Abstract
Background
Diabetic nephropathy (DN) is a disease characterized by oxidative stress and apoptosis of renal tubular epithelial cells driven by hyperglycemia. Apigenin is a flavonoid compound that possesses potent anti-apoptotic properties. The present study aimed to explore the protective effects and underlying mechanisms of apigenin on renal tubular epithelial cells exposed to hyperglycemia.
Material/Methods
Human renal epithelial cell HK-2 were incubated to D-glucose to establish in vitro DN model. The cell viability, lactate dehydrogenase (LDH) release, apoptosis and oxidative stress were evaluated. qRT-PCR was performed to determine the mRNA levels of NF-E2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1). Western blot analysis was performed to measure the protein expressions of Nrf2.
Results
In HK-2 cells, high glucose reduced cell viability in a concentration- and time-dependent manner. Apigenin suppressed the decrease in cell viability and increase in supernatant LDH release at 100 and 200 μM after 48-h treatment. Apigenin reduced apoptotic rate and pro-inflammatory cytokines production. Apigenin suppressed oxidative stress and increased mRNA expressions of Nrf2 and HO-1. Inhibition of Nrf2 using small interfering RNA (siRNA), or cotreatment with LY294002, an inhibitor of PI3K/Akt, abolished the protective effect on high glucose-induced injury, oxidative stress, and pro-inflammatory cytokines production by apigenin. LY294002 also attenuated the increase in Nrf2 protein by apigenin in high glucose-treated HK-2 cells.
Conclusions
Apigenin protects renal tubular epithelial cells against high glucose-induced injury through suppression of oxidative stress and inflammation via activation of the Nrf2 pathway.
MeSH Keywords: Acidosis, Renal Tubular; Apigenin; Diabetic Nephropathies; Hyperglycemia; NF-E2-Related Factor 2; Oxidative Stress
Background
Diabetic nephropathy (DN) is a microvascular complication of diabetes mellitus, and is characterized by persistent clinical albuminuria and reduced glomerular filtration rate (GFR) [1]. DN affects long-term morbidity and mortality of DM patients, and is also a leading cause of end-stage renal disease [2]. The mechanisms of the pathology of DN have not yet been fully described, and hyperglycemia is currently regarded a driving factor. Hyperglycemia activates various cellular signaling pathways in different kidney cell types, thus contributing to glomerular hyperfiltration [3]. Recently, renal tubular injury has been found to be involved in the progression of DN, and correlates with deterioration of renal function [4]. Moreover, tubular injury has been proposed to be a critical contributor to early DN [5]. As DN is a condition of multiple stages before becoming clinically evident, renal tubular injury is an appropriate therapeutic target and its mechanisms warrant detailed investigation.
Oxidative stress is thought to be an important factor that links hyperglycemia with vascular complications, and this is achieved by altering cellular signaling pathways involving kidney metabolism, thereby contributing to deteriorated hemodynamics and renal function [6]. Hyperglycemia enhances the production of reactive oxygen species (ROS) via a variety of mechanisms, thus aggravating oxidative stress [7]. After counteracting the endogenous antioxidant defense system, excessive oxidative stress oxidizes various biomolecules such as DNA, proteins, and lipids [8]. NF-E2-related factor 2 (Nrf2) is a master regulator of response to oxidative stress. Under oxidative conditions, Nrf2 binds to antioxidant response elements (AREs) and thus activates transcription of its target genes, including heme oxygenase-1 (HO-1), quinone oxidoreductase-1 (NQO1), glutathione peroxidase (GSHPX), and glutamate-cysteine ligase modifier subunit (GCLM) [9]. There is evidence that renal tissue of diabetes mellitus rats undergoes excessive oxidative stress at 4 weeks, and after 4 weeks the protein expression of Nrf2 was upregulated compared with the control group and further increased with DM progression [10]. This indicates that enhanced Nrf2 expression is a response to oxidative stress and provides a protective effect against hyperglycemic injury. In fact, upregulation of Nrf2 expression can inhibit oxidative stress and ameliorate diabetic nephropathy progression [11].
Apigenin is a flavonoid compound from common fruits and vegetables. Recently, increasingly studies have reported its various pharmacological activities, such as anticancer [12], antioxidation [13] and anti-inflammation [14] effects. Given these potent antioxidative effects, we hypothesized that apigenin may have therapeutic implications for DN. HK-2 is a human renal tubular epithelial cell line and has been used as an in vitro hyperglycemic injury DN model. In the present study, we used this model to examine the protective activity of apigenin against high glucose-induced cell viability, apoptosis, and oxidative stress, and we assessed the underlying mechanism.
