Abstract
Background:
Data regarding reperfusion strategies, adherence to national guidelines, and in‐hospital mortality in ST‐elevation myocardial infarction (STEMI) patients age ≥80 years are limited. The aim of this study was to determine current reperfusion trends, medical treatment, and in‐hospital mortality during STEMI in older adults.
Hypothesis:
Among patients aged 80 or above presenting with STEMI, adherence to guidelines, length of stay, and in‐hospital mortality would be better in those receiving reperfusion versus those who did not.
Methods:
Using the Get With The Guidelines‐Coronary Artery Disease (GWTG‐CAD) database, we examined care and in‐hospital outcomes of STEMI patients ≥80 years old. Use of evidence‐based therapies and quality measures were analyzed by reperfusion strategies.
Results:
A total of 5339 patients age ≥80 years hospitalized with STEMI were included. Of these, 42.8% (n = 2285) underwent primary percutaneous coronary intervention (PPCI), 4.8% (n = 255) underwent thrombolysis (TL), and 52.4% (n = 2799) received no reperfusion (NR). Patients with NR were more likely to be older, female, have lower body mass index, and higher prevalence of renal insufficiency and heart failure compared with PPCI or TL patients. During the last decade, there was a significant increase in the use of PPCI compared with TL as the main reperfusion strategy in this population. Adjusted in‐hospital mortality in PPCI patients was lower compared with NR patients (odds ratio [OR]: 0.41, 95% confidence interval [CI]: 0.35‐0.49); also, patients undergoing PPCI or TL had lower mortality compared with NR patients (OR: 0.47, 95% CI: 0.40‐0.55).
Conclusions:
Among patients ≥80 years old admitted with STEMI to GWTG‐CAD hospitals, less than half undergo mechanical or pharmacological reperfusion. However, the proportion of patients undergoing PPCI has increased substantially over the 8‐year study period. Patients undergoing PPCI or TL had lower in‐hospital mortality compared with the NR strategy. Clin. Cardiol. 2012 doi: 10.1002/clc.22036
Get With The Guidelines‐Coronary Artery Disease (GWTG‐CAD) is a program of the American Heart Association and is supported in part by an unrestricted educational grant from Merck/Schering‐Plough Pharmaceutical and Pfizer. The analysis of registry data was performed at Duke Clinical Research Institute (Durham, NC), which receives funding from the American Heart Association. The sponsors were not involved in the design, analysis, preparation, review, or approval of this manuscript.
C.P.C.: research grants, Accumetrics, AstraZeneca, GlaxoSmithKline, Merck, Takeda; advisory board, Intekrin Therapeutics; honoraria, Bristol‐Myers Squibb/Sanofi, Novartis, Alnylam; clinical advisor, Pfizer and AstraZeneca; equity, Automedics Medical Systems. A.F.H.: research grants, Johnson & Johnson/Scios, GSK, Medtronic, Novartis; honoraria, Medtronic, Novartis, AstraZeneca. G.C.F.: research grant, NIH; consultant, Novartis. F.P.: research grants, Abbott, Alere, BAS, Brahms, EKR, Nanosphere, The Medicine's Company; consultant, Abbott, Alere, Beckman Coulter, Electrocore, The Medicine's Company; speaker's bureau, Abbott, Alere; ownership interest, Comprehensive Research Associates LLC, Vital Sensors, Emergencies in Medicine LLC. L.S.: chairman, Get With The Guidelines Steering Committee. E.D.P.: research grants, BMS/Sanofi Research, Eli Lilly Research, Merck Research, Johnson & Johnson. D.L.B.: advisory board, Medscape Cardiology; board of directors, Boston VA Research Institute, Society of Chest Pain Centers; chair, American Heart Association Get With The Guidelines Science Subcommittee; honoraria, American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); research grants, Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; unfunded research, FlowCo, PLx Pharma, Takeda. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Older adults (defined as ≥80 years old) are a rapidly growing population in the United States,1 and coronary artery disease (CAD) is, similar to younger patients, the most common cause of mortality among this age strata.2 In this population, acute myocardial infarction (AMI) carries a worse prognosis as patients' mean average survival post‐AMI ranges between 2 and 6 years.3
Additionally, older patients have been under‐represented in AMI clinical trials. From 583 randomized studies published between 1991 and 2000, just 9% of patients were 75 years or older.4 This is particularly important as the main stay of therapy for ST‐elevation acute myocardial infarction (STEMI)5 includes early reperfusion, which has particular risks in older adults such as intracranial hemorrhage during thrombolysis (TL).6
Data regarding contemporary reperfusion strategies, adherence to national guidelines, and in‐hospital mortality in STEMI patients age ≥80 years are limited. Therefore, we performed a comprehensive analysis of the care patterns during STEMI in older adults using a national database from 2001 to 2009. The differences in the use of evidence‐based reperfusion strategies, recent revascularization trends, adherence to pharmacological guidelines, and in‐hospital death were analyzed based on the reperfusion strategy used on admission.
