Abstract
Background
Angiographic coronary lesion complexity has been reported to predict plaque vulnerability. It is important to develop a noninvasive blood biomarker for accurate prognostication of angiographically complex lesions in patients with coronary artery disease (CAD).
Hypothesis
Serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 (sLOX‐1) levels may be correlated with coronary lesion complexity in patients with CAD.
Methods
We measured serum sLOX‐1 levels in 180 consecutive patients undergoing coronary angiography for the evaluation of CAD. Coronary lesions were classified as simple or complex lesions based on coronary plaque morphology.
Results
Stable CAD patients with complex lesions (n = 50) had significantly higher serum sLOX‐1 levels than those with simple lesions (n = 72), at 0.914 ng/mL (range, 0.489–1.296 ng/mL) vs 0.426 ng/mL (range, 0.195–1.075 ng/mL), respectively, P < 0.01. Multivariate logistic regression analysis revealed that sLOX‐1 levels were independently associated with the presence of complex lesions in patients with stable CAD (odds ratio [OR]: 1.964, 95% confidence interval [CI]: 1.149–3.356, P < 0.05). Among patients with acute coronary syndrome (n = 58), who had significantly higher circulating sLOX‐1 levels than stable CAD patients (n = 122) at 1.610 ng/mL (range, 0.941–2.264 ng/mL) vs 0.579 ng/mL (range, 0.265–1.172 ng/mL), respectively, P < 0.01, sLOX‐1 levels were independently associated with the presence of multiple complex coronary lesions (OR: 1.967, 95% CI: 1.075–3.600, P < 0.05).
Conclusions
Serum sLOX‐1 levels were associated with complex lesions that might predict vulnerable plaques. This study suggested sLOX‐1 might be a useful biomarker of coronary plaque vulnerability in patients with CAD. © 2011 Wiley Periodicals, Inc.
This work is supported in part by the Science Research Foundation of the Ministry of Health & United Fujian Provincial Health and Education Project for Tackling Key Research, People's Republic of China (No. WKJ2008‐2‐62), and by the key program of scientific research of Fujian Medical University (09ZD011). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Lectin‐like oxidized low‐density lipoprotein receptor‐1 (LOX‐1), a type II membrane glycoprotein, is the major receptor for oxidized low‐density lipoprotein (ox‐LDL) in endothelial cells.1 It also has an inducible expression in macrophages and vascular smooth‐muscle cells (VSMCs).2 In addition to ox‐LDL, LOX‐1 can also be activated by other risk factors of coronary artery disease (CAD), including hemodynamic stress, angiotensin II, proinflammatory cytokines, and C‐reactive protein.3., 4., 5., 6. LOX‐1 activation is involved in endothelial cells dysfunction,7 ox‐LDL–induced VSMC apoptosis,8 accumulation of lipids in macrophages,9 and production of matrix metalloproteinases (MMPs).10 All of these pathophysiologic processes play crucial roles in atherosclerotic plaque formation, progression, and destabilization.11
Like many cell‐surface receptors with a single transmembrane domain, LOX‐1 can convert into soluble molecules by proteolytic cleavage at its membrane proximal extracellular domain.12 Circulating soluble LOX‐1 (sLOX‐1) levels may reflect the expression of LOX‐113 and are associated with inflammatory and oxidative stress markers in patients with CAD.14., 15. Moreover, circulating sLOX‐1 levels are elevated in patients with acute coronary syndrome (ACS),16 which may reflect its prominent expression and enhanced protease activities in vulnerable atherosclerotic plaques in situ.17 However, the association between sLOX‐1 levels and intracoronary plaque vulnerability in CAD patients, which may be reflected by angiographically complex coronary lesions,18 remains to be fully elucidated. We therefore sought to clarify the link between circulating sLOX‐1 levels and angiographic coronary lesion complexity in CAD patients.