Material and Methods
Reagents and chemicals
Apigenin (purity >99%), tert-butyl hydroperoxide (tBHP), and N-Acetyl-L-cysteine (NAC) were purchased from Sigma-Aldrich (Saint Louis, MO). PI3K/Akt inhibitor LY294002 was purchased from MedChem Express (Princeton, NJ). Low glucose DMEM media and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). Cell counting kit-8 (CCK-8) kits were obtained from Beyotime Institute of Biotechnology (Shanghai, China). LDH assay kit (CAS: A020-3), malondialdehyde (MDA) assay kit (CAS: A003-1), SOD assay kit (CAS: A001-1), and CAT kit (CAS: A007-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The primary antibody against Nrf2 (CAS: sc-365949) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ELISA kits for TNFα, IL-1β, and IL-6 were purchased from eBioscience (San Diego, CA).
Cell culture and treatment
The human renal tubular epithelial cell line (HK-2) was purchased from the cell bank of the Chinese academy of sciences (Shanghai, China), and cultured in low glucose DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a 5% CO2 incubator. HK-2 cells were incubated with D-glucose (5.5, 10, 15, 20, 25, 30, 40 mM) for up to 72 h. Then, 30 mM D-glucose was chosen as the appropriate concentration for further experiments.
Experiment grouping
The HK-2 cells were seeded, and after reaching to 80% confluence, cells were randomly divided into 4 groups: a control group (5.5 mM D-glucose), a high glucose group (30 mM D-glucose), and an apigenin group (cells were incubated with 30 mM D-glucose and 100 or 200 μM apigenin). After 48 h, the cells and the culture supernatant were collected for further experiments.
Cell viability assay
HK-2 cells were seeded at a density 2×104 cells/mL in a 96-well plate, with 100 μL media per well. Then, cells were randomly divided into various groups according to different experiments. After 48 h, CCK-8 solution (10 μL) was added to each well, followed by incubation at 37°C for 2 h. To evaluate cell viability, a microplate reader was used to measure the absorbance at 450 nm.
LDH release assay
HK-2 cells were seeded at a density 2×104 cells/mL in a 96-well plate, and treated D-glucose (30 mM) and apigenin (100, 200 μM) for 48 h. Then, the LDH content in the media was assessed using an LDH activity kit.
Flow cytometric analysis of apoptosis
HK-2 cells were incubated with 30 mM glucose and apigenin (100, 200 μM) for 48 h. Then, cells were harvested and washed twice with cold PBS, and were incubated with 200 μL 1×binding buffer (contains 5 μL Annexin V-FITC and 5 μL PI (KeyGEN Annexin V Apoptosis Detection Kit) for 15 min in the dark. Finally, cellular apoptosis was analyzed using a FACScan flow cytometer (Becton Dickinson, SF, CA) (Ex 488 nm, and Em 530 nm), and data were analyzed by CELLQuest software. At least 10 000 events were analyzed for each sample.
Determination of MDA, SOD, and CAT
HK-2 cells were harvested and protein was extracted. MDA content was measured at 532 nm absorbance by a microplate reader, SOD was measured at 520 nm absorbance, and CAT was measured at 240 nm absorbance.
Measurement of pro-inflammatory cytokines
ELISA assays were performed in triplicate to determine the levels of the pro-inflammatory cytokines TNFα, IL-1β, and IL-6. The concentrations of TNFα, IL-1β, and IL-6 in each group were determined based on the standard curve, which was established by their recombinant proteins.
Cell transfection with Nrf2 siRNA
Nrf2 siRNA was synthesized by Shanghai GenePharma Co. (Shanghai, China), with the primers as follows: forward: 5′-CGU GAA UCC CAA UGU GAA ATT-3′, reverse: 5′-UUU CAC AUU GGG AUU CAC GTT-3′. After plating in 24-well plates, HK-2 cells were added with 1000 μL transfection complex solution (containing 0.5 μg of Nrf2 or control siRNA plasmids) at 37°C for 8 h, using a lip2000 transfection kit (Invitrogen, USA). Then, cells were incubated for an additional 16 h in complete medium.
Quantitative real-time PCR
Total RNA was isolated using TRIzol (Invitrogen, USA) and reverse transcribed into cDNA. Specific primers were as follows: Nrf2: forward, 5′-TCC GGG TGT GTT TGT TCC AA-3′, reverse, 5′-CGC CCG CGA GAT AAA GAG TT-3′; HO-1: forward, 5′-CAG GCA ATG GCC TAA ACT TC-3′, reverse, 5′-GCT GCC ACA TTA GGG TGT CT-3′; GAPDH: forward, 5′-CCT CAA GAT CAT CAG CAA TG-3′, reverse, 5′-CCA TCC ACA GTC TTC TGG GT-3′. Real-time quantification was performed using the SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA) on an Applied Biosystems 7300 system (ABI 7300, USA). The PCR parameters were as follows: 95°C for 30 s, followed by 40 cycles of 95°C denaturation for 5 s, and 60°C annealing for 30 s. The data were analyzed using the 2-ΔΔCt method [15].