Methods
The data were extracted form the American Heart Association's Get With The Guidelines‐Coronary Artery Disease (GWTG‐CAD) registry. Such initiatives, which promote strategies to increase adherence to published guidelines for the management of AMI,7, 8, 9 took place in several hospitals in the United States. The GWTG‐CAD program encompasses didactic information, workshops, patient follow‐up, and an online Patient management tool (Outcome Sciences, Inc., Cambridge, MA). All participating hospitals were instructed to provide data without financial compensation. Data were collected in each center by experienced hospital staff. Cases were found after clinical identification of patients with STEMI according to the National Center for Health and Statistics International Classification of Diseases, Ninth Revision identification of CAD diagnoses. The Duke Clinical Research Institute (DCRI) has an agreement to analyze the aggregate data for research purposes and served as the data analysis center.
The patient enrollment took place between January 2000 to March 2009 in 438 medical centers in the United States. A total of 280 155 individuals were potentially eligible to be included. From the initial cohort, we limited our patient population to those age≥80 (n = 60 541) admitted with STEMI (n = 7532), including patients with new left bundle branch block (LBBB). Patients transferred from a different hospital outside the GWTG‐CAD network (n = 1541) or from an acute care facility (n = 442), and those with incomplete hospital reperfusion data (n = 210) were excluded. The final cohort included 5339 patients from 319 hospitals. Reperfusion times (including door‐to‐needle [DTN] and door‐to‐balloon [DTB] times) were reported in all patients.
The main reperfusion strategy, stratified as primary percutaneous coronary intervention (PPCI), TL, or no reperfusion (NR), was the primary independent variable. The primary outcomes of our analysis were clinical performance measures, reperfusion times, and length of stay. Performance measures included strategies known to improve the outcome in patients with STEMI.5 These include aspirin (ASA) and β‐blocker (BB) use on admission; reperfusion times; and ASA, BB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB) and statins use at discharge on those who survived. Measures are applied only to eligible patients without documented contraindications or other exceptions to treatment. Mortality was also analyzed as an outcome in a univariate fashion. According to national guidelines, DTN time goal was defined as ≤30 minutes and DTB time goal as ≤90 minutes.5 All invasive procedures due to STEMI, including cardiac catheterization, percutaneous coronary intervention (PCI) other than PPCI, and coronary artery bypass graft (CABG) were registered. Also, time trends regarding reperfusion strategies were done during the last decade.
Statistical Analysis
Baseline characteristics, procedure use, and clinical outcomes were compared across the 3 groups specified above (PPCI, TL, and NR). Continuous variables are presented as means with standard deviation or medians with interquartile percentiles; categorical variables are expressed as frequencies with percentages. To test for independence of patient baseline characteristics, in‐hospital care patterns, outcomes with respect to reperfusion strategy group,χ 2 rank‐based group means score statistics (equivalent to Kruskal‐Wallis test), and χ 2 1 df rank correlation statistics were used for all continuous/ordinal variables. The association between reperfusion strategy and predefined outcomes, the compliance with individual quality of care measures, the summary defect‐free care measure (coded 0, 1 for each patient), and mortality were examined using multivariable logistic regression models. The Generalized estimating equation method was used to account for within‐hospital clustering. Prespecified potential confounders adjusted in the models were: age, gender, race, hypertension, prior AMI, high lipids, peripheral vascular disease, heart failure, history of stroke/transient ischemic attack, diabetes, chronic obstructive pulmonary disease, and renal insufficiency. The association of reperfusion strategy with the predefined outcomes was presented using odds ratios (ORs) with 95% confidence intervals (CIs). Changes in patterns of reperfusion were analyzed from 2001 to 2009. A probability value of <0.05 was considered statistically significant for all tests.
All analyses were performed by the DCRI with the Statistical Analysis System (SAS) software version 9.2 (SAS Institute, Cary, NC) and Outcomes Sciences, Inc. served as the data collection and coordination center. The authors had full access to the data and take responsibility for the data's integrity.
Results
Patient Characteristics
The study population included 5339 STEMI patients age ≥80 years. From these, 42.8% (2285) underwent PPCI, 4.8% (255) underwent TL, and 52.4% (2799) had NR. Baseline characteristics are presented in Table 1. Patients in the NR group were more likely to be older, female, and have lower body mass index (BMI) compared with patients who did undergo PPCI or TL. On clinical presentation, NR patients had lower BMI, diastolic blood pressure, low‐density lipoprotein levels, left ventricular ejection fraction, and higher heart rate compared to those who underwent early reperfusion.
Table 1.