Methods
Study Subjects
We prospectively asked 312 consecutive patients undergoing coronary angiography for the evaluation of CAD in our hospital from September 2009 to February 2010 to participate in this study. The exclusion criteria were patients with a history of coronary intervention or coronary artery bypass graft surgery, suspected myocarditis or pericarditis, diabetes mellitus (DM), malignant disease, active inflammatory disease, and advanced renal disease. In total, 180 consecutive patients with CAD were enrolled in this study. Of these patients, 122 had stable CAD and 58 had ACS. Patients with ACS included 34 with acute myocardial infarction and 24 with unstable angina. Stable CAD was defined as no recent deterioration or rest pain in the previous 6 months but angiographically documented prior coronary artery stenosis >50% and no previous myocardial infarction. The definition of acute myocardial infarction was typical chest pain with or without ST‐segment elevation on the electrocardiogram and an increase in the serum level of cardiac troponin T to >2× the upper limit of the normal range. The definition of unstable angina was ischemic chest pain at rest within the preceding 48 hours or within the past month (Braunwald classes IIB or IIIB),19 with transient ST‐T segment depression or T‐wave inversion and normal serum level of cardiac troponin T. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive drugs. Hypercholesterolemia was defined as total cholesterol ≥ 6.22 mmol/L, low‐density lipoprotein cholesterol (LDL‐C) ≥4.14 mmol/L, or use of a statin.
Written informed consents were obtained from all patients for their participation. This study was approved by the Ethics Committee of Fujian Medical University and conducted in accordance with the principles outlined in the Declaration of Helsinki.
Coronary Angiography Analysis
Conventional invasive coronary angiography was performed according to standard protocols. Two experienced interventional cardiologists, blind to the patients' clinical characteristics and biochemical results, reviewed all the angiographic images. Coronary lesion morphology was assessed as previously reported20., 21. and was classified as either simple or complex. Briefly, complex lesions were defined according to the presence of at least 1 of the following features: (1) irregular morphology or scalloped borders, or both; (2) overhanging or abrupt edges perpendicular to the vessel wall; (3) plaque ulceration; and (4) the presence of filling defects consistent with intracoronary thrombus.22 Coronary lesions with no complex features were classified as simple lesions.
Blood Chemistry Analysis
Venous blood was drawn from all patients just before the angiographic procedure. The blood samples were immediately centrifuged at 3000 g for 10 minutes and frozen at −80°C until analysis. Fasting blood glucose and fasting plasma lipids (ie, total cholesterol [TC], triglycerides [TG], high‐density lipoprotein cholesterol [HDL‐C], and LDL‐C) were measured by commercially available kits using an autoanalyzer (LX‐20; Beckman Coulter, Inc., Brea, CA). Serum sLOX‐1 levels were measured by a commercially available enzyme‐linked immunosorbent assay kit (USCN Life Science, Wuhan, China). The detection limit for serum sLOX‐1 level was 2.4 pg/mL with a coefficient of variation <5%.
Statistical Analysis
Sample size was determined by power analysis using preliminary data obtained in our laboratory with the following assumptions: in patients with stable CAD, an expected complex lesion rate of 40%, difference in sLOX‐1 levels between patients with and without complex lesions of 0.5 ng/mL, and a standard deviation of 0.8 ng/mL. Therefore, a minimum of 86 patients would detect a difference in the sLOX‐1 levels with a power of 80% and a significance level (2‐tailed) of 0.05.23 The assumptions in patients with ACS were an expected multiple complex lesion rate of 40%, difference in sLOX‐1 levels between patients with and without multiple complex lesions of 0.8 ng/mL, and a standard deviation of 0.8 ng/mL. Therefore, a minimum of 35 patients would detect a difference in the sLOX‐1 levels with a power of 80% and a significance level (2‐tailed) of 0.05.23 Data normality was analyzed using the Kolmogorov‐Smirnov test. Normally distributed continuous variables were expressed as the mean value ± SD, and non‐normally distributed continuous variables were expressed as the median value (interquartile range). Differences between the 2 groups were analyzed using unpaired t test, Mann‐Whitney U test, or χ 2 test as indicated. Differences among the 3 groups were analyzed using 1‐way analysis of variance test followed by the Tukey post‐hoc test, Kruskal‐Wallis test, or χ 2 test as indicated. As the sLOX‐1 levels were not normally distributed, logarithmic transformed values were performed for multiple comparisons among the 3 groups. Univariate analysis was performed, and the variables with a P value <0.20 as well as the traditional risk factors were then entered into a stepwise multivariate logistic regression model to assess the independent predictors for the presence of complex lesions in patients with stable CAD or the independent predictors for the presence of multiple complex lesions in patients with ACS. Statistical significance was defined as P < 0.05. All of the statistical analyses were performed using SPSS version 10.0 (SPSS Inc., Chicago, IL).
