Abstract
Background:
Both the Tpeak‐Tend interval (Tp‐e) and the Tp‐e/QT ratio have been linked to increased risk for arrhythmia. Patient Tp‐e/QT ratios were investigated prior to primary percutaneous coronary intervention (pPCI) in patients with ST‐segment elevation myocardial infarction (STEMI).
Hypothesis:
Tp‐e/QT ratio maybe asscioated with the prognosis in patients with ST‐segment elevation.
Methods:
A total of 338 patients (N = 338) with STEMI treated by pPCI were included. The Tp‐e and Tp‐e/QT ratio were determined using electrocardiograms in the subjects exhibiting ST‐segment elevation.
Results:
The Tp‐e/QT ratio was correlated with both short‐ and long‐term outcomes. Analysis of the receiver operating characteristic curve demonstrated that the optimal cutoff value for outcome prediction was a Tp‐e/QT ratio of 0.29. Of the 388 patients enrolled, 115 (34.0%) exhibited a Tp‐e/QT ratio ≥0.29. Patients with a Tp‐e/QT ratio ≥0.29 showed elevated rates of both in‐hospital death (21.9% vs 2.3%; P < 0.001) and main adverse cardiac events (MACE) (48.1% vs 15.3%; P < 0.005). After discharge, Tp‐e/QT ratios ≥0.29 remained an independent predictor of all‐cause death (35.5% vs 5.2%, P < 0.001) and cardiac death (32.3% vs 2.6%, P < 0.001).
Conclusions:
The Tp‐e/QT ratio may serve as a prognostic predictor of adverse outcomes after successful pPCI treatment in STEMI patients. Clin. Cardiol. 2012 doi: 10.1002/clc.22022
This work was supported by grants from the Henan Provincial People's Hospital. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Myocardial infarction (MI), a type of coronary artery disease, is a major cause of morbidity and mortality in contemporary populations. A variety of studies have focused on the electrophysiological characterization of arrhythmogenic substrates in the myocardium of MI patients, such as prolonged QT intervals and T wave alternans. These studies have shown clinical promise for predicting malignant arrhythmias and sudden cardiac death (SCD).1., 2., 3. Alternatively, other research groups have focused on the QT interval as an index for predicting fatal arrhythmia and SCD. A longer QT interval has been shown to be closely associated with increased risk for SCD in some congenital and acquired channelopathies. Even in certain organic diseases, including myocardial infarction, these elongated QT intervals have been used to successfully indicate SCD risk.4., 5., 6.
Recently, the interval from the peak to the end the T wave (Tpeak‐Tend interval [Tp‐e]) has been proposed for use in the prediction of malignant arrhythmia and SCD in some ion channel diseases.7., 8., 9. Previous studies10., 11., 12. involving canine and rabbit left ventricular wedge models have indicated that the Tp‐e interval in an electrogram measured across the wedge correlates well with the transmural dispersion of cellular repolarization (TDR). Additionally, the Tp‐e interval may serve as an index of total dispersion of repolarization (transmural, apico‐basal, and global). As body weight increases, however, the linear increase in the QT interval is accompanied by a parallel increase in the Tp‐e interval. Furthermore, as heart rate (HR) increases from 60 to 100 bpm, the Tp‐e interval decreases linearly from 40 to 110 ms. Despite these changes in HR, the Tp‐e/QT ratio remains relatively constant within the narrow range of 0.15 to 0.25.
In many patients with congenital long QT syndrome (LQTS) or short QT syndrome, disproportionate prolongation of the Tp‐e interval (TDR at the cellular level) relative to the QT interval play an important role in arrhythmogenesis. Therefore, the Tp‐e/QT ratio may serve as an accurate index for the dispersion of ventricular repolarization, independent of dynamic changes in HR. Contemporary research has suggested that the Tp‐e/QT ratio is a more accurate predictor of ventricular arrhythmias than the QT interval, corrected QT (QTc), or Tp‐e. Additionally, a higher Tp‐e/QT ratio has been associated with arrhythmic events associated with many clinical conditions.13 In the past year, 1 novel study14 also demonstrated that the Tp‐e/QT ratio has a notable association with malignant ventricular arrhythmia in patients with ST‐segment elevation myocardial infarction (STEMI). This study, although notable, did not provide information on the number of patients requiring coronary artery revascularization. Therefore, little is known about this index in patients with STEMI undergoing primary percutaneous coronary intervention (pPCI). Therefore, the present study aimed to evaluate the Tp‐e/QT ratio immediately before pPCI in patients with STEMI to determine both its short‐ and long‐term prognostic value.
