines, UMUC3, SW780, 5637, and 724, and the normal human ureteric epithelial cell line, SVHUC-1 were purchased from the Type Culture Collection Cell Bank, Chinese Academy of Science Committee, Shanghai, China. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Gibco-BRL, Carlsbad, CA, USA) at 37°C in a 5% CO2 incubator.
Plasmids and small interfering RNA (siRNA)
TSGA10 siRNA (siRNA sense: 5′-GAGCUGGCAACUUAUGAGUTT-3′; siRNA antisense: 5′-ACUCAUAAGUUGCCAGCUCTT-3′) and control siRNA were synthesized by GenePharma Co., Ltd. (Shanghai, China). The negative control, NC/pcDNA3.1-TSGA10 specifically targeting TSGA10 was obtained from GenePharma Co., Ltd (Shanghai, China). Lipofectamine 2000 (Thermofisher Scientific, Waltham, MA, USA) was used for transient transfection, according to the manufacturer’s protocol.
Immunohistochemistry
Bladder cancer tissue and adjacent normal bladder tissue was fixed in formalin and embedded in paraffin wax and 4 μm sections were cut onto glass slides. Following de-waxing, antigen retrieval was performed by heating the sections in citrate buffer (pH 6.0). Then, 3% hydrogen peroxide was used to block endogenous peroxidase. Tissue sections were incubated overnight at 4°C with the polyclonal anti-TSGA10 antibody (dilution 1: 200) (Abcam, Cambridge, UK) or pre-immune serum as the negative control. The next day, the slides were brought to room temperature, followed by incubation with biotinylated secondary antibodies (ZSGB-Bio, China). After washing, the tissue sections were incubated with the brown chromogen, 3,3′-diaminobenzidine (DAB) (Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China), and the sections were counterstained with hematoxylin.
Western blot
Total cellular protein was extracted by lysis in RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA) with the proteinase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) on ice for 30 min. The supernatants were collected by centrifuging for 15 min at 12,000 rpm, followed by determination of the protein concentration using the Bradford reagent (Bio-Rad, Hercules, CA, USA). Samples were boiled with Laemmli sample buffer (Bio-Rad, Hercules, CA, USA). Equal amounts of protein samples underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), were transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Burlington, MA, USA) and blocked with 5% dried skimmed milk powder. The membranes were incubated with primary antibodies overnight at 4°C, followed by reaction with horseradish peroxidase (HRP)-conjugated secondary antibodies. The blots were visualized with enhanced chemiluminescent (ECL) reagent (Thermofisher Scientific, Waltham, MA, USA).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells and tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNAs were reverse transcribed to cDNA by using the PrimeScript RT reagent Kit (Takara, Minato-ku, Tokyo, Japan), according to the manufacturer’s protocol. The SYBR PrimeScript RT-PCR kit (Takara, Minato-ku, Tokyo, Japan) was purchased for qRT-PCR according to the manufacturer’s protocol. The following primers were used:
TSGA10, forward: 5′-CTGTTAAGGTGGTGGCATTG-3′;
TSGA10, reverse: 5′-GGAGGCTCATACTGGCTGAT-3′;
GAPDH, forward: 5′-CTCGCTTCGGCAGCACA-3′;
GAPDH, reverse: 5′-AACGCTTCACGAATTTGCGT -3′.
All assays were performed in triplicate. The relative expression levels were calculated using the 2−ΔΔCt method, followed by normalization against the mRNA expression of GAPDH.
Flow cytometry
Ice-cold PBS was used to wash the detached cells twice, followed by suspension in 1 ml of Dulbecco’s phosphate-buffered saline (DPBS) and dropwise addition of 4% paraformaldehyde (PFA). Cells were then stained with Annexin V-fluorescein isothiocyanate (FITC) and 7-aminoactinomycin D (7-AAD) (Beckman Coulter, Brea, CA, USA), and with propidium iodide (PI)/RNase staining buffer, and incubated at room temperature for 30 min in the dark.