Western blotting
Cells were rinsed with cold PBS and lysed by RIPA buffer to extract protein, followed by determination of protein concentration by bicinchoninic acid assay. Total protein (50 μg per lane) was loaded in 12% SDS-PAGE to perform electrophoresis, and then the protein was transferred to a nitrocellulose membrane (Millipore). The membrane was blocked with 5% skim milk for 1 h, followed by incubation with primary antibody against human Nrf2 and GAPDH (1: 1000 dilution) overnight at 4°C. Subsequently, the membrane was incubated with HRP-conjugated secondary antibody (1: 2000) for 1 h at room temperature. Finally, protein bands on the membrane were visualized by enhanced chemiluminescence (ECL) system (Thermo Scientific, MA, USA), and were analyzed using ImageJ software.
Statistical analysis
Data are expressed as means ± standard deviation (SD) from at least 3 independent experiments, and the results were analyzed using SPSS 19.0 statistical software. The significance of difference was determined by analysis of variance (ANOVA), followed by the post hoc Dunnett’s test. Two-tailed P value <0.05 was considered as statistical significance.
Results
High glucose decreased cell viability
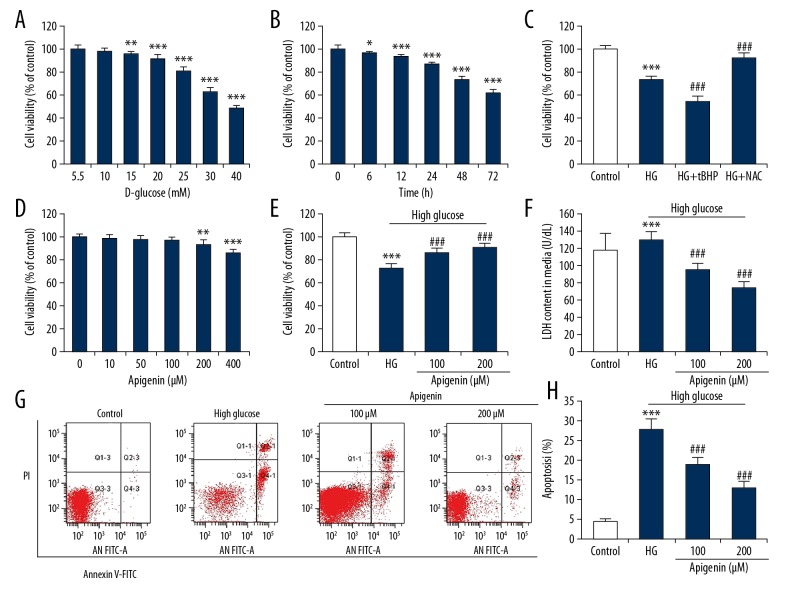
To establish an in vitro DN model to simulate injury of renal tubular epithelial cells induced by hyperglycemia, HK-2 cells were exposed to different concentrations of D-glucose (5.5, 10, 15, 25. 30, 40 mM) for 72 h to determine the appropriate glucose concentration. CCK-8 assay showed that D-glucose reduced the cell viability in a concentration-dependent manner (Figure 1A). We then incubated HK-2 cells with 30 mM D-glucose for 6, 12, 24, 48, and 72 h. There was a gradual decrease in viability over time, with significant differences at 48 and 72 h (Figure 1B). To explore the role of oxidative stress induced by high glucose, HK-2 cells were simultaneously incubated with pro-oxidant tBHP or antioxidant NAC. We found that 30 mM cytotoxicity induced by D-glucose was enhanced by tBHP and suppressed by NAC (Figure 1C).
Figure 1.
Apigenin increased viability and reduced apoptosis in renal tubular epithelial cell with high glucose (HG). CCK-8 assay was performed to assess cell viability. (A) HK-2 cells cultured in DMEM containing 5.5, 10, 15, 20, 25, 30, or 40 mM D-glucose for 72 h. ** P<0.01, *** P<0.001 vs. 5.5 mM group. (B) Cell viability was assessed in HK-2 cells cultured in DMEM containing 30 mM D-glucose for 6, 12, 24, 48, and 72 h. * P<0.05, *** P<0.001 vs. control group (0 h). (C) HK-2 cells were incubated with 30 mM D-glucose in combination with tBHP (200 μM) or NAC (1 mM). (D) HK-2 cells were treated with different concentrations (0, 10, 50, 100, 200, 400 μM) of apigenin for 48 h. HK-2 cells were treated with 30 mM D-glucose with or without apigenin (100, 200 μM) for 48 h. (E) CCK-8 analysis was used to measure cell viability. (F) LDH content in media was measured by assay kit. (G) Apoptosis was determined through annexin V-FITC and PI double staining. The apoptotic rate was calculated from the sum of cells in the right lower quadrant (early apoptosis) and cells in the right upper quadrant (late apoptosis). (H) Apigenin attenuated high glucose-induced increase in apoptotic rate. ** P<0.01, *** P<0.001 vs. control group; ### P<0.001 vs. HG group.
