Abstract
Background:
Tako‐tsubo cardiomyopathy (TTC) is typically triggered by an acute emotional or physical stress event. The aim of this study was to investigate the impact of stressor patterns on clinical features, laboratory parameters, and electrocardiographic and echocardiographic findings in patients with TTC.
Hypothesis:
Clinical features are different according to stressor patterns.
Methods:
Of 137 patients enrolled from the TTC registry database, 14 patients had emotional triggers (E group), 96 had physical triggers (P group), and 27 had no triggers (N group).
Results:
Most clinical presentations and in‐hospital courses were similar among the groups. However, the E group had a higher prevalence of chest pain (P = 0.006) and palpitation (P = 0.006), whereas the P group had a higher prevalence of cardiogenic shock (P = 0.040), than other groups. The P group had a significantly higher heart rate (P = 0.001); higher high‐sensitivity C‐reactive protein (P = 0.006), creatine kinase MB fraction (P = 0.045), and N terminal‐probrain natriuretic peptide (P = 0.036) levels; higher left ventricular end‐diastolic pressure (P = 0.019) and left ventricular end‐systolic diameter (P = 0.002); but lower left ventricular ejection fraction (P = 0.018). The E group had lesser prevalence of apical ballooning pattern (P = 0.038) than other groups. The P group required more frequent use of inotropics (P = 0.041) and diuretics (P = 0.047) and had significantly longer intensive care unit (P = 0.014) and in‐hospital stays (P = 0.001).
Conclusions:
The clinical features of TTC are different according to preceding stressor patterns. The TTC group with preceding physical stressors was less likely to have preserved cardiovascular reserve and more likely to require hemodynamic support than other groups. The overall prognosis of TTC is excellent, regardless of triggering stressors. Clin. Cardiol. 2011 DOI: 10.1002/clc.22053
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Tako‐tsubo cardiomyopathy (TTC), also known as transient left ventricular (LV) ballooning syndrome or stress‐induced cardiomyopathy, is characterized by transient LV dysfunction in the absence of significant angiographic coronary stenoses, typically triggered by preceding emotional or physical stress.1., 2., 3., 4. It has been typically observed in postmenopausal females experiencing emotionally stressful events, inspiring the nickname “broken heart syndrome,” and is increasingly being observed under diverse circumstances, including medical/surgical procedures or diagnostic testing.5., 6., 7. Differences in these clinical presentations have been observed, suggesting the possibility of diverse clinical features according to triggering stressors.7., 8. However, there have been few data to review and analyze the similarities and differences of clinical features between patients with TTC presenting with and without preceding stressors.9 In this study, we investigated the impact of stressor patterns on clinical characteristics, laboratory parameters, and electrocardiographic and echocardiographic findings in patients with TTC.
Methods
Study Subjects
We approached 137 consecutive patients enrolled from the TTC registry database at the Konkuk University Medical Center and Samsung Changwon Hospital from January 2004 to December 2010. From 6078 consecutive patients with a diagnosis of an acute coronary syndrome, including ST‐ and non–ST‐elevation myocardial infarction, who had an urgent coronary angiography, 137 (2%) patients were diagnosed with TTC. The enrolled 137 patients with TTC were divided into 3 subgroups according to stressor patterns: 14 patients had emotional triggers (E group), 96 had physical triggers (P group), and 27 had no triggers (N group). The criteria for inclusion were as follows: (1) transient akinesia/dyskinesia beyond a single major coronary artery vascular distribution; (2) absence of significant coronary artery disease on coronary angiograms (diameter stenosis <50% by visual estimation) or absence of angiographic evidence of acute plaque rupture; and (3) new electrocardiographic (ECG) changes (ST‐segment changes, T‐wave inversion, or Q wave).10 Cardiogenic shock was defined as a systolic blood pressure <90 mm Hg for ≥30 minutes that was not responsive to fluid administration alone, accompanied by evidence of tissue hypoperfusion in the setting of clinically adequate or elevated LV filling pressures.11 Pulmonary edema was defined as the presence of rales at pulmonary examination or a radiographic report of pulmonary alveolar/interstitial congestion at initial chest x‐ray.11 Hypertension was defined as repeated measurements of ≥140 mm Hg systolic blood pressure or ≥90 mm Hg diastolic blood pressure, or previous antihypertensive drug treatment. Diabetes mellitus (DM) was defined as serum glucose level ≥125 mg/dL, a history of DM, or current use of antidiabetic therapy. Smoking persons were defined as those who currently smoke and those who quit smoking less than a year prior to presentation. The protocol was approved by the Institutional Research Ethics Committee. The recommendations of the revised version of the Declaration of Helsinki were met.