Baseline Characteristics Stratified by Reperfusion Strategy
| Description | Overall [n = 5339] | PPCI [n = 2285] | Thrombolysis [n = 255] | None [n = 2799] | P Value |
|---|---|---|---|---|---|
| Age, mean ± SD, y | 85.6 ± 4.5 | 84.3 ± 3.7 | 85.4 ± 4.3 | 86.6 ± 4.8 | <0.001 |
| Men, no (%) | 2120 (39.7) | 959 (42.0) | 109 (42.8) | 1052 (37.6) | 0.005 |
| Race, no (%) | <0.001 | ||||
| White | 4294 (80.4) | 1914 (83.8) | 192 (75.3) | 2188 (78.2) | |
| Hispanic | 248 (4.7) | 104 (4.6) | 24 (9.4) | 120 (4.3) | |
| Black | 219 (4.1) | 97 (4.3) | 10 (3.9) | 112 (4.0) | |
| Asian | 226 (4.2) | 43 (1.9) | 20 (7.8) | 163 (5.8) | |
| American Indian or Alaska Native | 3 (0.1) | 30.1 (0.13) | 0 (0.0) | 40.1 (0.14) | |
| Native Hawaiian or Pacific Islander | 6 (0.1) | 3 (0.1) | 0 (0.0) | 3 (0.1) | |
| UTD | 224 (4.2) | 82 (3.6) | 8 (3.1) | 134 (4.8) | |
| Health insurance, no (%) | <0.001 | ||||
| Medicare | 2717 (50.9) | 1140 (49.9) | 106 (41.6) | 1471 (52.6) | |
| Medicaid | 240 (4.5) | 68 (3.0) | 15 (5.9) | 157 (5.6) | |
| Other | 1635 (30.6) | 779 (34) | 82 (32.2) | 774 (27.7) | |
| No insurance | 127 (2.4) | 66 (2.9) | 9 (3.5) | 52 (1.9) | |
| Medical history, no (%) | |||||
| Atrial fibrillation | 74 (14.7) | 260(11.9) | 35 (14.6) | 450 (17.1) | <0.001 |
| Atrial flutter | 18 (0.4) | 4 (0.2) | 1 (0.4) | 1 (0.5) | 0.193 |
| TIA/stroke | 67 (13.3) | 217 (9.9) | 22 (9.2) | 433 (16.4) | <0.001 |
| Chronic obstructive pulmonary disease | 667 (13.2) | 239 (10.9) | 29 (12.1) | 399 (15.1) | <0.001 |
| Coronary artery disease | 707 (14.0) | 318 (14.5) | 13 (5.4) | 376 (14.3) | <0.001 |
| Previous MI | 956 (18.9) | 357 (16.3) | 39 (16.3) | 560 (21.2) | <0.001 |
| Prior CABG | 79 (1.6) | 38 (1.7) | 0 (0.0) | 41 (1.6) | 0.121 |
| Diabetes/insulin treated | 136 (2.7) | 53 (2.4) | 2 (0.8) | 81 (3.1) | 0.073 |
| Diabetes/not insulin treated | 326 (6.4) | 152 (6.9) | 11 (4.6) | 163 (6.2) | 0.282 |
| Heart failure | 1110 (21.9) | 256 (11.7) | 51 (21.3) | 803 (30.4) | <0.001 |
| Hypertension | 3652 (72.1) | 1584 (72.3) | 166 (69.5) | 1902 (72.1) | 0.654 |
| Hyperlipidemia | 1742 (34.4) | 886 (40.4) | 61 (25.5) | 795 (30.1) | <0.001 |
| Peripheral vascular disease | 450 (8.9) | 186 (8.5) | 13 (5.4) | 251 (9.5) | 0.073 |
| Renal insufficiency | 627 (12.4) | 169 (7.7) | 27 (11.3) | 431 (16.3) | <0.001 |
| Smoking | 354 (6.6) | 162 (7.1) | 21 (8.2) | 171 (6.1) | <0.001 |
| Clinical characteristics, mean ± SD | |||||
| BMI | 25.2 ± 5.4 | 25.7 ± 5.2 | 25.5 ± 6.7 | 24.5 ± 5.4 | <0.001 |
| Systolic BP, mm Hg | 132.4 ± 31.3 | 133.9 ± 31.5 | 141.2 ± 28.5 | 130.5 ± 31.1 | 0.08 |
| Diastolic BP, mm Hg | 71.5 ± 18.6 | 73.0 ± 18.6 | 75.0 ± 14.7 | 69.9 ± 18.7 | <0.001 |
| LDL, mg/dL | 95.6 ± 36.7 | 96.6. ± 38.4 | 103.9 ± 36.2 | 93.4 ± 34.4 | <0.001 |
| Ejection fraction, mg/dL | 42.8 ± 14.6 | 45.5 ± 14.1 | 42.7 ± 13.9 | 40.4 ± 14.8 | <0.001 |
Abbreviations: BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; LDL, low‐density lipoprotein; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention; SD, standard deviation; TIA, transient ischemic attack; UTD, unable to define.
Clinical Performance Measures
Although the use of ASA and BB within the first 24 hours was high overall, patients who underwent PPCI were more likely to be treated with these guideline‐recommended therapies compared with those who had TL and NR (Table 2). This difference was still present after multivariate adjustment (Table 3). The median DTB time in those undergoing PPCI was 90 minutes (interquartile range [IQR]: 63–135), and the percentage of patients achieving optimal DTB time was 36.2%. The median DTN time in those undergoing TL was 54.5 minutes (IQR: 28–83), and the percentage of patients achieving optimal DTN time was 21.9% (Table 2).
Table 2.