Apigenin increased cell viability and reduced apoptosis of HK-2 cells with high glucose
We then examined the effect of different doses of apigenin (10, 50, 100, 200, 400 μM) on cell viability of HK-2 cells. Results showed that apigenin had little effect on cell proliferation at 10~100 μM at 48 h. However, apigenin at 200 and 400 μM significantly reduced cell viability (Figure 1D). Thus, we chose to use apigenin at 100 and 200 μM for further experiments. To investigate the effect of apigenin on cell injury, HK-2 cells were treated with 30 mM D-glucose and 30 mM D-glucose plus apigenin (100, 200 μM) for 48 h co-culture. The results showed that apigenin (100 and 200 μM) significantly reversed the decrease in viability and increase in LDH release of HK-2 cells exposed to high glucose (P<0.05) (Figure 1E, 1F). To explore whether apoptosis involves reduced cell viability, cells were stained with Annexin V/PI to detect the apoptotic rate. High glucose (30 mM D-glucose) significantly increased the apoptotic rate, which was significantly attenuated by apigenin at 100 and 200 μM (Figure 1G, 1H).
Apigenin inhibited oxidative stress and inflammatory response in high glucose-induced HK-2 cells
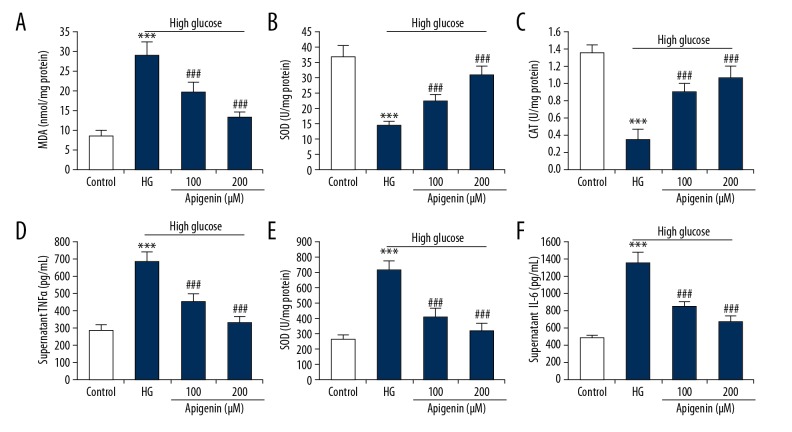
To investigate the effect of apigenin on oxidative stress, we measured intracellular oxidative stress indicators by colorimetric analysis. High glucose significantly increased MDA content and decreased SOD and CAT activities, which was reversed by apigenin treatment (P<0.05) (Figure 2A–2C). To investigate whether apigenin regulates inflammatory response in high glucose-induced HK-2 cells, we measured TNFα, IL-1β, and IL-6 contents in supernatant media by ELISA. Apigenin significantly attenuated the increase of TNFα, IL-1β, and IL-6 in culture supernatant of HK-2 cells induced by 30 mM D-glucose (P<0.05) (Figure 2D–2F).
Figure 2.
Effect of apigenin on oxidative stress and inflammatory cytokine production in high glucose-induced HK-2 cells. HK-2 cells were incubated with 30 mM D-glucose, and (or) apigenin (100, 200 μM) for 48 h. Apigenin decreased intracellular MDA (A), and increased SOD (B) and CAT (C). ELISA was performed to determine the production of pro-inflammatory cytokines. Apigenin decreased TNFα (D), IL-1β (E), and IL-6 (F) contents in supernatant media of HK-2 cells with 30 mM D-glucose. *** P<0.001 vs. control group; ### P<0.001 vs. HG group.