Patient Data
The medical history and coronary risk factors were obtained from medical records combined with a patient questionnaire. Any physical or emotional stresses prior to the onset of this syndrome were specifically investigated. Electrocardiographic and laboratory data including cardiac enzymes (creatine kinase [CK], creatine kinase MB fraction [CK‐MB], and troponin I) were recorded during the acute phase and were followed until the abnormalities disappeared. Follow‐up data were collected by direct telephone interviews and a detailed review of all medical records. The cause and data of death were confirmed by information from the National Population Registry of the Korea National Statistical Office, together with a review of all available clinical records at the time of death (by telephone interviews and a detailed review of all medical records).
All patients underwent coronary angiography concomitant with LV angiography at the time of presentation. To exclude vasospastic angina, a spasm provocation test was performed and showed negative result in 87 (64%) patients by intracoronary ergonovine infusion as described previously, with minor modification.12
Echocardiography
Transthoracic echocardiographic examinations were performed in all patients with a 2.5‐MHz transducer attached to a commercially available Doppler echocardiography machine, on the first hospital day or within 24 hours of coronary angiography and follow‐up. Left ventricular end‐diastolic diameter and left ventricular end‐systolic diameter (LVESD), along with septal and posterior wall thickness at end‐diastole, were measured in the parasternal long‐axis view using 2D‐guided M‐mode echocardiography according to the recommendations of chamber quantification.13 The LV ejection fraction (LVEF) was calculated by modified Simpson's method. The valvular regurgitation (VR) was assessed by color Doppler flow mapping of spatial distribution of the regurgitant jet in accordance with the American Society of Echocardiography recommendation.14 In our study, significant VR was defined as regurgitation of more than a mild degree. Reversible VR was defined as significant VR at initial echocardiogram that disappeared at follow‐up echocardiogram. In our study, we assessed the presence of systolic anterior motion of the mitral valve using 2‐dimensional imaging.15 Left atrial volume was determined by the prolate ellipsoid method and indexed by body surface area (left atrial volume index). As the definition of ballooning pattern in our study, typical TTC was defined as showing apical and/or midventricular akinesia or hypokinesia with normal contractility or hypercontractility in basal segments, inverted TTC was defined as showing basal akinesia or hypokinesia with preserved contractility of apical and midventricular segments, midventricular TTC was defined as showing midventricular akinesia or hypokinesia sparing the base and the apex, and localized TTC was defined as affecting a segment of the LV wall.10., 15., 16., 17.
N‐terminal Probrain Natriuretic Peptide Assay
We took blood samples from the antecubital vein using lithium heparin, and the blood samples were then centrifuged. The blood samples were stored at −70°C until further analysis. Plasma N‐terminal probrain natriuretic peptide (NT‐proBNP) levels were measured using an Elecsys pro BNP reagent kit (Roche Diagnostics, Indianapolis, IN) and an Elecsys 2010 (Roche Diagnostics). In all cases, the time interval between blood sampling for NT‐proBNP and echocardiography was not longer than 1 day.
Statistical Analysis
Statistical analyses were performed using SPSS statistical software version 11.0 (SPSS Inc., Chicago, IL). Quantitative data are presented as mean ± standard deviation. Age, heart rate, corrected QT interval, high sensitivity C‐reactive protein (hs‐CRP) levels, peak CK levels, peak CK‐MB levels, peak troponin I levels, left ventricular end‐diastolic pressure (LVEDP), duration of hospitalization, and duration of intensive care unit (ICU) stay are given in terms of the median and interquartile range. Qualitative data are presented as frequencies. The 1‐way ANOVA or the Kruskal‐Wallis test was used to compare the continuous variables and the χ 2 test was used to compare the categorical variables. All P values are 2‐tailed and differences were considered significant when the P value was <0.05.
Results
Comparison of Clinical Characteristics Among the Subgroups
The clinical characteristics and initial presentations of the subgroups are compared in Table 1. The E group had a significantly higher prevalence of chest pain (P = 0.006) and palpitation (P = 0.006), whereas the P group had a higher prevalence of cardiogenic shock (P = 0.040), than other groups. The groups did not differ significantly in terms of age; male sex; body surface area; the prevalence of underlying diseases such as stroke, liver cirrhosis, hypertension, and DM; and current smoking status. Also, there were no significant differences in clinical presentations such as dyspnea, loss of consciousness, nausea/vomiting, and pulmonary edema. Emotional stressors were documented in 10% of patients (14/137), whereas physical stressors were documented in 70% of patients (96/137). Twenty percent of patients (27/137) had no triggering stressors (Figure 1). Among physical stressors, acute medical illness, surgery/procedure, and intravenous drug administration including chemotherapy were the precipitants in 54% of patients (52/96), 43% of patients (41/96), and 3% of patients (3/96), respectively (Figure 1).
Table 1.