Performance Measures, Quality Measures, and Mortality Stratified by Reperfusion Strategy
| Measurement/Treatment | Overall [n = 5339] | PPCI [n = 2285] | Thrombolysis [n = 255] | None [n = 2799] | P Value |
|---|---|---|---|---|---|
| Early medical therapy, no. % | |||||
| Aspirin within <24 hours | 4365 (93.4) | 2026 (96.1) | 209 (94.9) | 2130 (90.9) | <0.001 |
| β‐Blockers within <24 hours | 3526 (86.9) | 1619 (90.4) | 177 (86.3) | 1730 (83.9) | <0.001 |
| Timelines of reperfusion | |||||
| DTN ≤ 30 minutes, % | N/A | N/A | 56 (21.9) | N/A | |
| DTB ≤ 90 minutes, % | N/A | 828 (36.2) | N/A | N/A | |
| DTN time, median (25th–75th), min | N/A | N/A | 54.5 (28.0–83.0) | N/A | |
| DTB time, median (25th–75th), min | N/A | 90.0 (63.0–135.0) | N/A | N/A | |
| Surgical revascularization, no. % | |||||
| CABG | 208 (4.8) | 33 (1.4) | 7 (4.7) | 168 (8.8) | <0.001 |
| Discharge medications, no. % | |||||
| ACEI/ARB in documented LVSD | 880 (78.9) | 390 (85.3) | 35 (76.1) | 455 (74.4) | <0.001 |
| ASA | 3672 (95.0) | 1889 (97.8) | 176 (98.9) | 1607 (91.5) | <0.001 |
| β‐Blockers | 3529 (94.3) | 1778 (96.8) | 168 (94.3) | 1583 (91.5) | <0.001 |
| Statins | 3109 (75.9) | 1735 (87.7) | 141 (71.2) | 1233 (64.3) | <0.001 |
| Clopidogrel | 2509 (67.8) | 1665 (91.7) | 127 (70.6) | 717 (42.1) | <0.001 |
| Other QCA measures, no. % | |||||
| Weight management | 1371 (77.8) | 830 (84.4) | 65 (69.2) | 476 (69.5) | <0.001 |
| Diabetes treatment | 291 (82.4) | 155 (86.1) | 9 (81.8) | 127 (78.4) | 0.1739 |
| Diabetes teaching | 29 (8.2) | 12 (6.7) | 1 (9.1) | 16 (9.9) | 0.5562 |
| Smoking cessation | 228 (80.9) | 129 (87.8) | 12 (66.7) | 87 (74.7) | 0.0067 |
| Blood pressure control | 2935 (79.1) | 1438 (81.1) | 148 (82.7) | 1349 (76.7) | 0.0026 |
| Cardiac rehabilitation | 3242 (77.1) | 1741 (86.2) | 137 (68.8) | 1364 (68.7) | <0.001 |
| In‐hospital mortality, no. % | 929 (17.4) | 226 (9.9) | 49 (19.2) | 654 (23.4) | <0.001 |
| LOS, median (25th–75th), d | 5 (3‐7) | 4 (3‐7) | 5 (3‐8) | 5 (3‐8) | 0.0045 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CABG, coronary artery bypass graft; DTB, door‐to‐balloon; DTN, door‐to‐needle; LOS, length of stay; LVSD, left ventricular systolic dysfunction; N/A, not applicable; QCA, quality control assessment.
Table 3.
Adjusted Odds Ratios for Clinical Performance Measures, Quality Measures, and In‐Hospital Death Comparing Primary Percutaneous Coronary Intervention vs No Reperfusion
| Measurement Treatment | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)a | P Value |
|---|---|---|---|---|
| Early medical treatment | ||||
| Aspirin within <24 hours | 1.98 (1.45‐2.70) | <0.0001 | 1.90 (1.40‐2.57) | <0.0001 |
| β‐blockers within <24 hours | 1.45 (1.14‐1.84) | 0.002 | 1.35(1.07‐1.71) | 0.0128 |
| Discharge medications | ||||
| ACEI/ARB | 1.82 (1.40‐2.38) | <0.0001 | 1.57 (1.14‐2.15) | 0.0052 |
| ASA | 3.37 (2.44‐4.64) | <0.0001 | 3.04 (2.17‐4.27) | <0.0001 |
| β‐Blockers | 2.26 (1.68‐3.06) | <0.0001 | 2.01 (1.47‐2.74) | <0.0001 |
| Statins | 3.17 (2.66‐3.78) | <0.0001 | 2.71 (2.27‐3.24) | <0.0001 |
| Clopidogrel | 15.1 (11.63‐19.60) | <0.0001 | 14.76 (11.36‐19.19) | <0.0001 |
| Other QCA measures | ||||
| Weight management | 1.75 (1.42‐2.15) | <0.0001 | 1.64 (1.34‐2.02) | <0.0001 |
| Diabetes treatment | 1.67 (0.96‐2.91) | 0.0680 | 1.46 (0.83‐2.54) | 0.1870 |
| Diabetes teaching | 0.72 (0.32‐1.61) | 0.4225 | 0.64 (0.26‐1.54) | 0.3179 |
| Smoking cessation | 2.43 (1.24‐4.76) | 0.0094 | 2.63 (1.39‐4.96) | 0.0029 |
| Blood pressure control | 1.23 (1.05‐1.45) | 0.0113 | 1.19 (1.01‐1.41) | 0.0389 |
| Cardiac rehabilitation | 2.01 (1.69‐2.39) | <0.0001 | 1.82 (1.53‐2.16) | <0.0001 |
| Mortality | 0.36 (0.31‐0.42) | <0.0001 | 0.41 (0.35‐0.49) | <0.0001 |
| LOS >4 days | 0.77 (0.69‐0.86) | <0.0001 | 0.77 (0.68‐0.86) | <0.0001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; ASA, aspirin; CI, confidence interval; LOS, length of stay; OR, odds ratio; QCA, quality control assessment.