Wound healing and cell migration assay
In the wound healing assay, the cells were seeded into a 24-well plate at a density of 5×106/ml, after successful transfection. After the cells reached about 90% confluence, a sterile 10 μl pipette tip was used to perform a single scrape of the cells. After washing three times with PBS to remove the adherent cells, the cells were cultured in DMEM without FBS and photographed in the same field at 0 h and 24 h. Image-Pro Plus version 6.0 software was used to determine the wound width (μm). The wound closure rate was calculated as, the wound width (at 0 h) – the wound width (at 24 h)/wound width (0 h)×100%.
The transwell cell migration and invasion assay were performed with transwell chambers (Corning, NY, USA) and Matrigel invasion gel (Becton Dickinson, Franklin Lakes, NJ, USA) were used to measure cell migration and invasion, respectively. Cells cultured in FBS-free medium were transferred into the upper chamber with or without 10 μg/ml Matrigel, 48 h after transfection. Medium containing 10% FBS was added into the lower chamber. After incubation for 24 h, a cotton swab was used to wipe off the cells on the upper surface of chamber while cells migrating to the lower chamber were fixed in methanol, stained with 0.1% crystal violet and counted under the light microscope by randomly selecting three fields.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). Comparison between groups was performed using the Student’s t-test, one-way analysis of variance (ANOVA), or the chi-squared (χ2) test. Spearman’s correlation analysis was used to determine the correlation between TSGA10 expression levels in tissues from patients with bladder cancer compared with adjacent normal bladder tissue, and bladder cancer cells with controls. The Kaplan-Meier method was performed and patient survival curves were analyzed. Multivariate analysis of prognostic parameters in patients with bladder cancer was analyzed by the Cox proportional hazards model. A P-value <0.05 was considered to be statistically significant.
Results
The expression of testis-specific gene antigen 10 (TSGA10) was increased in patients with bladder cancer and was significantly associated with survival
To determine the role of TSGA10 in bladder cancer, we first searched the expression of TSGA10 in the OncoMine database. According to the Cancer Genome Atlas (TCGA) database, the expression of TSGA10 DNA was significantly increased in patients with bladder cancer when compared with normal bladder and peripheral blood (p=0.042) (Supplementary Figure 1). Also, the mRNA level of TSGA10 was significantly increased in bladder cancer when compared with normal bladder (p=0.003) (Supplementary Figure 1).
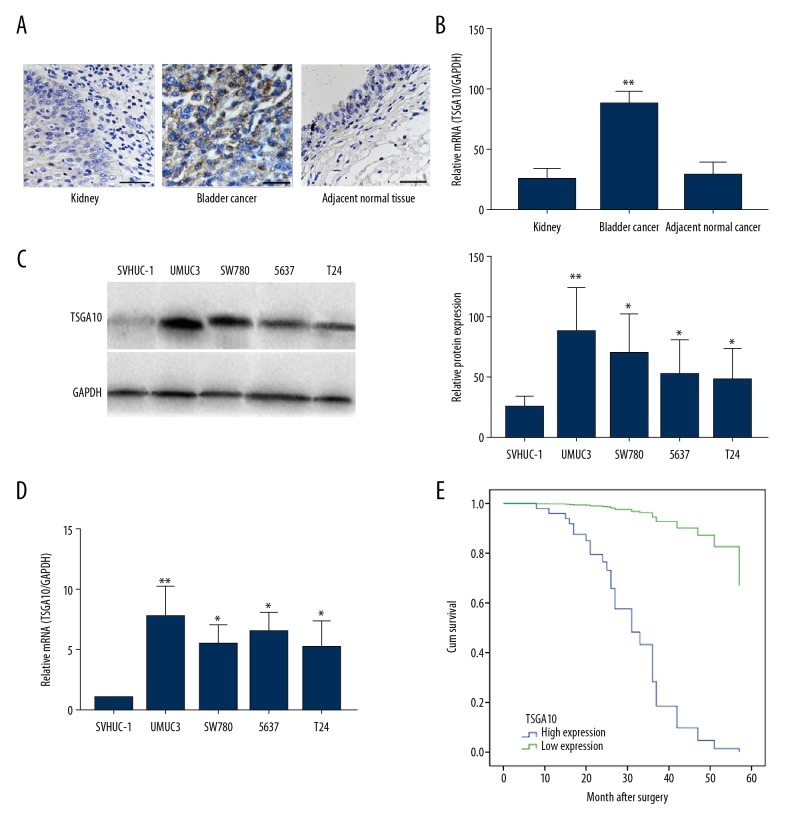
To verify these results, tumor tissue and adjacent normal bladder tissue were collected from 41 patients. Immunohistochemistry confirmed the increased expression of TSGA10 in tissues from patients with bladder cancer, when compared with normal kidney tissues or adjacent normal tissues (Figure 1A), and a similar increase in TSGA10 mRNA is shown in Figure 1B. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot showed overexpression of TSGA10 in the four bladder cancer cell lines compared with SVHUC-1 cell line (Figure 1C, 1D). Due to the gene expression limit in the cells, the 5637 cell line and the UMUC3 and T24 cell lines were not chosen for the subsequent experiments.