Comparison of Clinical Characteristics Among the Subgroups
| Total (N = 137) | E Group (n = 14) | P Group (n = 96) | N Group (n = 27) | P Value | |
|---|---|---|---|---|---|
| Age, ya | 59.0 (53.0–72.0) | 58.0 (56.0–79.5) | 58.0 (52.0–71.0) | 63.0 (53.0–75.0) | 0.315 |
| Female sex, n (%) | 101 (74) | 12 (86) | 68 (71) | 21 (78) | 0.431 |
| Body surface area (m2) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.1 | 0.579 |
| Hypertension, n (%) | 47 (34) | 2 (14) | 32 (33) | 13 (48) | 0.090 |
| DM, n (%) | 25 (18) | 2 (14) | 21 (22) | 2 (7) | 0.210 |
| Current smoker, n (%) | 8 (6) | 0 (0) | 6 (6) | 2 (7) | 0.601 |
| Underlying diseases, n (%) | |||||
| Stroke/TIA | 8 (6) | 0 (0) | 8 (8) | 0 (0) | 0.163 |
| Liver cirrhosis | 2 (2) | 0 (0) | 2 (2) | 0 (0) | 0.648 |
| Chronic renal failure | 8 (6) | 0 (0) | 8 (8) | 0 (0) | 0.163 |
| Malignancy | 18 (13) | 0 (0) | 16 (17) | 2 (7) | 0.139 |
| Clinical presentation, n (%) | |||||
| Chest pain | 71 (52) | 12 (86) | 42 (44) | 17 (63) | 0.006b |
| Dyspnea | 77 (56) | 10 (71) | 55 (57) | 12 (44) | 0.237 |
| Palpitation | 11 (8) | 4 (29) | 4 (4) | 3 (11) | 0.006b |
| Loss of consciousness | 2 (2) | 0 (0) | 2 (2) | 0 (0) | 0.648 |
| Nausea/vomiting | 14 (10) | 0 (0) | 12 (13) | 2 (7) | 0.306 |
| Cardiogenic shock | 48 (35) | 2 (14) | 40 (42) | 6 (22) | 0.040b |
| Pulmonary edema | 57 (42) | 6 (44) | 39 (41) | 12 (44) | 0.934 |
Abbreviations: DM, diabetes mellitus; TIA, transient ischemic attack.
Presented as median (interquartile range).
Significant finding.
Figure 1.
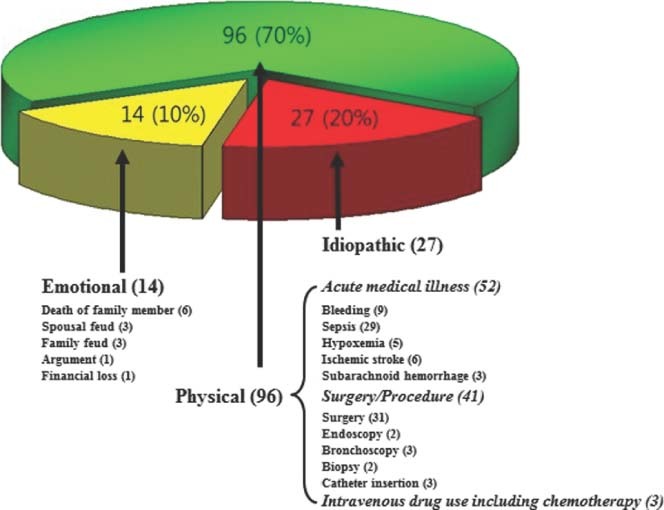
Preceding stressful events of 137 patients with tako‐tsubo cardiomyopathy. Emotional stressors were documented in 10% of patients (14/137), whereas physical stressors were documented in 70% of patients (96/137). Twenty percent of patients (27/137) had no triggering stressors.
Comparison of Electrocardiographic Changes and Laboratory and Angiographic Findings Among the Subgroups
The ECG changes and laboratory and angiographic findings of subgroups are compared in Table 2. The P and N groups had significantly higher heart rate (P = 0.001) than the E group. The P and N groups had significantly higher hs‐CRP (P = 0.006), peak CK‐MB (P = 0.045), NT‐proBNP levels (P = 0.036), and LVEDP (P = 0.019) than the E group. However, there were no significant differences in ECG findings with regard to rhythm abnormalities such as atrial fibrillation, life‐threatening arrhythmias, corrected QT interval, ST‐segment elevation, T‐wave inversion, and Q wave among the 3 groups.
Table 2.