Methods
Study Subjects
From January 2007 to January 2010, 483 consecutive Chinese patients admitted for STEMI who received pPCI were enrolled in the current study. Patients were included if they possessed1 relevant chest discomfort,2 electrocardiograph (ECG) changes fulfilling the criteria for STEMI,3., 15. a significant stenosis or occlusion requiring placement of at least 1 intracoronary stent, and successful pPCI with thrombolysis in myocardial ischemia flow grade 2 to 3.4 After pPCI, patients received a standard of care in accordance with clinical guidelines, including treatment with aspirin for life and clopidogrel for a minimum of 12 months. The study was approved by the institutional ethics committee prior to onset. All patients were informed of the study before data collection based on the procedure, and each participant provided written informed consent.
Patients were excluded if they exhibited atrial fibrillation (n = 53), a left bundle branch block (n = 23), a prior MI (n = 17), chronic treatment with type Ia or III antiarrhythmic drugs (n = 17), or noninterpretable ECG data (n = 18). Additionally, patients were excluded who were lost to follow‐up (n = 17). Based on the above criteria,6,16 145 subjects were excluded, resulting in the inclusion of a final total of 338 patients in the study.
Demographic and Clinical Data, Follow‐up, and End Points
Demographic and clinical data were obtained from the catheterization database of the Henan Provincial People's Hospital. The time to treatment was defined as the time from the first symptoms to the first balloon inflation, and it was divided into quartiles for the comparison of Tp‐e/QT ratios. The infarct‐related artery was classified as the left anterior descending artery, right coronary artery, circumflex artery, or another epicardial coronary artery >2 mm in diameter. The left ventricular ejection fraction (LVEF) was evaluated with echocardiography during the hospital stay. Primary short‐term end points were the incidence of death and main adverse cardiac events (MACE) during hospitalization. A MACE was defined as death, nonfatal myocardial infarction, or repeated coronary revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass graft), not including nonfatal heart failure. The primary long‐term end points were the incidence of death from all causes, cardiac death including cardiac failure, fatal myocardial infarction, and fatal arrhythmia and sudden death after hospital discharge.
ECG Recordings and Measurements
Just before pPCI, a digital 12‐lead standard ECG was recorded at 25 mm/s. Observers responsible for data recording were trained prior to the study in accordance with standard clinical procedures. To avoid difficulties in assessing T wave markers, the Tp‐e was evaluated only in subjects exhibiting ST‐segment elevation at the J point.8., 9. The QT interval was measured from the onset of the QRS complex to the point at which the tangent of the maximal downslope of the descending limb of the T wave crossed the isoelectric baseline. The QT interval was corrected for heart rate using Bazett's formula: QTc = QT/√(RR). All measured QT values were corrected using Bazett's formula, consistent with the approach widely accepted by other research groups. The QTp interval was measured from the onset of the QRS complex until the maximal deflection of the T wave. The Tp‐e was calculated as the difference between the QT interval and the QTp interval. The RR interval was taken as the average of 3 consecutive complexes, and all other measured intervals were also expressed as the average of measurements on 3 consecutive complexes. Furthermore, the measured data were additionally verified by a second physician to avoid inconsistencies before final inclusion. No analysis, however, was conducted to determine inter‐ and intraobserver variability.
Statistical Analysis
Statistical analyses were performed using SPSS 16.0 (IBM, Armonk, NY). Results were presented as the mean ± SD, frequencies, or mean and 95% confidence intervals (CI) for continuous variables. Categorical data are presented as the percent or alternatively the mean and range. Receiver operator characteristic (ROC) curves were computed for the Tp‐e/QT ratio to assess the optimal cutoff values to predict mortality after hospital discharge. The optimal cutoff value was defined as the value yielding the maximal Youden index (Youden index = b[sensitivity] + [specificity] − 1).17 Survival curves were calculated by the Kaplan‐Meier method, and the log‐rank test was used to compare different curves. Multivariate logistic regression analysis was performed to determine the predictors of in‐hospital outcomes. This analysis included variables with statistical significance in the univariate logistic regression analysis, and those with a known clinical impact. A Cox proportional hazards model was used to assess the impact of selected risk factors on the long‐term survival of patients after hospital discharge. P < 0.05 was considered statistically significant.