Adjusted for age (continuous), gender, race (whites vs others), medical history (chronic obstructive pulmonary disease or asthma, cerebrovascular accident/transient ischemic attack, diabetes/insulin, diabetes/not insulin, heart failure, hyperlipidemia, hypertension, peripheral vascular disease [PVD], prior myocardial infarction, renal failure [RF]).
For smoking cessation, the adjustment was only for age (continuous), gender, race (whites vs others), history of RF.
In‐Hospital Mortality
In‐hospital mortality of patients admitted with STEMI (Table 2) who had early reperfusion was lower compared with NR patients (PPCI: 9.9% vs TL: 19.2% vs NR: 23.4%; P< 0.001). Further univariate comparisons of PPCI vs NR and PPCI or TL vs NR showed lower mortality and shorter length of stay (LOS) as shown in Tables 3 and 4. Adjusted analyses for LOS and in‐hospital mortality of patients undergoing PPCI or TL also showed statistically significant reduction compared with patients undergoing no reperfusion (Tables 3 and 4) after adjustment for measured confounders.
Table 4.
Adjusted Odds Ratios for Clinical Performance Measures, Quality Measures, and In‐Hospital Death Comparing Primary Percutaneous Coronary Intervention or Thrombolysis vs No Reperfusion
| Measurement Treatment | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)a | P Value |
|---|---|---|---|---|
| Early medical treatment | ||||
| Aspirin within <24 hours | 2.05 (1.49‐2.81) | <0.0001 | 1.96 (1.44‐2.65) | <0.0001 |
| β‐Blockers within <24 hours | 1.45 (1.15‐1.83) | 0.0015 | 1.37 (1.09‐1.71) | 0.0070 |
| Discharge medications | ||||
| ACEI/ARB | 1.71 (1.33‐2.21) | <0.0001 | 1.47 (1.09‐1.99) | 0.0129 |
| ASA | 3.63 (2.62‐5.03) | <0.0001 | 3.31 (2.36‐4.64) | <0.0001 |
| β‐Blockers | 2.15 (1.61‐2.89) | <0.0001 | 1.93 (1.42‐2.61) | <0.0001 |
| Statins | 2.63 (1.75‐3.95) | <0.0001 | 2.47 (1.65‐3.71) | <0.0001 |
| Clopidogrel | 11.27 (8.94‐14.22) | <0.0001 | 10.86 (8.56‐13.79) | <0.0001 |
| Other QCA measures | ||||
| Weight management | 1.60 (1.31‐1.95) | <0.0001 | 1.49 (1.22‐1.82) | <0.0001 |
| Diabetes treatment | 1.64 (0.96‐2.80) | 0.0728 | 1.42 (0.83‐2.44) | 0.2053 |
| Diabetes teaching | 0.74 (0.31‐1.76) | 0.4942 | 0.65 (0.25‐1.64) | 0.3582 |
| Smoking cessation | 1.96 (1.05‐3.68) | 0.0358 | 2.05 (1.11‐3.78) | 0.0209 |
| Blood pressure control | 1.26 (1.07‐1.48) | 0.0058 | 1.21 (1.03‐1.43) | 0.0231 |
| Cardiac rehabilitation | 1.83 (1.56‐2.15) | <0.0001 | 1.67 (1.43‐1.95) | <0.0001 |
| Main outcomes | ||||
| Mortality | 0.40 (0.35‐0.47) | <0.0001 | 0.47 (0.40‐0.55) | <0.0001 |
| LOS >4 days | 0.81 (0.72‐0.90) | <0.0001 | 0.81 (0.73‐0.91) | 0.0003 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; ASA, aspirin; CI, confidence interval; LOS, length of stay; OR, odds ratio; QCA, quality control assessment.
Adjusted for age (continuous), gender, race (whites vs others), medical history (chronic obstructive pulmonary disease or asthma, cerebrovascular accident/transient ischemic attack, diabetes/insulin, diabetes/not insulin, heart failure, hyperlipidemia, hypertension, peripheral vascular disease [PVD], prior myocardial infarction, renal failure [RF]).
For smoking cessation, the adjustment was only for age (continuous), gender, race (whites vs others), history of RF.
Discharge Clinical Performance/Quality Measures
The rate of patients discharged on ACEI/ARB with left ventricular systolic dysfunction, ASA, BB, clopidogrel, and statins post‐STEMI was lower in patients in the NR arm compared with those who had early reperfusion (Table 2). The lowest adherence to routine post‐STEMI medications was observed with statins, particularly those in the NR group (64.3% compared with 87.7% in PPCI patients). After multivariate adjustment, these differences remained statistically significant, as shown in Tables 3 and 4. Other quality‐of‐care variables such as weight management, smoking cessation, blood pressure control, and cardiac rehabilitation were also higher when comparing PPCI and TL patients vs NR patients in univariate and multivariate analyses. Patients who did not undergo early revascularization were more likely to have CABG compared with those who did undergo early reperfusion (PCI: 1.4% vs TL: 4.7% vs NR 8.8%; P< 0.001).