Comparison of ECG Changes and Laboratory and Angiographic Findings Among the Subgroups
| Total (N = 137) | E Group (n = 14) | P Group (n = 96) | N Group (n = 27) | P Value | |
|---|---|---|---|---|---|
| ECG changes | |||||
| Life‐threatening arrhythmia, n (%)a | 18 (13) | 0 (0) | 16 (17) | 2 (7) | 0.139 |
| Heart rate (bpm)b | 80 (69–91) | 66 (58–72) | 84 (74–93) | 80 (70–91.0) | 0.001c |
| AF, n (%) | 7 (5) | 0 (0) | 5 (5) | 2 (7) | 0.592 |
| QTc (ms)b | 438.0 (417.0–486.5) | 462.0 (391.0–544.0) | 434.5 (412.0–473.0) | 470.0 (417.0–497.0) | 0.157 |
| ST‐segment elevation, n (%) | 102 (75) | 10 (71) | 67 (70) | 25 (93) | 0.060 |
| Q wave, n (%) | 17 (12) | 0 (0) | 13 (14) | 4 (15) | 0.326 |
| T‐wave inversion, n (%) | 104 (76) | 10 (71) | 73 (76) | 21 (78) | 0.902 |
| Laboratory findings | |||||
| hs‐CRP (mg/dL)b | 1.7 (0.6–9.9) | 0.1 (0.1–9.9) | 3.2 (1.0–11.1) | 1.8 (0.1–2.3) | 0.006c |
| Peak CK (ng/mL)b | 262.0 (96.5–533.5) | 267.5 (89.0–510.0) | 519.0 (100.0–987.0) | 406.0 (232.0–593.0) | 0.436 |
| Peak CK‐MB (ng/mL)b | 13.8 (2.9–39.8) | 10.4 (2.9–33.9) | 41.2 (3.4–95.1) | 19.6 (4.1–78.1) | 0.045c |
| Peak TnI (ng/mL)b | 1.8 (0.2–10.1) | 1.0 (0.2–3.3) | 2.5 (0.3–11.5) | 2.0 (0.2–10.6) | 0.113 |
| NT‐proBNP (pg/mL)b | 3210.0 (1399.0–16015.0) | 1102.0 (82.8–5059.0) | 7644.0 (1458.0–30181.0) | 3210.0 (1482.5–17167.0) | 0.036c |
| Angiographic findings | |||||
| LVEDP (mm Hg)b | 19.0 (13.0–25.0) | 15.0 (9.0–20.0) | 20.0 (17.5–34.0) | 19.0 (12.3–25.0) | 0.019c |
Abbreviations: AF, atrial fibrillation; CK, creatine kinase; CK‐MB, creatine kinase MB fraction; ECG, electrocardiographic; hs‐CRP, high‐sensitivity C‐reactive protein; LVEDP, left ventricular end‐diastolic pressure; NT‐proBNP, N‐terminal probrain natriuretic peptide; QTc, corrected QT interval; TnI, troponin I.
Life‐threatening arrhythmia includes 3rd‐degree atrioventricular block, ventricular tachycardia, ventricular fibrillation, and cardiac arrest.
Presented as median (interquartile range).
Significant finding.
Comparison of Echocardiographic Findings Among the Subgroups
The echocardiographic findings of subgroups are compared in Table 3. The E group had lesser prevalence of apical ballooning pattern (P = 0.038) than other groups. The P and N groups had significantly lower LVEF (P = 0.018) but higher LVESD (P = 0.002) than the E group. There were no significant differences in left ventricular end‐diastolic diameter, left atrial volume index, and E/E′ on the initial and follow‐up examinations among the 3 groups. Also, there were no significant differences in systolic anterior motion, significant mitral regurgitation, aortic regurgitation, and tricuspid regurgitation between the 3 groups on the initial and follow‐up examinations. All patients showed normalized regional wall motion in their follow‐up echocardiogram.
Table 3.