Results
Demographic and Baseline Characteristics
Demographic, clinical, and angiographic data of the 338 patients are summarized in Table 1. Before pPCI, the mean Tp‐e/QT ratio was 27.3, the median Tp‐e/QT ratio was 27.0, and the range of Tp‐e/QT ratios was 0.19 to 0.32.
Table 1.
Patient Demographics, Clinical Data, and Angiographic Data (N = 338)
| Age, y, mean (range) | 57.8 (32–78) |
|---|---|
| Male, n (%) | 232 (68.6) |
| BMI (kg/m2) | 25.1 ± 3.1 |
| Hypertension, n (%) | 197 (58.4) |
| Diabetes, n (%) | 80 (23.7) |
| Hypercholesterolemia, n (%) | 60 (17.8) |
| Positive family history of IHD, n (%) | 40 (11.9) |
| Previous and current smoking, n (%) | 163 (48.3) |
| Alcohol habit, n (%) | 117 (34.7) |
| Infarct‐related artery, n (%) | |
| LAD | 166 (49.2) |
| RCA | 126 (37.3) |
| LCX | 34 (10.1) |
| Other | 12 (3.5) |
| Stents | |
| No. of stents, mean (range) | 1.3 (1–4) |
| Drug‐eluting stent (%) | 77 |
| Bare‐metal stent (%) | 7 |
| Multiple stents (%) | 16 |
| Lesions | |
| Occlusion, n (%) | 269 (79.7) |
| No. of lesions, mean (range) | 1.3 (1–5) |
| Time to treatment (min) | 169 (30∼724) |
| Heart rate (bpm) | 87 ± 22 |
| LVEF (%) | 55 ± 12 |
| Medical therapy, n (%) | |
| Aspirin | 324 (96.0) |
| Clopidogrel | 331 (97.9) |
| Nitrates | 291 (86.1) |
| ACE inhibitors | 180 (53.3) |
| β‐Blockers | 209 (61.8) |
| Calcium channel blockers | 46 (13.6) |
| Statins | 327 (96.7) |
| Digoxin | 86 (25.4) |
| Antiarrhythmic drugs | 26 (7.7) |
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index; IHD, ischemic heart disease; LAD, left anterior descending artery; LCX, left circumflex coronary artery; LVEF, left ventricular ejection fraction; RCA, right coronary artery.
In‐Hospital Outcomes
During hospitalization, 29 (8.6%) patients died and 99 (29.3%) patients experienced MACE (Table 2). The Tp‐e/QT ratio was significantly elevated in patients who died or suffered MACE, indicating that these subjects experienced an increased risk of death (area under ROC curve: 0.88; P < 0.001) and MACE (area under ROC curve: 0.88; P < 0.001). The sensitivity and specificity of a Tp‐e/QT ratio ≥0.29 for death were 86% and 83%, respectively; the sensitivity and specificity of a Tp‐e/QT ratio ≥0.29 for MACE were 88% and 81%, respectively (Figure 1).
Table 2.
Patient Outcomes During Hospitalization
| End Points | n (%) |
|---|---|
| Death | 29 (8.6) |
| Cardiac failure | 19 (5.6) |
| Ventricular fibrillation | 6 (1.8) |
| Bradyarrhythmia | 3 (0.9) |
| Cardiac rupture | 1 (0.3) |
| MACE | 99 (29.3) |
| Death | 29 (8.6) |
| Nonfatal MI | 14 (4.1) |
| Repeat PCI | 13 (3.8) |
| Repeat CABG | 5 (1.5) |
| Ventricular tachyarrhythmia | 38 (11.2) |
Abbreviations: CABG, coronary artery bypass graft; MACE, major adverse cardiac event; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 1.