Results
Baseline Clinical Characteristics
Patients with stable CAD were divided into 2 groups, simple lesion (n = 72) and complex lesion (n = 50), based on the presence or absence of angiographically complex coronary lesions. The baseline clinical characteristics of each group are listed in Table S1. Patients with ACS were divided into 3 groups according to the number of angiographically complex coronary lesions: no complex lesion (No‐CL; n = 11), 1 complex lesion (One‐CL; n = 23), and multiple complex lesions (Multi‐CLs; n = 24). The baseline clinical characteristics of each group are listed in Table S2.
Serum sLOX‐1 Levels in Patients With Stable CAD and ACS
The data in Figure 1 indicate that patients with ACS had significantly higher serum sLOX‐1 levels than those with stable CAD, at 1.610 ng/mL (range, 0.941–2.264 ng/mL) vs 0.579 ng/mL (range, 0.265–1.172 ng/mL) respectively, P < 0.01.
Figure 1.
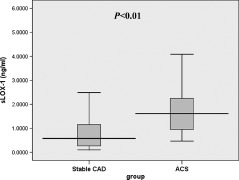
Box‐and‐whisker plot showing serum sLOX‐1 levels in patients with stable CAD (n = 122) and ACS (n = 58). Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; sLOX‐1, serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1.
sLOX‐1 and Presence of Complex Coronary Lesions in Patients With Stable CAD
As shown in Figure 2, stable CAD patients with complex lesions had significantly higher serum sLOX‐1 levels than patients with simple lesions, at 0.914 ng/mL (range, 0.489–1.296 ng/mL) vs 0.426 ng/mL (range, 0.195–1.075 ng/mL), respectively, P < 0.01. Associations between the presence of complex lesions and all other parameters were first analyzed by univariate logistic regression analysis (Table S3). Univariate analysis showed that body mass index, TG, LDL‐C, and sLOX‐1 levels showed a trend (P < 0.20) toward an association with the presence of complex lesions (Table S3). All these variables and traditional risk factors including HDL‐C, smoking, hypertension, and hypercholesterolemia were then entered into a stepwise multivariate logistic regression model. Multivariate logistic regression revealed that, after adjusting for other factors, sLOX‐1 level was a significant and independent predictor of complex lesions (odds ratio [OR]: 1.964, 95% confidence interval [CI]: 1.149–3.356, P < 0.05) (Table 1).
Figure 2.
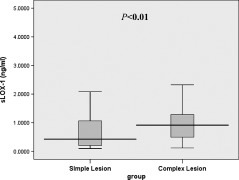
Box‐and‐whisker plot showing serum sLOX‐1 levels in stable CAD patients with simple lesions (n = 72) and complex lesions (n = 50). Abbreviations: CAD, coronary artery disease; sLOX‐1, serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1.
Table 1.
Multivariate Logistic Regression Analysis for the Presence of Complex Lesions in Patients With Stable CAD
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| BMI (per kg/mm2) | 1.085 | 0.929–1.267 | 0.304 |
| TG (per mmol/L) | 1.253 | 0.891‐1.764 | 0.195 |
| LDL‐C (per mmol/L) | 1.502 | 0.958–2.354 | 0.076 |
| sLOX‐1 (per ng/mL) | 1.964 | 1.149–3.356 | 0.014 |
| HDL‐C (per mmol/L) | 0.362 | 0.059–2.221 | 0.272 |
| Smoking (yes) | 0.853 | 0.358–2.032 | 0.719 |
| Hypertension (yes) | 1.203 | 0.539–2.684 | 0.653 |
| Hypercholesterolemia (yes) | 1.342 | 0.567–3.176 | 0.503 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; sLOX‐1, serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1; TG, triglycerides.