Comparison of Echocardiographic Findings Among the Subgroups
| Total (N = 137) | E Group (n = 14) | P Group (n = 96) | N Group (n = 27) | P Value | |
|---|---|---|---|---|---|
| Initial TTE findings | |||||
| Ballooning pattern | |||||
| Typical TTC pattern, n (%) | 106 (77) | 8 (57) | 74 (77) | 24 (89) | 0.038a |
| Inverted TTC pattern, n (%) | 24 (18) | 6 (43) | 15 (16) | 3 (11) | |
| Midventricular pattern, n (%) | 7 (5) | 0 (0) | 7 (7) | 0 (0) | |
| Localized pattern, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| LVEF (%) | 40.5 ± 8.1 | 46.2 ± 4.7 | 38.3 ± 8.9 | 40.5 ± 7.9 | 0.018a |
| LVEDD (mm) | 52.1 ± 6.2 | 50.0 ± 2.2 | 53.7 ± 6.5 | 51.2 ± 6.2 | 0.236 |
| LVESD (mm) | 37.3 ± 7.0 | 31.3 ± 4.7 | 38.1 ± 7.2 | 37.8 ± 5.6 | 0.002a |
| LAVI (mL/m2) | 28.2 ± 14.7 | 21.9 ± 4.1 | 28.0 ± 15.1 | 30.2 ± 12.7 | 0.193 |
| E/E′ | 10.9 ± 5.4 | 9.8 ± 3.3 | 11.4 ± 6.8 | 11.0 ± 4.9 | 0.727 |
| SAM | 18 (13) | 4 (29) | 12 (13) | 2 (7) | 0.155 |
| Significant MR, n (%) | 31 (23) | 6 (43) | 19 (20) | 6 (22) | 0.156 |
| Significant AR, n (%) | 9 (7) | 0 (0) | 7 (7) | 2 (7) | 0.578 |
| Significant TR, n (%) | 18 (13) | 3 (21) | 12 (13) | 3 (11) | 0.464 |
| Follow‐up TTE findings | |||||
| LVEF (%) | 62.6 ± 4.7 | 66.3 ± 5.5 | 62.4 ± 4.1 | 61.9 ± 5.7 | 0.264 |
| LVEDD (mm) | 50.2 ± 5.2 | 52.3 ± 3.6 | 50.3 ± 5.4 | 52.2 ± 4.0 | 0.664 |
| LVESD (mm) | 31.6 ± 4.2 | 30.7 ± 5.0 | 31.1 ± 3.4 | 32.4 ± 5.9 | 0.187 |
| LAVI (mL/m2) | 27.2 ± 12.9 | 26.2 ± 3.8 | 26.0 ± 12.6 | 31.5 ± 15.0 | 0.172 |
| E/E′ | 10.2 ± 4.8 | 9.7 ± 3.3 | 11.6 ± 3.6 | 11.0 ± 7.7 | 0.439 |
| Significant MR, n (%) | 9 (7) | 2 (14) | 3 (3) | 4 (15) | 0.056 |
| Reversible MR, n (%) | 22 (16) | 4 (29) | 16 (17) | 2 (7) | 0.207 |
| Significant AR, n (%) | 7 (5) | 0 (0) | 5 (5) | 2 (7) | 0.592 |
| Significant TR, n (%) | 8 (8) | 3 (21) | 6 (6) | 7 (26) | 0.224 |
Abbreviations: AR, aortic regurgitation; E/E′, early diastolic mitral inflow velocity/early diastolic annular velocity; LAVI, left atrial volume index; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MR, mitral regurgitation; RVSP, right ventricular systolic pressure; SAM, systolic anterior motion of anterior mitral leaflet; TR, tricuspid regurgitation; TTC, tako‐tsubo cardiomyopathy; TTE, transthoracic echocardiography.
Significant finding.
Comparison of Management and Clinical Outcomes Among the Subgroups
There were no significant differences in use of an intra‐aortic balloon pump and β‐blockers during hospitalization among the 3 groups (Table 4). The P and N groups had significantly higher prevalence of inotropic (P = 0.041) and diuretic use (P = 0.047); significantly higher frequency of ICU stay (P = 0.007); and significantly longer in‐hospital stays (P = 0.001) and ICU stays (P = 0.014) than the E group. The E and N groups had significantly higher use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers than the E group (P = 0.001).
Table 4.
Comparison of Clinical Courses and Management Among the Subgroups
| Total (N = 137) | E Group (n = 14) | P Group (n = 96) | N Group (n = 27) | P Value | |
|---|---|---|---|---|---|
| Use of inotropics, n (%) | 51 (37) | 2 (14) | 42 (44) | 7 (26) | 0.041a |
| Use of IABP, n (%) | 16 (12) | 0 (0) | 14 (15) | 2 (7) | 0.211 |
| Use of ACEI or ARB, n (%) | 79 (58) | 12 (86) | 44 (46) | 23 (85) | 0.001a |
| Use of β‐blocker, n (%) | 26 (19) | 4 (29) | 15 (16) | 7 (26) | 0.303 |
| Use of diuretic, n (%) | 61 (45) | 2 (14) | 45 (47) | 14 (52) | 0.047a |
| Temporal pacemaker, n (%) | 10 (7) | 0 (0) | 8 (8) | 2 (7) | 0.534 |
| Cardioversion, n (%) | 8 (6) | 0 (0) | 7 (7) | 1 (6) | 0.163 |
| ICU hospitalization, n (%) | 92 (67) | 8 (57) | 59 (62) | 25 (93) | 0.007a |
| ICU hospitalization, db | 2.0 (0–5.0) | 1.0 (0–2.0) | 3.0 (0–5) | 2.0 (2–4) | 0.014a |
| Hospitalization, db | 14.0 (7.0–28.0) | 3.0 (3.0–9.0) | 17.0 (8.0–32.0) | 8.0 (5.0–14.0) | 0.001a |
| In‐hospital cardiac mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
| Cardiac mortality during follow‐up, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
| Mortality during follow‐up, n (%) | 12 (9) | 1 (7) | 10 (10) | 1 (4) | 0.538 |
| Recurrence, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; IABP, intra‐aortic balloon pump; ICU, intensive care unit; NS, not significant.