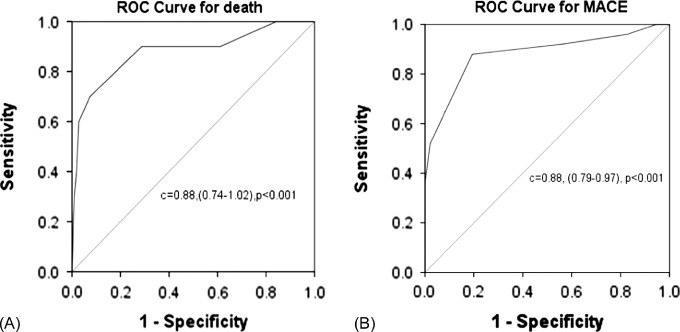
Receiver operating characteristic (ROC) curves of the Tp‐e/QT ratio for death (a) and major adverse cardiac event (MACE) (b) during hospitalization. A cutoff value of 0.29 resulted in optimal sensitivity and specificity.
In univariate logistic regression analysis, a Tp‐e/QT ratio ≥0.29 increased the risk of both death (21.9% vs 2.3%; P < 0.001) and MACE (48.1% vs 15.3 %; P < 0.001), as shown in Table 3. Multivariate logistic regression analysis included the following variables: age, diabetes mellitus, hypertension, heart rate, LVEF, angiotensin‐converting enzyme inhibitors, β‐blockers, calcium channel blockers, statins, and the use of antiarrhythmic drugs. In this analysis, a Tp‐e/QT ratio ≥0.29 was the only independent predictor of MACE.
Table 3.
Prognostic Implications of a Tp‐e/QT Ratio ≥0.29 During Hospitalization Based on Univariate Logistic Regression Analysis
| % | Odds Ratio | |
|---|---|---|
| Death | 21.9 vs 2.3 | 7.8 (95% CI: 1.6‐38.5); P < 0.001 |
| MACE | 48.1 vs 15.3 | 5.4 (95% CI: 1.5‐9.9); P < 0.001 |
Abbreviations: CI, confidence interval; MACE, major adverse cardiac event.A total of 115 patients exhibited a Tp‐e/QT ratio ≥0.29, and 34.0% exhibited a ratio <0.29.
Out‐of‐Hospital Outcomes
After discharge, during a mean follow‐up of 17 ± 8 months (median, 17 months), 36 patients (12.0%) died of any cause, 33 patients (11.1%) died of cardiac causes, and 18 (5.9%) patients suffered a new nonfatal myocardial infarction (Table 4). A Tp‐e/QT ratio ≥0.29 was associated with increased risk of death from both any cause and cardiac death. The area under the ROC curve was 0.75 (95% CI: 0.63‐0.81, P < 0.001) for death from any cause and 0.78 (95% CI: 0.64‐0.82, P < 0.001) for cardiac death A Tp‐e/QT ratio ≥0.29 had a sensitivity of 82% and a specificity of 67% to predict death from any cause, and a sensitivity of 83% and specificity of 71% to predict cardiac death (Figure 2). Bradyarrhythmias included in this figure include both sinus bradycardia and sinus arrest. Second‐ or third‐degree atrioventricular block was primarily associated with cardiac death.
Table 4.
Patient Outcomes During Follow‐up
| Outcomes After Hospital Discharge | n (%) |
|---|---|
| Death from all causes | 36 (12.0) |
| Cardiac death | 33 (10.7) |
| Cardiac failure | 22 (6.5) |
| Fatal arrhythmia and sudden death | 7 (2.1) |
| Fatal myocardial infarction | 4 (1.2) |
| New nonfatal myocardial infarction | 18 (5.9) |
Figure 2.