Apigenin activated Nrf2-ARE pathway in high glucose-induced HK-2 cells
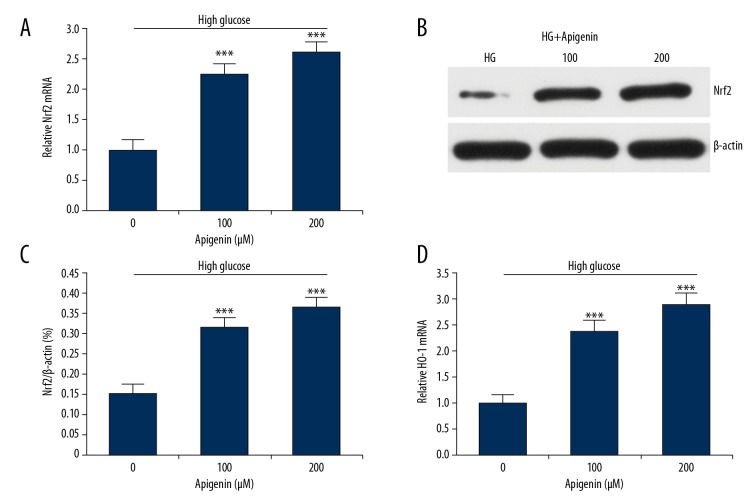
To explore the molecular mechanism underlying suppressed oxidative stress by apigenin, we performed qRT-PCR and Western blot analysis to measure the expression of Nrf2 and HO-1. Treatment with apigenin significantly increased the mRNA and protein expressions of Nrf2 in HK-2 cells after exposure to 30 mM D-glucose (P<0.05) (Figure 3A–3C). Apigenin also increased the mRNA expression of a Nrf2 response gene, HO-1 (Figure 3D).
Figure 3.
Apigenin activated the Nrf2-HO-1 signaling pathway in high glucose-induced cells. HK-2 cells were treated with 30 mM D-glucose with or without apigenin (100, 200 μM) for 48 h. (A) qRT-PCR showed that apigenin enhanced mRNA expression of Nrf2 in HK-2 cells treated with 30 mM D-glucose. (B) Western blot showed that apigenin enhanced protein expression of Nrf2 in HK-2 cells. (C). LY294002 pretreatment significantly increased Nrf2 protein levels compared to HK-2 cells with D-glucose. (D) Apigenin also enhanced mRNA expression of HO-1. *** P<0.001 vs. HG group. HG – high glucose.
Apigenin protected against high glucose-induced HK-2 cell injury via Nrf2 pathway
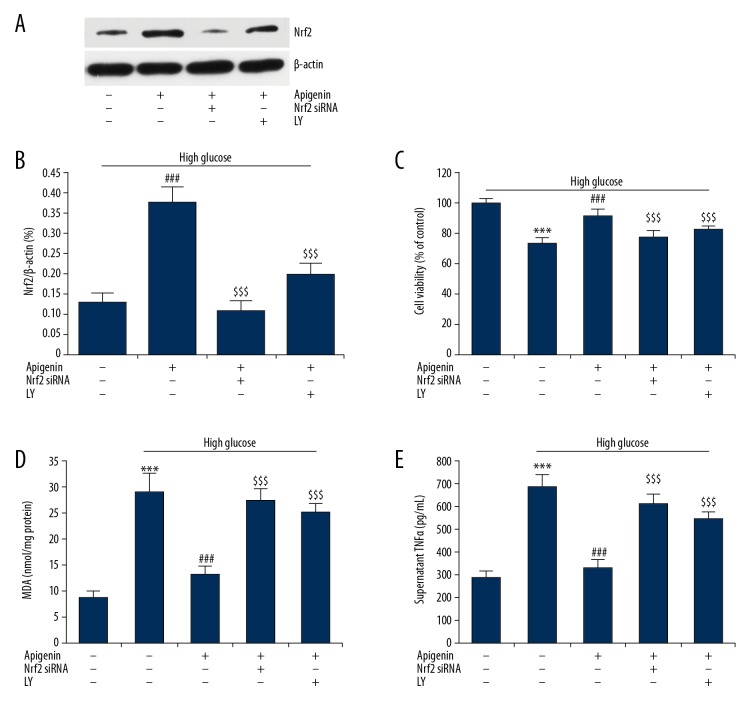
To investigate whether Nrf2 mediates the protective effect of apigenin on hyperglycemia injury, HK-2 cells were transfected with Nrf2 siRNA for 48 h, or pretreated with PI3K inhibitor LY294002 (LY, 50 μM) for 2 h, followed by incubation with D-glucose (30 mM) and apigenin (200 μM) for 48 h. Knocking down Nrf2 sharply downregulated expression of Nrf2 protein (71% reduction rate) in HG-2 cells after apigenin treatment (Figure 4A, 4B). Nrf2 protein expression was also downregulated by pretreatment with LY294002 in HK-2 cells. We then investigated the role of Nrf2 and LY294002 in cell viability, oxidative stress, and inflammatory cytokine production. Compared with HK-2 cells with D-glucose and apigenin, cells with Nrf2 siRNA transfection or pretreatment with LY294002 had significantly reduced cell viability (Figure 4C), as well as increased MDA content (Figure 4D) and supernatant TNFα level (Figure 4E). Taken together, these data indicate that Nrf2 play critical roles in the protection of hyperglycemia damage to renal tubular epithelial cells by apigenin, and PI3K may lie upstream of the Nrf2 pathway.