Significant finding.
Presented as median (interquartile range).
During follow‐up (median, 5.7 years; interquartile range, 5.0–6.9 years), 12 (9%) patients died: 6 (50%) patients died of malignancy, 2 of stroke, 2 of chronic renal failure with panperitonitis, 1 of liver cirrhosis with variceal bleeding, and 1 of with pneumonia with empyema. However, cardiac deaths associated with LV ballooning syndrome itself were not noted in the 3 groups. Also, recurrence of the LV ballooning syndrome was not noted in the 3 groups.
Discussion
As has been widely reported in the past, TTC usually is precipitated by an acute episode of emotional and or physiological stress.1., 2., 3., 4. Recently, diverse clinical features of TTC have been observed, suggesting the possibility of ≥1 clinical phenotype, according to triggering stressors.5., 6., 7., 8. However, a limited amount of literature currently exists on the similarities and differences of clinical features between patients with TTC according to preceding stressor patterns.9
To the best of our knowledge, this is one of the largest studies investigating the similarities and differences of clinical features, laboratory parameters, and electrocardiographic and echocardiographic findings between patients with TTC presenting with and those without stressors. In the present study, the E group had significantly higher prevalence of chest pain and palpitation, whereas the P group had significantly higher prevalence of cardiogenic shock; higher hs‐CRP, CK‐MB, and NT‐proBNP levels; and higher LVEDP and LVESD, but lower LVEF. The E group had lesser prevalence of the apical ballooning pattern than other groups. The P group required more frequent hemodynamic support and had significantly longer ICU and in‐hospital stays.
In contrast with the previously published data,5., 6., 7., 8. our study showed that physical stress, rather than emotional stress, was the predominant triggering event for TTC. Tako‐tsubo cardiomyopathy associated with sepsis and respiratory failure without obstructive coronary artery disease was already reported,6., 7., 8. and several hypotheses have been proposed, including catecholamine‐mediated cardiotoxicity, spasm of the epicardial and/or microvascular coronary circulation, and endothelial‐cell dysfunction.18., 19., 20. It was also surprising to know that TTC could occur during or after uneventful elective procedures or surgery performed under regional or general anesthesia.
A notable finding in our study was that 24 (18%) of 137 enrolled patients had a history of cancer at the index hospitalization for TTC. Our finding may support the results of recently published reports showing 23.6% of patients with TTC had cancer, which greatly exceeds the expected prevalence of cancer in age‐matched populations in the United States (8.2%), Germany (11.2%), and all European countries combined (7.8%).21., 22. Burgdorf et al suggested increased basal sympathetic tone in patients with cancer may be associated with susceptibility to TTC with additional stress, and that paraneoplastic mediators may directly alter cardiac adrenoreceptors.21., 22. In the present study, most clinical features were similar among the subgroups. The overlapping clinical features in all these presentations may suggest that myocardial stunning resulting from emotional stress may share a common mechanism with that from physical stress or without triggering stressors, which has been described after subarachnoid hemorrhage and stroke and which is believed to be mediated by catecholamine.23
Our study showed that the E group had significantly higher prevalence of chest pain and palpitation than the other groups. Some studies reported that catecholamine‐mediated myocardial injury has been observed postmortem in people who died under terrifying circumstances such as violent assault, suggesting that catecholamine may be an important link between emotional stress and cardiac injury.23 Therefore, we reasoned that these subjective symptoms predominant in the E group might reflect the emotionally stressful event triggering an excessive release of catecholamine, resulting in the presentation of these subjective symptoms; these patients appear to be more vulnerable to sympathetically mediated myocardial stunning.
Interestingly, in our study, the P group had a significantly higher prevalence of cardiogenic shock, but lower LVEF than the other groups. Also, the P group had significantly higher hs‐CRP, peak CK‐MB, NT‐proBNP, and LVEDP levels than the other groups. These observations may suggest that patients with physical stressors have more cardiovascular impairment and that perhaps other factors such as significant underlying comorbidities contribute to myocardial stunning. The possible explanations may include myocardial stunning, inflammation, and microvascular spasm in physically stressed TTC patients.1., 23., 24. Therefore, we reasoned that patients with physical stressors had the greater extent of affected myocardium, and cardiac markers such as CK‐MB may reflect this extent of affected myocardium. On the other hand, our patients with preceding emotional stressors had relatively preserved myocardial function with higher LVEF and lower LVEDP on initial presentation. These findings may suggest a transient catecholamine‐induced myocardial stunning in this group.