Figure 1.
Testis-specific gene antigen 10 (TSGA10) expression was increased in patients with bladder cancer and was significantly associated with patient survival. (A) Photomicrograph of the immunohistochemistry (IHC) staining for testis-specific gene antigen 10 (TSGA10) in kidney, bladder cancer tissue, and adjacent normal bladder tissues. (B) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) for TSGA10 mRNA expression in kidney, bladder cancer tissue, and adjacent normal bladder tissues. (C) Western blot for the protein expression of TSGA10 in four breast cancer cell lines and the human bladder epithelial cell immortalized cell line, SVHUC-1. (D) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay for mRNA expression of TSGA10 in four breast cancer cell lines and the human bladder epithelial cell immortalized cell line, SVHUC-1. The 5637 cell line was selected to perform subsequent experiments. (E) The overall survival (OS) in patients with bladder cancer presented by Kaplan–Meier curves. * p<0.05, ** p<0.01. Bar, 20 μm. The columns show the mean for three separate experiments.
Following the above findings, we assessed the prognostic role of TSGA10 in bladder cancer using Kaplan–Meier survival analysis. Overexpression of TSGA10 was significantly associated with gender, lymphovascular invasion, and tumor stage (Table 1). Also, Cox multivariate analysis showed that only lymphovascular invasion and TSGA10 expression were significant independent prognostic factors (Table 2). Patients with high expression levels of TSGA10 had significantly reduced overall survival (OS) in months compared with patients with low expression levels, and the patients in the higher expression group had reduced OS in months (Figure 1E). Further studies are required to study the expression of TSGA10 to determine its potential role as an independent prognostic biomarker for patients with bladder cancer.
Table 2.
Cox proportional hazards model was used to analyze the effect of testis-specific gene antigen 10 (TSGA10) expression on the survival of patients with bladder cancer.
| Variable | Category | P-value | OR | HR (95%CI) |
|---|---|---|---|---|
| Gender | Male | 0.109 | 0.464 | 0.181–1.187 |
| Female | ||||
| Age | <60 | 0.674 | 1.014 | 0.949–1.084 |
| ″61 | ||||
| Smoking | Yes | 0.745 | 1.166 | 0.461–2.950 |
| No | ||||
| Pathological | <IV | 0.202 | 2.417 | 0.623–9.382 |
| ≥IV | ||||
| Lymphovascular | <70 | 0.020** | 7.172 | 1.357–37.915 |
| ≥70 | ||||
| Tumor range | T1–T3 | 0.684 | 0.790 | 0.254–2.459 |
| T4 | ||||
| TSGA10 expression | High | 0.005** | 22.356 | 2.512–199.005 |
| Low |
Univariate analysis was performed using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. HR, hazard ratio; 95% CI, 95% confidence interval for relative risk.
Statistically significant (p<0.05);
statistically significant (p<0.01).