sLOX‐1 and the Number of Complex Coronary Lesions in Patients With ACS
In patients with ACS, Figure 3 shows that a significant positive correlation was found between increments in sLOX‐1 levels and number of complex lesions. The sLOX‐1 levels were 1.003 ng/mL (range, 0.783–1.668 ng/mL) in the No‐CL group, 1.456 ng/mL (range, 0.923–2.124 ng/mL) in the One‐CL group, and 2.171 ng/mL (range, 1.067–3.247 ng/mL) in the Multi‐CLs group; P < 0.05. Multiple comparisons among the 3 groups showed that the levels of sLOX‐1 were significantly higher in both the Multi‐CLs (P < 0.01) and One‐CL groups (P < 0.05) when compared with the No‐CL group. Although the levels of sLOX‐1 in the Multi‐CLs group tended to be higher than those in the One‐CL group, the difference was not statistically significant (P = 0.14). Associations between the presence of multiple complex lesions and all other parameters were first analyzed by univariate logistic regression analysis (Table S4). Multivariate regression model including fasting blood glucose, LDL‐C, sLOX‐1, and traditional risk factors (HDL‐C, smoking, hypertension, and hypercholesterolemia) demonstrated that sLOX‐1 levels were independently associated with the presence of multiple complex coronary lesions (OR: 1.967, 95% CI: 1.075–3.600, P < 0.05) (Table 2).
Figure 3.
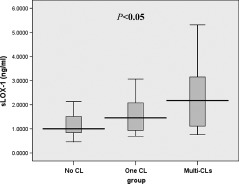
Box‐and‐whisker plot showing numbers of complex lesions and serum sLOX‐1 levels in patients with ACS. Abbreviations: ACS, acute coronary syndrome; CL, complex lesion; sLOX‐1, serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1.
Table 2.
Multivariate Logistic Analysis for the Presence of Multiple Complex Lesions in Patients With ACS
| Variables | OR | 95% CI | P Value |
|---|---|---|---|
| FBG (per mmol/L) | 1.981 | 0.805–4.871 | 0.137 |
| LDL‐C (per mmol/L) | 1.140 | 0.490–2.653 | 0.761 |
| sLOX‐1 (per ng/mL) | 1.967 | 1.075–3.600 | 0.028 |
| HDL‐C (per mmol/L) | 0.768 | 0.036–6.368 | 0.866 |
| Smoking (yes) | 1.269 | 0.335–4.805 | 0.726 |
| Hypertension (yes) | 1.494 | 0.404–5.524 | 0.547 |
| Hypercholesterolemia (yes) | 0.880 | 0.256–3.027 | 0.840 |
Abbreviations: ACS, acute coronary syndrome; CI, confidence interval; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; sLOX‐1, serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1.
Discussion
The results of this study showed that among patients with stable CAD, those with complex coronary lesions had significantly higher circulating sLOX‐1 levels than those with simple coronary lesions, and sLOX‐1 level was an independent predictor of the presence of complex coronary lesions. Moreover, among patients with ACS who had significantly higher circulating sLOX‐1 levels than stable CAD patients, sLOX‐1 levels are correlated with the number of complex coronary lesions. To the best of our knowledge, this is the first study to show that the serum level of sLOX‐1 represents a marker of atherosclerotic plaque complexity in CAD patients.
Rupture of vulnerable plaques with subsequent thrombus formation has been implicated as the most common pathogenic mechanism responsible for the development of ACS.24 It has been reported that vulnerable plaques prone to disruption or truly disrupted plaques are often manifested as angiographically complex lesions.25., 26., 27., 28. As the angiographic hallmark of plaque vulnerability, angiographically complex lesions correlate with pathologic plaque rupture and thrombus29 and have been shown to provide prognostic information.20., 30., 31. Therefore, it is important to develop a noninvasive blood biomarker to provide incremental predictive value for accurate prognostication of angiographically complex lesions in patients with CAD when added to other traditional risk factors.