Temporal Trends
These analyses were done for percentage of patients age ≥80 years undergoing PPCI vs TL vs NR, including those with and without contraindications for either type of reperfusion strategy, and were performed on a year‐by‐year basis from 2001 to 2009, as shown in the Figure. It was noticed that during the last 8 years the percentage of patients having PPCI as the primary reperfusion strategy for STEMI increased from 26.7% in 2001 to 53.3% in 2009. At the same time, the number of patients having TL and NR has decreased from 20.0% to 1.8% and 53.3% to 44.9%, respectively, within the same time frame (P for trend < 0.0001).
Figure 1.
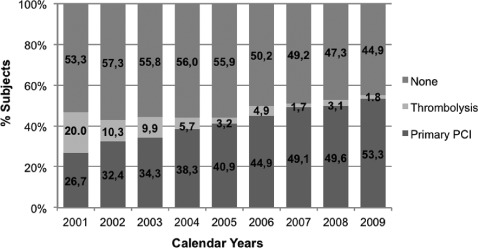
Reperfusion strategy by calendar years from 2001 to 2009. P for trend <0.0001. Abbreviation: PCI, percutaneous coronary intervention.
Discussion
In the present study based on a large national database, less than half of STEMI patients ≥80 years old received reperfusion therapy with either PPCI or TL. The use of evidence‐based medical therapies for these patients was high, particularly in those receiving mechanical or pharmacological reperfusion. The use of PPCI as the reperfusion strategy provided to patients age 80 years and above has substantially increased during the last decade compared with TL. Finally, the use of PPCI or TL was associated with lower in‐hospital death and LOS compared with patients who had NR.
As the population of older adults continues to increase, acute coronary events are of paramount importance as this is the top etiology for mortality in this age group.2 In these older patients, STEMI has particular epidemiological characteristics that frequently make them ineligible for early reperfusion including late presentation, atypical electrocardiographic features (up to one‐third of these patients present with LBBB vs typical ST‐elevation), and high prevalence of contraindications for reperfusion.10, 11 Also, there may be a common belief regarding the applicability of national guidelines5, 12 for acute reperfusion during STEMI to patients age ≥80 years, due to the low number of patients in this age group who have participated in landmark AMI trials.4, 13 For instance, the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology and American Heart Association Guidelines) investigators found that those patients who were ineligible to receive reperfusion during STEMI were older compared to those who were eligible in a large national database.14 In addition, there was a significant 13% increased risk of not receiving reperfusion per every 10‐year increase in age. In this study, inability to receive reperfusion therapy without a clear contraindication resulted in higher odds of death and occurred in 7% of the total population across all ages. Data from the Thrombolysis In Myocardial Infarction 9B trial showed a similar trend in terms of low use of primary reperfusion for patients in a similar age strata; among those patients eligible who were not enrolled in the trial eligible for reperfusion, only 60% had TL and 12% had PPCI.15 Our study determined the dramatic low rate (47.6%) of older patients receiving reperfusion during STEMI, although precise determination of individuals eligible for such therapies is not possible with our database. However, such a gap should be explored further as many older patients may be eligible for early reperfusion therapy during STEMI, and age per se may be the only reason for withholding these therapies, which can potentially impact short‐ and long‐term mortality.
In this study, the used of TL was very low and decreased over time. The high incidence of TL‐related complications in older adults, such as intracranial hemorrhage16 compared with younger patients, may be an important factor to explain the low rate of use of TL therapy seen in this and prior cohorts.10, 17 Prior STEMI studies have stratified patients by age analyzing the effects of TL compared with placebo. In particular, an age subgroup analysis from the Fibrinolytic Therapies Trialists Group (FTT)18 analyzed outcomes of 58600 STEMI patients from 9 randomized trials. In that analysis, there was a benefit of TL vs placebo in short‐term mortality in the overall population, although such benefit was not seen in patients ≥75 years old. Prior nonrandomized data have shown conflicting results, with the latest FTT report indicating lack of benefit from TL in older patients during STEMI and even potential detrimental effects.11, 19, 20 These results raise an ongoing debate regarding the benefit of TL in older adults presenting with STEMI.10 Most importantly, data regarding the benefit of TL vs placebo in this particular age stratum are derived only from subgroup analyses of randomized trials and observational studies.