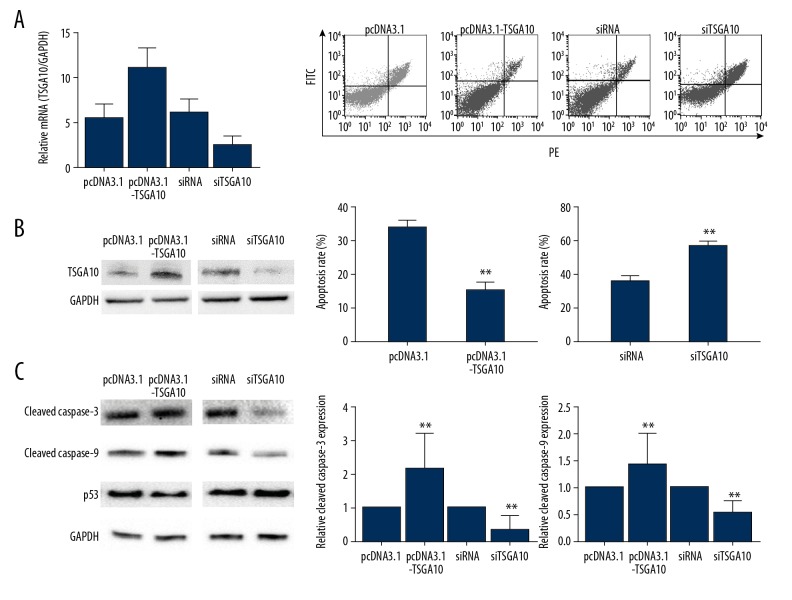
Down-regulation of TSGA10 increased bladder cancer cell apoptosis in vitro
To further investigate the role of TSGA10 in bladder cancer, the 5637 cell line was chosen to study biological behavior induced by abnormal expression of TSGA10. Briefly, the 5637 cell line was transfected with either pcDNA3.1-TSGA10 (pcDNA3.1 as control) or siTSGA10 (siRNA as control), which achieved satisfactory transfection efficiency after 48 hours (Figure 2A). Flow cytometry was performed to examine the influence of TSGA10 on apoptosis. As shown in Figure 2B, the total apoptotic rate decreased in the TSGA10 overexpression group (p<0.05), whereas the total apoptotic rate increased in the TSGA10 underexpression group (p<0.05). To identify the underlying mechanism, the expression of cleaved caspase-3 and cleaved caspase-9 were determined, which are both apoptotic protein markers. As shown in Figure 2.C, cleaved caspase-3 and cleaved caspase-9 were significantly increased following transfection with pcDNA3.1-TSGA10 compared with the control group (Figure 2C). Down-regulation of TSGA10 resulted in significantly reduced expression of cleaved caspase-3 and cleaved caspase-9 compared with the control group (p<0.01). These findings indicated that TSGA10 reduced cell apoptosis of the bladder cancer cell lines by increasing the expression of cleaved caspase-3 and caspase-9.
Figure 2.
Down-regulation of testis-specific gene antigen 10 (TSGA10) increased bladder cancer cell apoptosis in vitro. (A) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay for mRNA expression of TSGA10 and Western blot show the level of TSGA10 in 5637 cells transfected with pcDNA3.1 or pcDNA3.1-TSGA10, siRNA, or siTSGA10. (B) Flow cytometry analysis of cell apoptosis and the percentage of total apoptotic cells show that TSGA10 inhibits apoptosis in 5637 cells. (C) Western blot assay shows the effects of down-regulation or upregulation of TSGA10 on 5637 cells and apoptosis protein expression levels, including cleaved caspase-3, cleaved caspase-9, and p53. The data are presented as the mean ± standard deviation (SD). * p<0.05 and ** p<0.01.