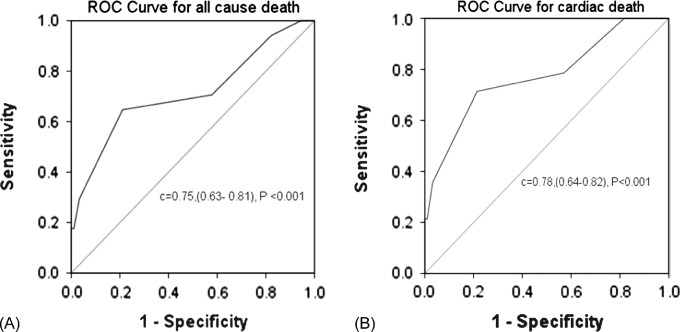
Receiver operating characteristic (ROC) curves of the Tp‐e/QT ratio for death from any cause (a) and cardiac death (b) after discharge. A cutoff value of 0.29 resulted in optimal sensitivity and specificity
In the Kaplan‐Meier analysis, a Tp‐e/QT ratio ≥0.29 increased the incidence of both death from any cause (35.5% vs 5.2%, P < 0.001) and cardiac death (32.3% vs 2.6%, P < 0.001) as shown in Figure 3. A Tp‐e/QT ratio ≥0.29 remained an independent predictor of death from any cause and cardiac death in the Cox regression analysis (Table 5).
Figure 3.
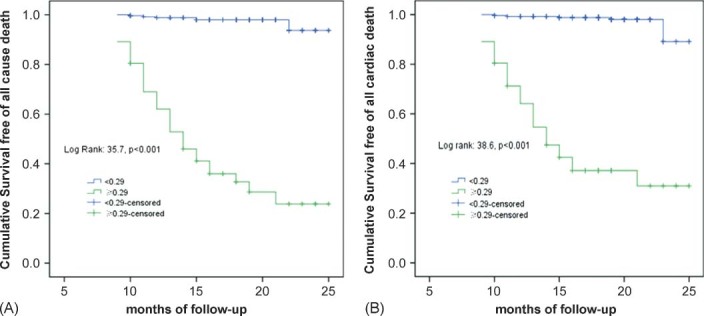
Kaplan‐Meier plots of the incidence of death from any cause (A) and cardiac death (B).
Table 5.
Independent Predictors of Major Events During Follow‐up in Cox Regression Analysis
| Hazard Ratio | P Value | |
|---|---|---|
| Cause of death | ||
| Any cause, age >65 years | 3.1 (95% CI: 1.1‐13.4) | 0.047 |
| Tp‐e/QT ratio ≥0.29 | 5.2 (95% CI: 1.9‐14.0) | <0.001 |
| LVEF ≤0.45 | 2.7 (95% CI: 1.4‐6.3) | |
| Cardiac cause of death | ||
| Age >65 years | 2.1 (95% CI: 1.1‐3.3) | 0.043 |
| Tp‐e/QT ratio ≥0.29 | 7.2 (95% CI: 2.3‐22.9) | <0.001 |
| LVEF ≤0.45 | 1.8 (95% CI: 1.2‐3.0) | 0.005 |
Abbreviations: CI, confidence interval; LVEF, left ventricular ejection fraction; Tp‐e, interval from the peak to the end of the T wave.
Discussion
The utility of the Tp‐e/QT ratio as a marker of prognosis in patients with STEMI undergoing pPCI was investigated based on the previous indication that certain Tp‐e/QT ratios may correlate with increased risk to patients experiencing myocardial infarction. Elongated Tp‐e/QT ratios have been associated with death and MACE during the hospital stay for these patients, and also with death from any cause or cardiac death associated with second‐ or third‐degree atrioventricular block during the follow‐up period. Use of the Tp‐e/QT ratio as an index for assessing risk in patients undergoing pPCI may allow for future improvements in prognosis and clinical assessment of these patients, leading to decreased risk of death both during and after the hospital stay.