A remarkable finding of our study was that the P and N groups had a higher prevalence of the typical ballooning pattern, whereas the E group had a higher prevalence of the inverted ballooning pattern. The hypothesis is that the extent of affected myocardium could be smaller and wall‐motion abnormalities resolve more rapidly in the E group than in the P group. Classic TTC involves the apical and/or midventricular segments, whereas the apical segment is spared in inverted TTC.25., 26. It has also been suggested that wall‐motion abnormalities in inverted TTC resolve more rapidly than in TTC.27., 28. It is therefore reasonable to suppose that differences of myocardial insults with triggering stressor patterns may effect different ballooning patterns between the 2 groups. Further research is required to study our hypothesis about these differences.
In contrast with a recently published study showing the emotional‐stress group was younger than the idiopathic/physical‐stress group,9 our data reported that the groups did not differ significantly in terms of age. The possible hypothesis for this discrepancy is that racial differences may affect emotional stressor and susceptibility to TTC with aging. Also, this discrepancy may be related to differences in activated sympathetic tones after a stressful event according to racial differences.
Notably, in our study, 51 (37%) of 137 enrolled patients needed treatment with inotropics during the index hospitalization. Moreover, the P group had a significantly higher prevalence of inotropic use and diuretic use, higher frequency of ICU stay, and had significantly longer durations of in‐hospital and ICU stays than the E group. These findings are in contrast with results of the previous studies showing the use of inotropic agents, particularly in patients with cardiogenic shock, may increase the LV outflow tract pressure gradient and worsen cardiogenic shock in patients with TTC.4., 29. It seems likely that cardiogenic shock due to heart failure is treated with standard therapies such as inotropes and intra‐aortic balloon pump, although a cautious trial of intravenous fluids and β‐blockers may help by reducing pressure gradients, thereby reducing the LV outflow obstruction in the absence of shock. Based on findings of aforementioned studies,18., 19., 20. in addition to the results of the present study, we reasoned that clinicians should monitor physically stressed TTC patients carefully for hemodynamic compromise. In our study, in‐hospital and follow‐up cardiac mortalities were 0% and 0% respectively, in TTC patients, irrespective of stressor patterns. These findings may emphasize that the prognosis of TTC itself may be excellent if a meticulous therapeutic strategy under careful monitoring is performed in these physically stressed patients, particularly in hemodynamic instability.
In the present study, the overall mortality (9%) was relatively higher than in previous studies.1., 2., 3., 4. However, the overall mortality associated with TTC itself was 0%. It was comparable with results of published reports in other areas of the world.1., 2., 3., 4. During the follow‐up period of 5.7 years, most patients (50%) died of malignancy in our study. According to aforementioned reports, malignancies may be associated with TTC, potentially as a result of paraneoplastic phenomena.21., 22. These findings may indicate that patients with TTC have an excellent prognosis in the absence of significant underlying comorbidities such as malignancy or stroke.
Study Limitations
There are some limitations that should be considered in our study. First, this was a retrospective analysis. Second, the results of our study may be limited by the relatively small number of patients. However, this is one of the largest studies yet published to compare clinical characteristics, echocardiographic findings, and NT‐proBNP levels between TTC subgroups in detail. Third, we did not perform systemic investigations, such as catecholamine measurements, magnetic resonance imaging, viral antibody titers, or pathology. Because TTC is a kind of exclusion diagnosis, some patients with other diseases, such as myocarditis, may be misdiagnosed in our study. However, diagnosis of TTC on the basis of clinical presentation and characteristic wall‐motion abnormality is realistic and often used in many studies.
Conclusion
The clinical features of TTC are different between groups with and without preceding emotional stressors. The TTC group with preceding physical stressors was lesser likely to have preserved cardiovascular reserve and more likely to require hemodynamic support than other groups. The overall prognosis of TTC is excellent, regardless of triggering stressors.