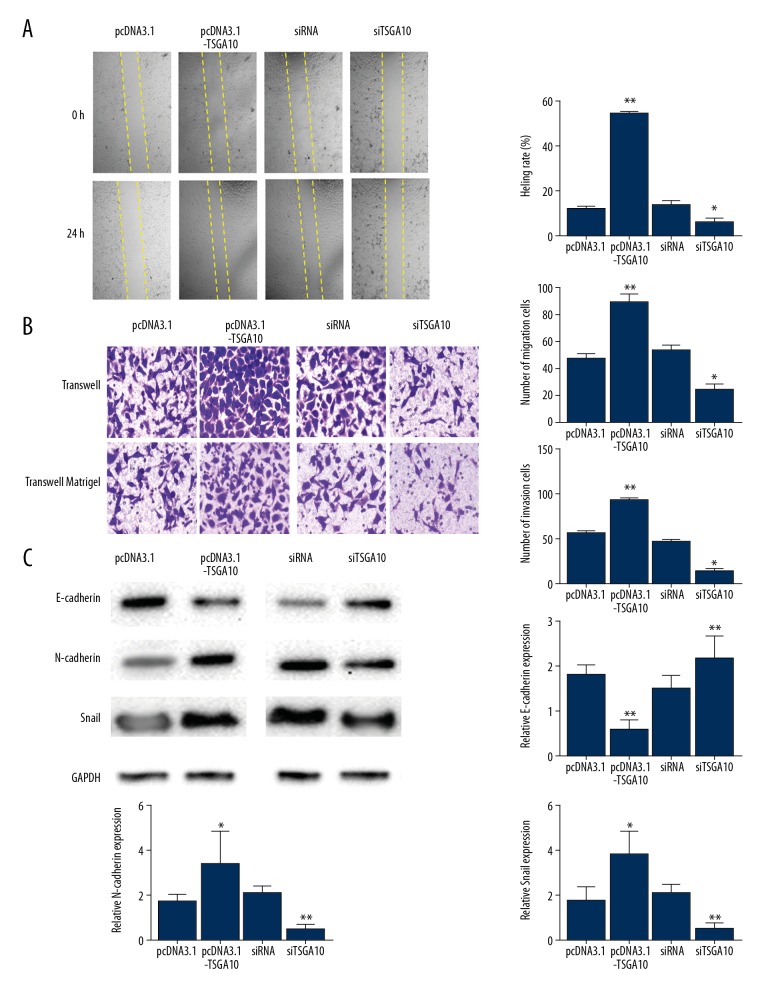
Down-regulation of TSGA10 reduced the cell migration of bladder cancer cells in vitro
Cell migration assays were performed after upregulation and down-regulation of the expression of TSGA10 in human bladder cancer cells. First, as shown in the wound healing assays, closure of the defect was more rapid in the TSGA10 overexpression group compared with the control group (Figure 3A). Also, the wound healing ability of cells transfected with short interfering (si)TSGA10 were significantly decreased compared with the control group (Figure 3A). The cell migration and invasion assays showed that fewer cells migrated into the lower chamber following TSGA10 upregulation when compared with the control group (Figure 3B). Also, the number of cells transported into the lower chamber was significantly reduced following transfection with siTSGA10 (Figure 3B).
Figure 3.
Down-regulation of testis-specific gene antigen 10 (TSGA10) increased apoptosis of bladder cancer cells in vitro. (A) Wound healing assay shows that testis-specific gene antigen 10 (TSGA10) accelerated cell migration ability. Magnification ×50. (B) Transwell migration assay and invasion assay show that TSGA10 promotes migration capacity and invasion potential. Magnification ×50. (C) Western blot shows the effects of TSGA10 down-regulation or upregulation on the expression of proteins associated with epithelial-mesenchymal transition (EMT) in 5637 cells, including E-cadherin, N-cadherin, and Snail. The data are presented as the mean ± standard deviation (SD). * p<0.05 and ** p<0.01.
Epithelial-mesenchymal transition (EMT) is associated with cancer cell [9]. To understand the relationship between expression of TSGA10 and cell motility, the most representative EMT markers were selected that included E-cadherin, N-cadherin, and Snail, and the protein levels were detected after the bladder cancer cells were transfected with either pcDNA3.1-TSGA10 (pcDNA3.1 as the control) or siTSGA10 (siRNA as the control). As shown in Figure 3.C, overexpression of TSGA10 significantly increased the expression levels of N-cadherin (p<0.05) and Snail (p<0.05), whereas E-cadherin levels were significantly was decreased (p<0.01). Also, significantly increased levels of E-cadherin were identified in the TSGA10 down-regulation group when compared with the control group (p<0.01). However, levels of N-cadherin and Snail were significantly decreased (both, p<0.01). These findings supported that down-regulation of TSGA10 enhanced apoptosis of bladder cancer cells in vitro by regulating the process of EMT.