Although vulnerable plaques are more common in ACS patients than in stable CAD patients, the incidence of the presence of vulnerable plaques is also high in the latter.32 Studies have recently supported the emerging role of LOX‐1 interaction in atherosclerotic plaque destabilization and rupture; LOX‐1 is significantly expressed in VSMCs and macrophages in advanced atherosclerotic plaques,33 resulting in apoptosis of VSMCs in the shoulder region of the atherosclerotic plaque8., 33. and production of MMPs.10 Local release of MMPs may degrade the extracellular matrix, further destabilizing the atherosclerotic plaque.34 Animal studies have also clarified the relationship between LOX‐1 expression levels and histological plaque vulnerability in vivo. An experimental study using hypercholesterolemic rabbit models demonstrated that LOX‐1 expression was associated with histological markers of plaque vulnerability, such as fibromuscular cap thickness and plaque instability index.35 Moreover, a recent study showed that imaging of a labeled anti–LOX‐1 antibody could sensitively evaluate atherosclerotic plaque vulnerability in a rabbit model of spontaneous atherosclerosis.36 Proteolytic cleavage of LOX‐1 from vulnerable plaques or thrombosis releases a soluble form of the receptor, thus releasing sLOX‐1.13 The major finding of the present study was that serum sLOX‐1 levels were independently correlated with the presence of angiographically complex coronary lesions in patients with stable CAD. These results concurred with those of earlier studies linking LOX‐1 to vulnerable plaques and suggested that sLOX‐1 might be a potential biomarker of providing valuable information about atherosclerotic plaque vulnerability in patients with stable CAD.
Similar to the previous study by Hayashida et al,16 our data confirmed that circulating sLOX‐1 levels were significantly higher in patients with ACS than in patients with stable CAD. The increased sLOX‐1 levels in ACS patients were most probably the result of the enhanced protease activity in vulnerable plaques or thrombosis. We also observed a striking association between increments in sLOX‐1 levels and number of complex lesions. On multivariate analysis, sLOX‐1 levels were independently associated with the presence of multiple complex coronary lesions. These results suggested that sLOX‐1 levels were correlated with the number of complex lesions and the burden of vulnerable plaques in patients with ACS. As multiple complex lesions are associated with adverse clinical outcomes in patients with ACS,37 it could be speculated that patients with higher sLOX‐1 levels might harbor multiple complex lesions and have higher incidence of adverse clinical outcomes. More recently, a pilot study gave support to this hypothesis and demonstrated that sLOX‐1 levels predicted prognosis after ACS.38
There are still some important limitations needed to be considered in this study. First, coronary angiography provides insufficient information regarding true lesion complexity and coronary thrombus, which can be evaluated in humans by updated vascular imaging modalities including virtual histology intravascular ultrasound, optical coherence tomography, and magnetic resonance imaging. However, a recent study has shown no significant differences in assessing progression/regression of coronary atherosclerotic plaques when using quantitative coronary angiography or intravascular ultrasound.39 Second, this cross‐sectional study has a relatively small sample size. Therefore, it is necessary to validate our data in prospective studies with a larger sample size. Last, we excluded diabetic patients to avoid the contribution of DM as a potential confounder, which might include some bias because complex lesions appear to be more prominent in patients with DM than in patients without DM.40
Conclusion
Our study demonstrated that serum sLOX‐1 levels were associated with angiographically complex coronary lesions that might predict vulnerable plaques. These results suggested that the sLOX‐1 might be a useful biomarker of plaque vulnerability in patients with CAD. For patients who have no opportunity to undergo angiography, sLOX‐1 appears to be a potential biomarker for stratifying vulnerable patients into risk categories, which could lead to improved patient or physician adherence to risk‐reducing behaviors or interventions. However, further studies are needed to define more clearly the significance of these findings.