As opposed to TL during STEMI in older adults, there is a limited amount of prospective data comparing mechanical vs pharmacological reperfusion. In a small randomized trial, de Boer et al compared the outcome of older adults during STEMI using PPCI vs TL (mean age, 80 and 81, respectively).21 The study was terminated early due to an overwhelming increase in death, stroke, and reinfarction at 30 days in patients undergoing TL. The differences in outcomes were even more impressive considering the relatively small sample size allocated to each treatment (PPCI: 46 patients and TL: 41 patients). The second study by Goldenberg et al22 was a prospective nonrandomized comparison of PPCI vs TL in patients >70 years old in 2 hospitals, each with a preference for mechanical vs pharmacologic reperfusion. The in‐hospital and 6‐month mortalities in both groups were similar, but the composite outcome of death, need for revascularization, or recurrent AMI was higher in the TL group, driven by need of revascularization. More recently, the TRIANA (Tratamiento del Infarto Agudo de Miocardio en Ancianos) investigators compared similar strategies (PPCI vs TL) in STEMI patients ≥75 years old.23 The trial was stopped early due to slow enrollment, and only 266 patients were included. There was no significant difference in death alone or major adverse events (defined as death, reinfarction, or disabling stroke) between the 2 groups. However, the authors also performed a meta‐analysis with prior studies testing the same strategies in similar populations. After pooling all the available data, there was a 36% decrease in the odds of major events in patients undergoing PPCI vs TL (OR: 0.64, 95% CI: 0.45‐0.91) and a nonsignificant trend toward decrease in mortality favoring PPCI (OR: 0.74, 95% CI: 0.74‐1.13). Also, subgroup analyses of multiple randomized trials24, 25 and large observational studies26, 27, 28 have shown consistent benefit of PPCI over TL. Our study demonstrated the overwhelming increase in the number of patients undergoing PPCI among hospitals enrolled in the GWTG‐CAD database, acknowledging that these time‐trend analyses include a combination of patients eligible for reperfusion and also those with contraindications. For instance, in 2001 only 26.7% of patients underwent PPCI compared with 53.3% in 2009. Also, the number of patients receiving some type of reperfusion increased from 46.7% in 2001 to 55% in 2009. Despite the paucity of dedicated trials examining exclusively older adults, the existent data reflect the relative safety profile of PPCI in this population, especially when considering the bleeding risks associated with TL.21, 22 In a prior analysis from the GWTG‐CAD database, older adults were found to have higher mortality compared with younger adults when pooling all acute coronary syndromes.29 Although the present 5339 patients were part of that analysis, the current study focuses only on STEMI patients and compares their adherence to guidelines and outcomes depending on the revascularization method during the index event.
The overall use of medications on admission and discharge, which have demonstrated benefit during STEMI, was high in older adults. However, patients receiving PPCI or TL had higher rates of use of ASA and BB on admission and were discharged more often on ASA, BB, ACEI/ARB, clopidogrel, and statins compared with NR patients. This pattern was consistent in univariate and multivariate analyses. Overall, differences in the prescription of all these medications can be due to sicker patients in the NR arm resulting in unmeasured confounding not amendable to multivariate adjustment, or due to variability in blood pressure and heart rate during the STEMI hospitalization. These variables may have played a role in the clinical judgment to prescribe or withhold these medications. However, differences in these gaps need to be explored further, as it may be related to local practice patterns in which lower use of medications known to decrease mortality during STEMI is associated with lower use of early reperfusion. Also, it was noticed that the percentage of patients achieving optimal DTB times during PPCI (36.2%) was higher compared with the percentage of patients with optimal DTN times during TL (21.9%). Also, the median DTB in our cohort was exactly 90.0 minutes, which is defined as optimal compared with the median DTN of 54.5 minutes, which is almost twice the duration recommended by current national guidelines.5
Our analysis has multiple strengths including the enrollment of a large and diverse population treated in many medical centers in the United States. Furthermore, it constitutes a detailed depiction of presentation and treatment patterns of STEMI in older adults with strict inclusion criteria and a high degree of quality control assessment. The index diagnosis was confirmed both on admission and discharge to improve accuracy. However, there are a number of inherent limitations. There may be residual measured and unmeasured confounding in the regression analyses, despite recording multiple comorbidities and checking for several clinical characteristics on presentation. In particular, the observations about lower mortality with reperfusion may be due, in whole or in part, to residual measured and unmeasured confounding. Also, the hospitals participating in the GWTG‐CAD database are self‐selected and may not represent the pattern of general practice. Finally, we cannot comment on the clinical situation at presentation that would potentially pose ethical conflicts and reasonably obviate candidacy for invasive or lytic therapy (eg, severe dementia, cancer, or other major comorbidities) and thus explain the low rate of reperfusion therapy in older adults. The lack of this particular clinical information may explain why offering or withholding reperfusion therapy is more appropriate depending on the baseline characteristics of each specific patient. Finally, all clinical data were taken from medical records and are dependent on the quality and accuracy of chart abstraction.
Conclusion
Among adults age 80 years and older admitted with STEMI to medical centers enrolled in the GWTG‐CAD program, less than half the patients received reperfusion therapy. However, the portion of patients undergoing PPCI has increased dramatically during the 8‐year study period. Medications that have demonstrated benefit during STEMI are used more often in patients undergoing PPCI or TL compared with NR patients. The remaining treatment gaps in reperfusion and other evidence‐based treatments represent potential opportunities for improving quality of care and outcomes for older adults with STEMI. The hypotheses generated by the present study, suggesting that PPCI or TL may be associated with lower in‐hospital death and LOS in older patients, need to be tested in a prospective randomized trial.
References
- 1. The state of aging and health in America report 2007. Centers for Disease Control and Prevention and The Merck Company Foundation. Whitehouse Station, NJ: The Merck Company Foundation; 2007.. http://www.cdc.gov/aging/pdf/saha_2007.pdf.
- 2. National Center for Health Statistics. U.S. deaths down sharply in 2006.. December 21, 2011. http://www.cdc.gov/media/pressrel/2008/r080611.htm.