Discussion
Cancer-testis antigens (CTAs) are presently considered to be a potential target for cancer immunotherapy due to their immunogenicity and restricted expression pattern [10]. Kanehira et al. showed that the MPHOSPH1/PRC1 complex might potentially play a critical role in bladder tumorigenesis and that suppression of MPHOSPH1/PRC1 expression or their interaction might be therapeutic targets for bladder cancer [11]. Also, Tsuruta et al. showed that a DEPDC1-derived and MPHOSPH1-derived short peptide vaccine showed promising efficacy in reducing relapse of bladder cancer in a phase I/II clinical trial [12]. These findings indicate the potential role for CTAs as immunotherapeutic targets in bladder cancer. Testis-specific gene antigen 10 (TSGA10) has been shown to be overexpressed and to be a potential target for antigen-specific immunotherapy in brain tumors [13], esophageal squamous cell carcinoma [14], breast cancer [15] and locoregionally invasive nasopharyngeal carcinoma [16].
In the present study, we initially detected the expression of TSGA10 at the mRNA and protein level in bladder cancer tissues and cell lines, which was significantly increased when compared with adjacent normal bladder tissues and the normal cell line. However, the expression trends were not exactly the same between mRNA and protein level in bladder cancer cells, which may be due to obstacles in the protein translation pathway, which resulted in the failure of translating all mRNAs into proteins.
To identify the mechanism of TSGA10 in tumorigenesis of bladder cancer, flow cytometry showed that TSGA10 represented an oncogene that could inhibit the apoptosis of bladder cancer cells, as shown by increased expression of the apoptotic marker, cleaved caspase-3 [17], and cleaved caspase-9, an apoptotic protease of the mitochondrial or intrinsic apoptotic pathway [18]. However, whether the FAS or caspase-8 apoptosis markers, which are involved in the extrinsic pathway, are involved in anti-apoptosis induced by TSGA10, require further study. Therefore, it is not possible to confirm that TSGA10 expression induced anti-apoptosis by regulating the mitochondrial apoptotic pathway.
In epithelial-mesenchymal transition (EMT), sessile epithelial cells switch to motile cells that may trigger the expansion of malignant cellular subpopulation following expression of the mesenchymal phenotype [19]. Bryan et al. regarded E-cadherin and N-cadherin switching to indicate the malignant progression of bladder cancer [20]. Also, the aberrant expression level of Snail has previously been shown to be crucial for the molecular pathogenesis of bladder cancer [19]. In the present study, we determined the change in Snail, N-cadherin, and E-cadherin, the most representative EMT markers, in TSGA10 upregulated and down-regulated cells to show that TSGA10 might have a role in promoting bladder cancer cell metastasis by modulating the EMT pathway.
In conclusion, in the present study, we first assessed the oncogenic roles of TSGA10 in bladder cancer, which was significantly increased, and therefore might be a possible indicator of poor prognosis in patients with bladder cancer. Also, TSGA10 expression reduced cell apoptosis and facilitated cell migration as well as EMT in vitro. Therefore, targeting TSGA10 expression might be a novel therapeutic approach in patients with bladder cancer in the future. These findings indicate that TSGA10 acts as an oncogene and might be a potential prognostic biomarker and therapeutic target in bladder cancer. However, the regulatory mechanism of TSGA10 in bladder cancer remains to be determined.
Conclusions
The findings from the present study showed that the testis-specific gene antigen 10 (TSGA10) deserves further study as a potential novel prognostic biomarker in bladder cancer.
Supplementary
The expression of testis-specific gene antigen 10 (TSGA10) in the Cancer Genome Atlas (TCGA) database. According to the Cancer Genome Atlas (TCGA) database, the expression of testis-specific gene antigen 10 (TSGA10) DNA (A) was significantly increased in bladder cancer tissue compared with normal adjacent bladder tissue and blood (p=0.042). Also, the mRNA expression level of TSGA10 (B) was significantly increased in bladder cancer tissue compared with normal bladder tissue (p=0.003).