References
- 1. Kurisu S, Sato H, Kawagoe T, et al. Tako‐tsubo‐like left ventricular dysfunction with ST‐segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143:448–455. [DOI] [PubMed] [Google Scholar]
- 2. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. [DOI] [PubMed] [Google Scholar]
- 3. Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008;124:283–292. [DOI] [PubMed] [Google Scholar]
- 4. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako‐Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 5. Lee YP, Poh KK, Lee CH, et al. Diverse clinical spectrum of stress‐induced cardiomyopathy. Int J Cardiol. 2009;133:272–275. [DOI] [PubMed] [Google Scholar]
- 6. Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako‐tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–341. [DOI] [PubMed] [Google Scholar]
- 7. Singh NK, Rumman S, Mikell FL, et al. Stress cardiomyopathy: clinical and ventriculographic characteristics in 107 North American subjects. Int J Cardiol. 2010;141:297–303. [DOI] [PubMed] [Google Scholar]
- 8. Vidi V, Rajesh V, Singh PP, et al. Clinical characteristics of tako‐tsubo cardiomyopathy. Am J Cardiol. 2009;104:578–582. [DOI] [PubMed] [Google Scholar]
- 9. Yaqub Y, Jenkins LA, Suarez J, et al. Emotional stress and tako‐tsubo cardiomyopathy: observations on 2 distinct clinical phenotypes. J Investig Med. 2010;58:298–302. [DOI] [PubMed] [Google Scholar]
- 10. Song BG, Hahn JY, Cho SJ, et al. Clinical characteristics, ballooning pattern, and long‐term prognosis of transient left ventricular ballooning syndrome. Heart Lung. 2010;39:188–195. [DOI] [PubMed] [Google Scholar]
- 11. Menon V, White H, LeJemtel T, et al. The clinical profile of patients with suspected cardiogenic shock due to predominant left ventricular failure: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1071–1076. [DOI] [PubMed] [Google Scholar]
- 12. Hackett D, Larkin S, Chierchia S, et al. Induction of coronary artery spasm by a direct local action of ergonovine. Circulation. 1987;75:577–582. [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 14. Zoghbi WA, Enriquez‐Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 15. Song BG, Park SJ, Noh HJ, et al. Clinical characteristics, and laboratory and echocardiographic findings in takotsubo cardiomyopathy presenting as cardiogenic shock. J Crit Care. 2010;25:329–335. [DOI] [PubMed] [Google Scholar]
- 16. Marechaux S, Fornes P, Petit S, et al. Pathology of inverted Takotsubo cardiomyopathy. Cardiovasc Pathol. 2008;17:241–243. [DOI] [PubMed] [Google Scholar]
- 17. Kimura S, Mitsuma W, Ito M, et al. Inverted Takotsubo contractile pattern caused by pheochromocytoma with tall upright T waves, but not typical deep T wave inversion. Int J Cardiol. 2010;139:e15–e17. [DOI] [PubMed] [Google Scholar]
- 18. Cocco G, Chu D. Stress induced cardiomyopathy: a review. Eur J Intern Med. 2007;18:369–379. [DOI] [PubMed] [Google Scholar]
- 19. Lindsay J, Paixao A, Chao T, et al. Pathogenesis of the Takotsubo syndrome: a unifying hypothesis. Am J Cardiol. 2010; 106:1360–1363. [DOI] [PubMed] [Google Scholar]
- 20. Pernicova I, Garg S, Bourantas CV, et al. Takotsubo cardiomyopathy: a review of the literature. Angiology. 2010;61:166–173. [DOI] [PubMed] [Google Scholar]
- 21. Burgdorf C, Nef HM, Haghi D, et al. Takotsubo (stress‐induced) cardiomyopathy and cancer. Ann Intern Med. 2010;152:830–831. [DOI] [PubMed] [Google Scholar]
- 22. Burgdorf C, Kurowski V, Bonnemeier H, et al. Long‐term prognosis of the transient left ventricular dysfunction syndrome (Tako‐Tsubo cardiomyopathy): focus on malignancies. Eur J Heart Fail. 2008;10:1015–1019. [DOI] [PubMed] [Google Scholar]
- 23. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 24. Sadamatsu K, Tashiro H, Maehira N, et al. Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn Circ J. 2000;64:789–792. [DOI] [PubMed] [Google Scholar]
- 25. Haghi D, Papavassiliu T, Flüchter S, et al. Variant form of the acute apical ballooning syndrome (takotsubo cardiomyopathy): observations on a novel entity. Heart. 2006;92:392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Movahed MR, Donohue D. Review: transient left ventricular apical ballooning, broken heart syndrome, ampulla cardiomyopathy, atypical ballooning, or Tako‐Tsubo cardiomyopathy. Cardiovasc Revasc Med. 2007;8:289–292. [DOI] [PubMed] [Google Scholar]
- 27. Hahn JY, Gwon HC, Park SW, et al. The clinical features of transient left ventricular nonapical ballooning syndrome: comparison with apical ballooning syndrome. Am Heart J. 2007;154:1166–1173. [DOI] [PubMed] [Google Scholar]
- 28. Hurst RT, Askew JW, Reuss CS, et al. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol. 2006;48:579–583. [DOI] [PubMed] [Google Scholar]
- 29. El Mahmoud R, Mansencal N, Pilliere R, et al. Prevalence and characteristics of left ventricular outflow tract obstruction in Tako‐Tsubo syndrome. Am Heart J. 2008;156:543–548. [DOI] [PubMed] [Google Scholar]