Figure 4.
Nrf2 mediates the protective effects of apigenin on high glucose-induced injury. HK-2 cells were transfected with Nrf2 siRNA for 48 h, or pretreated with PI3K inhibitor LY294002 (LY, 50 μM) for 2 h, followed by incubation with D-glucose (30 mM) and apigenin (200 μM) for 24 h. (A) Western blotting was performed to determine protein levels of Nrf2 in HK-2 cells. (B) The increase of Nrf2 protein by apigenin was reversed by Nrf2 siRNA or LY294002. Nrf2 siRNA or LY294002 reversed the increase in cell viability (C), decrease in MDA (D), and supernatant TNFα (E) by apigenin. *** P<0.001 vs. control group; ### P<0.001 vs. HG group; $$$ P<0.001 vs. crocin group. LY – LY294002.
Discussion
This study investigated the protective effects of apigenin on high glucose-induced human renal tubular epithelial cells. High glucose reduced HK-2 cell viability in a concentration- and time-dependent manner, which was attenuated by apigenin treatment. Apigenin reduced apoptotic rate and suppressed oxidative stress and pro-inflammatory cytokine production in high glucose-induced HK-2 cells. Apigenin also increased mRNA expressions of Nrf2 and HO-1. The protective effects of apigenin in high glucoses-induced HK-2 cells were mediated by Nrf2 and PI3K, as the protective effect on cell viability, oxidative stress, and pro-inflammatory cytokine production could be abolished by Nrf2 siRNA or cotreatment with LY294002. Therefore, apigenin protects renal tubular epithelial cells against high glucose-induced injury through modulation of Nrf2 and HO-1 pathway.
Several studies showed the protective effect of apigenin on the progression of renal diseases. Apigenin can ameliorate nephrotoxicity of human renal proximal tubular epithelial cells induced by cisplatin [16]. Another study indicated that apigenin improved diabetic nephropathy in rats by suppressing oxidative stress and fibrosis [17]. However, the role of apigenin in hyperglycemic injury of renal tubular epithelial cells remains unclear. This study found that apigenin alleviated injury in HK-2 cells, and proved that the Nrf2 mediated its protective effect.
Oxidative stress, apoptosis, and inflammation are important mechanisms involved in hyperglycemic injury of DN [18]. Persistent hyperglycemia increased ROS production, which exceeded endogenous antioxidants, leading to MDA production and subsequent cellular damage [19]. SOD and CAT act as important antioxidant defense systems, and their expressions were reduced in DN rats compare with healthy rats [20]. Therefore, MDA, SOD, and CAT are 3 biomarkers for detection of oxidative stress in DN. In this study, exposure of HK-2 cells to high glucose resulted in reduced cell viability and enhanced oxidative stress, as evidenced by elevated MDA and decreased SOD and CAT levels in cellular supernatant. This confirms that our model is effective in simulating renal injury from oxidative stress through incubation of HK-2 cells with 30 mM D-glucose, and supports the concept that hyperglycemia promotes oxidative stress and aggravates diabetic nephropathy [7]. Furthermore, treatment with apigenin increased cell viability and suppressed oxidative stress, which is consistent with the previous reports that apigenin alleviated renal injury induce by cisplatin [21] and ischemia/reperfusion [22]. Therefore, agents targeting oxidative stress are promising therapeutic strategies against tubular epithelial injury in DN, and we showed apigenin is another molecule targeting oxidative stress.
This study showed that apigenin activates Nrf2 and its downstream antioxidant response gene, HO-1, in HK-2 cells with high glucose. As a transcription factor of the endogenous antioxidant defense system, Nrf2 protein can reduce renal cell injury in diabetes [23], thus slowing the progression of DN [24]. The mechanisms underlying the protective effects of Nrf2 were suppression of excessive inflammation and oxidative stress [25]. Nrf2 has been regarded as a therapeutic target of diabetic nephropathy [26], and mediated alleviation of diabetic nephropathy by various agents, such as Carnosic acid Notoginsenoside R1 [27,28]. Our study adds apigenin as a new Nrf2 activator in high glucose-induced renal tubular, which is in accordance with its wide modulation of Nrf2 in retinal pigmented epithelium with oxidative injury [29] and D-galactose-induced aging mice [30]. Moreover, Nrf2 seems to suppress inflammation, as evidenced by increased pro-inflammatory cytokine production in HK-2 cells with apigenin and Nrf2 siRNA, compared with cells with apigenin alone. Whether there are other upstream and downstream molecules regulated by apigenin needs further investigation.