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Afsharpad M, Nowroozi MR, Mobasheri MB, et al. Cancer-testis antigens as new candidate diagnostic biomarkers for transitional cell carcinoma of bladder. Pathol Oncol Res. 2019;25(1):191–99. doi: 10.1007/s12253-017-0313-4. [DOI] [PubMed] [Google Scholar]
- 3.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: Prime candidates for immunotherapy. Oncotarget. 2015;6:15772–87. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shida A, Futawatari N, Fukuyama T, et al. Frequent high expression of Kita-Kyushu lung cancer antigen-1 (KK-LC-1) in gastric cancer. Anticancer Res. 2015;35:3575–79. [PubMed] [Google Scholar]
- 5.Hanagiri T, Shigematsu Y, Shinohara S, et al. Clinical significance of expression of cancer/testis antigen and down-regulation of HLA class-I in patients with stage I non-small cell lung cancer. Anticancer Res. 2013;33:2123–28. [PubMed] [Google Scholar]
- 6.Modarressi MH, Cameron J, Taylor KE, Wolfe J. Identification and characterisation of a novel gene, TSGA10, expressed in testis. Gene. 2001;262:249–55. doi: 10.1016/s0378-1119(00)00519-9. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka R, Ono T, Sato S, et al. Over-expression of the testis-specific gene TSGA10 in cancers and its immunogenicity. Microbiol Immunol. 2004;48(4):339–45. doi: 10.1111/j.1348-0421.2004.tb03515.x. [DOI] [PubMed] [Google Scholar]
- 8.Hägele S, Behnam B, Borter E, et al. TSGA10 prevents nuclear localization of the hypoxia-inducible factor (HIF)-1alpha. FEBS Lett. 2006;580(15):3731–38. doi: 10.1016/j.febslet.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 9.Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnadas DK, Bai F, Lucas KG. Cancer testis antigen and immunotherapy. Immunotargets Ther. 2013;2:11–19. doi: 10.2147/ITT.S35570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanehira M, Katagiri T, Shimo A, et al. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Res. 2007;67:3276–85. doi: 10.1158/0008-5472.CAN-06-3748. [DOI] [PubMed] [Google Scholar]
- 12.Tsuruta M, Ueda S, Yew PY, et al. Bladder cancer-associated cancer-testis antigen-derived long peptides encompassing both CTL and promiscuous HLA class II-restricted Th cell epitopes induced CD4+ T cells expressing converged T-cell receptor genes in vitro. Oncoimmunology. 2018;7:e1415687. doi: 10.1080/2162402X.2017.1415687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babak B, Ali C, Jogi P, Jonathan W. TSGA10 is specifically expressed in astrocyte and over-expressed in brain tumors. Avicenna J Med Biotechnol. 2009;1:161–66. [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan X, He J, Sun F, Gu J. Effects and interactions of MiR-577 and TSGA10 in regulating esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2013;6:2651–67. [PMC free article] [PubMed] [Google Scholar]
- 15.Dianatpour M, Mehdipour P, Nayernia K, et al. Expression of testis specific genes TSGA10, TEX101 and ODF3 in breast cancer. Iranian Red Crescent Med J. 2012;14:722–26. doi: 10.5812/ircmj.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao L, You B, Shi S, et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene. 2018;37:2873–89. doi: 10.1038/s41388-018-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhary GS, Alharbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2015;1219:1–9. doi: 10.1007/978-1-4939-1661-0_1. [DOI] [PubMed] [Google Scholar]
- 18.Kim B, Srivastava SK, Kim SH. Caspase-9 as a therapeutic target for treating cancer. Expert Opin Ther Targets. 2015;19:113. doi: 10.1517/14728222.2014.961425. [DOI] [PubMed] [Google Scholar]
- 19.Yun SJ, Kim WJ. Role of the epithelial-mesenchymal transition in bladder cancer: From prognosis to therapeutic target. Korean J Urol. 2013;54:645–50. doi: 10.4111/kju.2013.54.10.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan RT, Tselepis C. Cadherin switching and bladder cancer. J Urol. 2010;184:423–31. doi: 10.1016/j.juro.2010.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of testis-specific gene antigen 10 (TSGA10) in the Cancer Genome Atlas (TCGA) database. According to the Cancer Genome Atlas (TCGA) database, the expression of testis-specific gene antigen 10 (TSGA10) DNA (A) was significantly increased in bladder cancer tissue compared with normal adjacent bladder tissue and blood (p=0.042). Also, the mRNA expression level of TSGA10 (B) was significantly increased in bladder cancer tissue compared with normal bladder tissue (p=0.003).