References
- 1. Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low‐density lipoprotein. Nature. 1997;386:73–77. [DOI] [PubMed] [Google Scholar]
- 2. Chen M, Masaki T, Sawamura T. LOX‐1, the receptor for oxidized low‐density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. [DOI] [PubMed] [Google Scholar]
- 3. Iwai‐Kanai E, Hasegawa K, Sawamura T, et al. Activation of lectin‐like oxidized low‐density lipoprotein receptor‐1 induces apoptosis in cultured neonatal rat cardiac myocytes. Circulation. 2001;104:2948–2954. [DOI] [PubMed] [Google Scholar]
- 4. Hu CP, Dandapat A, Liu Y, et al. Blockade of hypoxia‐reoxygenation‐mediated collagen type I expression and MMP activity by overexpression of TGF‐beta1 delivered by AAV in mouse cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1833–H1838. [DOI] [PubMed] [Google Scholar]
- 5. Moriwaki H, Kume N, Kataoka H, et al. Expression of lectin‐like oxidized low density lipoprotein receptor‐1 in human and murine macrophages: upregulated expression by TNF‐alpha. FEBS Lett. 1998;440:29–32. [DOI] [PubMed] [Google Scholar]
- 6. Shih HH, Zhang S, Cao W, et al. CRP is a novel ligand for the oxidized LDL receptor LOX‐1. Am J Physiol Heart Circ Physiol. 2009;296:H1643–H1650. [DOI] [PubMed] [Google Scholar]
- 7. Li D, Mehta JL. Antisense to LOX‐1 inhibits oxidized LDL‐mediated upregulation of monocyte chemoattractant protein‐1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. [DOI] [PubMed] [Google Scholar]
- 8. Kume N, Kita T. Apoptosis of vascular cells by oxidized LDL: involvement of caspases and LOX‐1 and its implication in atherosclerotic plaque rupture. Circ Res. 2004;94:269–270. [DOI] [PubMed] [Google Scholar]
- 9. Smirnova IV, Kajstura M, Sawamura T, et al. Asymmetric dimethylarginine upregulates LOX‐1 in activated macrophages: role in foam cell formation. Am J Physiol Heart Circ Physiol. 2004;287:H782–H790. [DOI] [PubMed] [Google Scholar]
- 10. Li D, Liu L, Chen H, et al. LOX‐1 mediates oxidized low‐density lipoprotein‐induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. [DOI] [PubMed] [Google Scholar]
- 11. Mehta JL, Chen J, Hermonat PL, et al. Lectin‐like, oxidized low‐density lipoprotein receptor‐1 (LOX‐1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. [DOI] [PubMed] [Google Scholar]
- 12. Murase T, Kume N, Kataoka H, et al. Identification of soluble forms of lectin‐like oxidized LDL receptor‐1. Arterioscler Thromb Vasc Biol. 2000;20:715–720. [DOI] [PubMed] [Google Scholar]
- 13. Brinkley TE, Kume N, Mitsuoka H, et al. Variation in the human lectin‐like oxidized low‐density lipoprotein receptor 1 (LOX‐1) gene is associated with plasma soluble LOX‐1 levels. Exp Physiol. 2008;93:1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lubrano V, Del Turco S, Nicolini G, et al. Circulating levels of lectin‐like oxidized low‐density lipoprotein receptor‐1 are associated with inflammatory markers. Lipids. 2008;43:945–950. [DOI] [PubMed] [Google Scholar]
- 15. Kamezaki F, Yamashita K, Tasaki H, et al. Serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 correlates with oxidative stress markers in stable coronary artery disease. Int J Cardiol. 2009;134:285–287. [DOI] [PubMed] [Google Scholar]
- 16. Hayashida K, Kume N, Murase T, et al. Serum soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812–818. [DOI] [PubMed] [Google Scholar]
- 17. Nomata Y, Kume N, Sasai H, et al. Weight reduction can decrease circulating soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 levels in overweight middle‐aged men. Metabolism. 2009;58:1209–1214. [DOI] [PubMed] [Google Scholar]
- 18. Levin DC, Fallon JT. Significance of the angiographic morphology of localized coronary stenoses: histopathologic correlations. Circulation. 1982;66:316–320. [DOI] [PubMed] [Google Scholar]
- 19. Braunwald E. Unstable angina: a classification. Circulation. 1989;80:410–414. [DOI] [PubMed] [Google Scholar]