Our study showed that Nrf2 expression induced by apigenin is dependent on the PI3K/Akt pathway, as evidenced by significantly decreased Nrf2 protein levels after cotreatment with PI3K/Akt inhibitor LY294002. PI3K/Akt is involved in renal cellular processes, including glucose uptake and cell survival, and PI3K/Akt activation showed protective effects in DN [31], and also promoted survival of renal tubular epithelial cells induced by high glucose [32]. This can explain how the protective effect of apigenin on high glucose-induced cellular injury could be abolished by LY294002. This indicates that PI3K/Akt lies upstream of the Nrf2 pathway, which is supported by recent reports that enhanced Nrf2 activation by berberine or zinc can be effectively reduced by PI3K/AKT inhibitors treatment in renal tubular epithelial cells with high glucose [33,34]. This suggests that PI3K/Akt mediates the cellular survival effect of apigenin in renal tubular epithelial cells [35].
Conclusions
Apigenin treatment can attenuate high glucose-induced injury in renal tubular epithelial cells, and can inhibit oxidative stress and inflammation through activation of the Nrf2 signaling pathway. Our study suggests that apigenin could be a safe therapeutic option for DN.
Abbreviations
- ANOVA
analysis of variance
- AREs
antioxidant response elements
- CCK-8
cell counting kit-8
- DN
diabetic nephropathy
- GCLM
glutamate-cysteine ligase modifier subunit
- GFR
glomerular filtration rate
- GSHPX
glutathione peroxidase
- HO-1
heme oxygenase-1
- LDH
lactate dehydrogenase
- MDA
malondialdehyde
- NAC
N-Acetyl-L-cysteine
- NQO1
quinone oxidoreductase-1
- Nrf2
NF-E2-related factor 2
- ROS
reactive oxygen species
- SD
standard deviation
- siRNA
small interfering RNA
- tBHP
tert-butyl hydroperoxide
Footnotes
Source of support: This work was supported by a project of the Discipline Construction of Health and Family Planning Commission in Pudong New District: the Key Discipline of Endocrine and Metabolic Diseases (PWZ2k2017-07); and a pilot project of Traditional Chinese Medicine (TCM) Care Service in Public Hospitals for Comprehensive Reform of Development of Traditional Chinese Medicine in Pudong New District (Nov. 2015)
Conflict of interest
None.
References
- 1.Martínez-Castelao A, Navarro-González JF, Górriz JL, de Alvaro F. The concept and the epidemiology of diabetic nephropathy have changed in recent years. J Clin Med. 2015;4(6):1207–16. doi: 10.3390/jcm4061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waanders F, Visser FW, Gans RO. Current concepts in the management of diabetic nephropathy. Neth J Med. 2013;71(9):448–58. [PubMed] [Google Scholar]
- 3.Wu CZ, Chang LC, Lin YF, et al. Chaperonin-containing t-complex protein-1 subunit β as a possible biomarker for the phase of glomerular hyperfiltration of diabetic nephropathy. Dis Markers. 2015;2015 doi: 10.1155/2015/548101. 548101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant. 2012;27(8):3049–56. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012;32(5):452–62. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 7.Yan LJ. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J Diabetes Res. 2014;2014 doi: 10.1155/2014/137919. 137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–54. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid Redox Signal. 2010;13(11):1665–78. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q, Shen ZY, Meng QT, et al. The role of DJ-1/Nrf2 pathway in the pathogenesis of diabetic nephropathy in rats. Ren Fail. 2016;38(2):294–304. doi: 10.3109/0886022X.2015.1120119. [DOI] [PubMed] [Google Scholar]
- 11.Neves KB, Montezano AC, Alves-Lopes R, et al. Upregulation of Nrf2 and decreased redox signaling contribute to renoprotective effects of chemerin receptor blockade in diabetic mice. Int J Mol Sci. 2018;19(8) doi: 10.3390/ijms19082454. pii: E2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madunić J, Madunić IV, Gajski G, et al. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Jung WW. Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. Int J Mol Med. 2014;33(5):1327–34. doi: 10.3892/ijmm.2014.1666. [DOI] [PubMed] [Google Scholar]
- 14.Arango D, Diosa-Toro M, Rojas-Hernandez LS, et al. Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol Nutr Food Res. 2015;59(4):763–72. doi: 10.1002/mnfr.201400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan S, Liu X, Zhu X, et al. The role of TLR4 on PGC-1α-mediated oxidative stress in tubular cell in diabetic kidney disease. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/6296802. 6296802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju SM, Kang JG, Bae JS, et al. The flavonoid apigenin ameliorates cisplatin-induced nephrotoxicity through reduction of p53 activation and promotion of PI3K/Akt pathway in human renal proximal tubular epithelial cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/186436. 186436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik S, Suchal K, Khan SI, et al. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK- fibronectin pathways. Am J Physiol Renal Physiol. 2017;313(2):F414–22. doi: 10.1152/ajprenal.00393.2016. [DOI] [PubMed] [Google Scholar]