- 3. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. [DOI] [PubMed] [Google Scholar]
- 4. Lee PY, Alexander KP, Hammill BG, et al. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286:708–713. [DOI] [PubMed] [Google Scholar]
- 5. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction; A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1–E211. [DOI] [PubMed] [Google Scholar]
- 6. White HD, Barbash GI, Califf RM, et al. Age and outcome with contemporary thrombolytic therapy. Results from the GUSTO‐I trial. Global Utilization of Streptokinase and TPA for Occluded coronary arteries trial. Circulation. 1996;94:1826–1833. [DOI] [PubMed] [Google Scholar]
- 7. LaBresh KA, Ellrodt AG, Gliklich R, et al. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164:203–209. [DOI] [PubMed] [Google Scholar]
- 8. LaBresh KA, Gliklich R, Liljestrand J, et al. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29:539–550. [DOI] [PubMed] [Google Scholar]
- 9. Hong Y, Labresh KA. Overview of the American Heart Association “Get With the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. [DOI] [PubMed] [Google Scholar]
- 10. Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part II: ST‐segment‐elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. [DOI] [PubMed] [Google Scholar]
- 11. Berger AK, Radford MJ, Wang Y, et al. Thrombolytic therapy in older patients. J Am Coll Cardiol. 2000;36:366–374. [DOI] [PubMed] [Google Scholar]
- 12. Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. [DOI] [PubMed] [Google Scholar]
- 13. Bhatt DL, Roe MT, Peterson ED, et al. Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292: 2096–2104. [DOI] [PubMed] [Google Scholar]
- 14. Gharacholou SM, Alexander KP, Chen AY, et al. Implications and reasons for the lack of use of reperfusion therapy in patients with ST‐segment elevation myocardial infarction: findings from the CRUSADE initiative. Am Heart J. 2010;159:757–763. [DOI] [PubMed] [Google Scholar]
- 15. Bahit MC, Cannon CP, Antman EM, et al. Direct comparison of characteristics, treatment, and outcomes of patients enrolled versus patients not enrolled in a clinical trial at centers participating in the TIMI 9 Trial and TIMI 9 Registry. Am Heart J. 2003;145: 109–117. [DOI] [PubMed] [Google Scholar]
- 16. Brass LM, Lichtman JH, Wang Y, et al. Intracranial hemorrhage associated with thrombolytic therapy for elderly patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. Stroke. 2000;31:1802–1811. [DOI] [PubMed] [Google Scholar]
- 17. Rojas CA, El‐Sherief A, Medina HM, et al. Embryology and developmental defects of the interatrial septum. AJR Am J Roentgenol. 2010;195:1100–1104. [DOI] [PubMed] [Google Scholar]
- 18. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 19. Sinnaeve PR, Huang Y, Bogaerts K, et al. Age, outcomes, and treatment effects of fibrinolytic and antithrombotic combinations: findings from Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT)‐3 and ASSENT‐3 PLUS. Am Heart J. 2006;152:684 e1–e9. [DOI] [PubMed] [Google Scholar]
- 20. Thiemann DR, Coresh J, Schulman SP, et al. Lack of benefit for intravenous thrombolysis in patients with myocardial infarction who are older than 75 years. Circulation. 2000;101:2239–2246. [DOI] [PubMed] [Google Scholar]
- 21. de Boer MJ, Ottervanger JP, van't Hof AW, et al. Reperfusion therapy in elderly patients with acute myocardial infarction: a randomized comparison of primary angioplasty and thrombolytic therapy. J Am Coll Cardiol. 2002;39:1723–1728. [DOI] [PubMed] [Google Scholar]
- 22. Goldenberg I, Matetzky S, Halkin A, et al. Primary angioplasty with routine stenting compared with thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2003;145:862–867. [DOI] [PubMed] [Google Scholar]
- 23. Bueno H, Betriu A, Heras M, et al. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. 2011;32:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grines C, Patel A, Zijlstra F, et al. Primary coronary angioplasty compared with intravenous thrombolytic therapy for acute myocardial infarction: six‐month follow up and analysis of individual patient data from randomized trials. Am Heart J. 2003; 145:47–57. [DOI] [PubMed] [Google Scholar]
- 25. Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in‐hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–788. [DOI] [PubMed] [Google Scholar]
- 26. Mehta RH, Sadiq I, Goldberg RJ, et al. Effectiveness of primary percutaneous coronary intervention compared with that of thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2004;147:253–259. [DOI] [PubMed] [Google Scholar]
- 27. Shelton RJ, Crean AM, Somers K, et al. Real‐world outcome from ST elevation myocardial infarction in the very elderly before and after the introduction of a 24/7 primary percutaneous coronary intervention service. Am Heart J. 2010;159:956–963. [DOI] [PubMed] [Google Scholar]
- 28. Berger AK, Schulman KA, Gersh BJ, et al. Primary coronary angioplasty vs thrombolysis for the management of acute myocardial infarction in elderly patients. JAMA. 1999;282: 341–348. [DOI] [PubMed] [Google Scholar]
- 29. Medina HM, Cannon CP, Zhao X, et al. Quality of acute myocardial infarction care and outcomes in 33,997 patients aged 80 years or older: Findings from Get With The Guidelines‐Coronary Artery Disease (GWTG‐CAD). Am Heart J. 2011;162: 283–290.e2. [DOI] [PubMed] [Google Scholar]