- 20. Kaski JC, Chester MR, Chen L, et al. Rapid angiographic progression of coronary artery disease in patients with angina pectoris: the role of complex stenosis morphology. Circulation. 1995;92:2058–2065. [DOI] [PubMed] [Google Scholar]
- 21. Kaski JC, Chen L, Chester M. Rapid angiographic progression of “target” and “nontarget” stenoses in patients awaiting coronary angioplasty. J Am Coll Cardiol. 1995;26:416–421. [DOI] [PubMed] [Google Scholar]
- 22. Ambrose JA, Winters SL, Stern A, et al. Angiographic morphology and the pathogenesis of unstable angina pectoris. J Am Coll Cardiol. 1985;5:609–616. [DOI] [PubMed] [Google Scholar]
- 23. Dupont WD, Plummer WD Jr. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11:116–128. [DOI] [PubMed] [Google Scholar]
- 24. Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992;326:242–250. [DOI] [PubMed] [Google Scholar]
- 25. Garcia‐Moll X, Coccolo F, Cole D, et al. Serum neopterin and complex stenosis morphology in patients with unstable angina. J Am Coll Cardiol. 2000;35:956–962. [DOI] [PubMed] [Google Scholar]
- 26. Otsuka F, Sugiyama S, Kojima S, et al. Plasma adiponectin levels are associated with coronary lesion complexity in men with coronary artery disease. J Am Coll Cardiol. 2006;48: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 27. Dubey L, Zeng H, Hashmi S, et al. Association of plasma leptin levels and complexity of the culprit lesion in patients with unstable angina. Int J Cardiol. 2008;126:183–189. [DOI] [PubMed] [Google Scholar]
- 28. Niccoli G, Ferrante G, Cosentino N, et al. Eosinophil cationic protein: a new biomarker of coronary atherosclerosis. Atherosclerosis. 2010;211:606–611. [DOI] [PubMed] [Google Scholar]
- 29. Goldstein JA, Chandra HR, O'Neill WW. Relation of number of complex coronary lesions to serum C‐reactive protein levels and major adverse cardiovascular events at one year. Am J Cardiol. 2005;96:56–60. [DOI] [PubMed] [Google Scholar]
- 30. Williams AE, Freeman MR, Chisholm RJ, et al. Angiographic morphology in unstable angina pectoris. Am J Cardiol. 1988; 62:1024–1027. [DOI] [PubMed] [Google Scholar]
- 31. Bugiardini R, Pozzati A, Borghi A, et al. Angiographic morphology in unstable angina and its relation to transient myocardial ischemia and hospital outcome. Am J Cardiol. 1991;67:460–464. [DOI] [PubMed] [Google Scholar]
- 32. Hong MK, Mintz GS, Lee CW, et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three‐vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110:928–933. [DOI] [PubMed] [Google Scholar]
- 33. Kataoka H, Kume N, Miyamoto S, et al. Expression of lectinlike oxidized low‐density lipoprotein receptor‐1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. [DOI] [PubMed] [Google Scholar]
- 34. Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5:265–282. [DOI] [PubMed] [Google Scholar]
- 35. Ishino S, Mukai T, Kume N, et al. Lectin‐like oxidized LDL receptor‐1 (LOX‐1) expression is associated with atherosclerotic plaque instability—analysis in hypercholesterolemic rabbits. Atherosclerosis. 2007;195:48–56. [DOI] [PubMed] [Google Scholar]
- 36. Ishino S, Mukai T, Kuge Y, et al. Targeting of lectinlike oxidized low‐density lipoprotein receptor 1 (LOX‐1) with 99mTc‐labeled anti‐LOX‐1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med. 2008;49:1677–1685. [DOI] [PubMed] [Google Scholar]
- 37. Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–922. [DOI] [PubMed] [Google Scholar]
- 38. Kume N, Mitsuoka H, Hayashida K, et al. Soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 predicts prognosis after acute coronary syndrome: a pilot study. Circ J. 2010;74: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 39. Berry C, L'Allier PL, Grégoire J, et al. Comparison of intravascular ultrasound and quantitative coronary angiography for the assessment of coronary artery disease progression. Circulation. 2007;115:1851–1857. [DOI] [PubMed] [Google Scholar]
- 40. Baris N, Akdeniz B, Uyar S, et al. Are complex coronary lesions more frequent in patients with diabetes mellitus? Can J Cardiol. 2006;22:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]