- 18.Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Díaz AG. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018;2018 doi: 10.1155/2018/1875870. 1875870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad A, Manjrekar P, Yadav C, et al. Evaluation of ischemia-modified albumin, malondialdehyde, and advanced oxidative protein products as markers of vascular injury in diabetic nephropathy. Biomark Insights. 2016;11:63–68. doi: 10.4137/BMI.S39053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzegar-Fallah A, Alimoradi H, Asadi F, et al. Tropisetron ameliorates early diabetic nephropathy in streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol. 2015;42(4):361–68. doi: 10.1111/1440-1681.12373. [DOI] [PubMed] [Google Scholar]
- 21.He X, Li C, Wei Z, et al. Protective role of apigenin in cisplatin-induced renal injury. Eur J Pharmacol. 2016;789:215–21. doi: 10.1016/j.ejphar.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wang L, Du Y, et al. Effects of apigenin pretreatment against renal ischemia/reperfusion injury via activation of the JAK2/STAT3 pathway. Biomed Pharmacother. 2017;95:1799–808. doi: 10.1016/j.biopha.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 23.Habib SL, Yadav A, Kidane D, et al. Novel protective mechanism of reducing renal cell damage in diabetes: Activation AMPK by AICAR increased NRF2/OGG1 proteins and reduced oxidative DNA damage. Cell Cycle. 2016;15(22):3048–59. doi: 10.1080/15384101.2016.1231259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao P, Li L, Ji L, et al. Nrf2 ameliorates diabetic nephropathy progression by transcriptional repression of TGFβ1 through interactions with c-Jun and SP1. Biochim Biophys Acta. 2014;1839(11):1110–20. doi: 10.1016/j.bbagrm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Mou Z, Feng Z, Xu Z, et al. Schisandrin B alleviates diabetic nephropathy through suppressing excessive inflammation and oxidative stress. Biochem Biophys Res Commun. 2019;508(1):243–49. doi: 10.1016/j.bbrc.2018.11.128. [DOI] [PubMed] [Google Scholar]
- 26.Landis RC, Quimby KR, Greenidge AR. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr Pharm Des. 2018;24(20):2241–49. doi: 10.2174/1381612824666180716163845. [DOI] [PubMed] [Google Scholar]
- 27.Xie Z, Zhong L, Wu Y, et al. Carnosic acid improves diabetic nephropathy by activating Nrf2/ARE and inhibition of NF-κB pathway. Phytomedicine. 2018;47:161–73. doi: 10.1016/j.phymed.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Zhang X, Zhang C, et al. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-mediated HO-1 expression. Molecules. 2019;24(2) doi: 10.3390/molecules24020247. pii: E247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Li M, Chen W, et al. Apigenin attenuates oxidative injury in ARPE-19 cells thorough activation of Nrf2 pathway. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/4378461. 4378461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sang Y, Zhang F, Wang H, et al. Apigenin exhibits protective effects in a mouse model of d-galactose-induced aging via activating the Nrf2 pathway. Food Funct. 2017;8(6):2331–40. doi: 10.1039/c7fo00037e. [DOI] [PubMed] [Google Scholar]
- 31.Jing D, Bai H, Yin S. Renoprotective effects of emodin against diabetic nephropathy in rat models are mediated via PI3K/Akt/GSK-3β and Bax/caspase-3 signaling pathways. Exp Ther Med. 2017;14(5):5163–69. doi: 10.3892/etm.2017.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Zhu X, Zhang J, Shi J. MicroRNA-25 inhibits high glucose-induced apoptosis in renal tubular epithelial cells via PTEN/AKT pathway. Biomed Pharmacother. 2017;96:471–79. doi: 10.1016/j.biopha.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Liang D, Lian X, et al. Berberine activates Nrf2 nuclear translocation and inhibits apoptosis induced by high glucose in renal tubular epithelial cells through a phosphatidylinositol 3-kinase/Akt-dependent mechanism. Apoptosis. 2016;21(6):721–36. doi: 10.1007/s10495-016-1234-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zhao Y, Chu Q, et al. Zinc modulates high glucose-induced apoptosis by suppressing oxidative stress in renal tubular epithelial cells. Biol Trace Elem Res. 2014;158(2):259–67. doi: 10.1007/s12011-014-9922-x. [DOI] [PubMed] [Google Scholar]
- 35.Chen F, Sun Z, Zhu X, Ma Y. Astilbin inhibits high glucose-induced autophagy and apoptosis through the PI3K/Akt pathway in human proximal tubular epithelial cells. Biomed Pharmacother. 2018;106:1175–81. doi: 10.1016/j.biopha.2018.07.072. [DOI] [PubMed] [Google Scholar]