The Tp‐e/QT ratio is a relatively new index of ventricular repolarization that remains constant despite differences in body mass or dynamic changes in heart rate within subjects, providing a valuable consistency and allowing longitudinal comparison of patient data. It is considered to be a more sensitive index of arrhythmogenesis compared with the sole use of either the Tp‐e or QT intervals, and it provides an estimate of dispersion of repolarization relative to total duration of repolarization.13 Some studies have shown that the Tp‐e/QT ratio is higher in certain congenitally acquired channelopathies and in hypertrophic cardiomyopathy. Furthermore, these studies have described the utility of the Tp‐e interval and the Tp‐e/QT ratio as an arrhythmogenic index in a variety of cardiac conditions associated with elevated risk for malignant arrhythmia and SCD. Yamaguchi et al18 demonstrated in patients with acquired LQTS that the Tp‐e/QT ratio is a superior predictor of Torsades de Pointes (TdP) than either the QTc interval or QT dispersion, demonstrating that a Tp‐e/QT ratio >0.28 was strongly associated with the risk of developing TdP in patients with acquired LQTS. In another study involving patients with hypertrophic cardiomyopathy, Shimizu et al19 demonstrated that the Tp‐e/QT ratio was significantly higher in patients who had an episode of SCD than in those without symptoms. Logistic regression analysis further revealed that the Tp‐e/QT ratio was a strong predictor of SCD in that group of patients. Letsas et al20 presented data addressing the problem of improving noninvasive risk stratification in patients with Brugada syndrome, indicating that an increased Tp‐e/QT ratio in lead V6 was associated with the inducibility of ventricular tachycardia and/or ventricular fibrillation (VF) in Brugada patients with a spontaneous or ajmaline‐induced type 1 ECG pattern (0.214 + 0.028 vs 0.180 + 0.014, P = 0.009). Shu et al14 evaluated 120 patients with STEMI and reported that the Tp‐e/QT ratio was significantly elevated in those who suffered malignant ventricular arrhythmias as compared with those who did not experience these events (0.32 ± 0.07 vs 0.26 ± 0.05, P < 0.001). No information, however, was provided to indicate the number of patients who experienced coronary artery revascularization with PCI or thrombolytic therapy, and no report on the onset period for malignant ventricular arrhythmia after STEMI was made. In contrast, the current study included patients with STEMI undergoing pPCI and established precise observational end points during hospitalization as well as over a relatively long follow‐up period (17 months). Furthermore, the prognostic implications of the Tp‐e/QT ratio during both hospitalization and long‐term follow‐up were evaluated to provide a clinically useful assessment scale.
Although studies of the Tp‐e/QT ratio have been performed in the context of specific channelopathies and some organic heart diseases, the current study is novel in its investigation of the broad association between prognosis and Tp‐e/QT ratio in Chinese patients with STEMI undergoing pPCI. In spite of the relatively small number of study subjects, the Tp‐e/QT ratio before pPCI was clearly demonstrated to have prognostic implications for STEMI patients during hospitalization and up to 17 months afterward, as exhibited by follow‐up studies. Furthermore, ROC curve analysis allowed for the establishment of an optical cutoff value for the Tp‐e/QT ratio, indicating that a Tp‐e/QT ratio ≥0.29 before pPCI was positively associated with increased incidence of death and MACE during hospitalization and an increased risk of all‐cause and cardiac death after discharge.
During acute MI, complex metabolic and electrochemical changes occur in cardiac muscle affecting tissue oxygen levels, pH, intercellular and intracellular ion channel status, electrochemical gradients, and current. These changes have a complex influence on the duration of action potentials in the ischemic zone and ischemic border zone; thus, the Tp‐e and QT intervals display changes that are modestly concordant. Yan et al21 found that phase 2 reentry caused by the heterogeneous loss of the transient outward potassium current during the epicardial action potential dome can produce a closely coupled extrasystole (R on T) leading to VF under conditions of ST‐segment elevation unrelated to ischemia.
The evidence outlined over the course of the current study clearly suggests the applicability of the Tp‐e/QT ratio as a potentially important clinical index of arrhythmogenesis under conditions of short, normal, or long QT intervals and in congenital and acquired channelopathies. Its potential applicability as an index of arrhythmogenesis in a variety of other cardiac conditions, such as heart failure, hypertrophy, or chronic MI, remains to be examined in future studies; however, it is likely that a correlation will be found based on the evidence presented in this study and its predecessors. Although all of these disorders have diverse genotypic and phenotypic features and different triggers for arrhythmia, the amplification of TDR (or repolarization dispersion) may serve as a final common substrate for arrhythmogenesis. More specifically, disproportionate amplification of TDR or global dispersion relative to the QT interval has pronounced arrhythmic effects. The direct validation of the Tp‐e interval from the body surface as an independent TDR index is still lacking, although several lines of evidence suggest the applicability of the Tp‐e interval as an index of arrhythmogenesis, indicating that further verification studies, as well as mechanistic studies, will be critical for improving the understanding of risk associated with myocardial events.
Study Limitations
The current observational study, by nature, was inclusive of nonstandardized treatment strategies. The study also, however, was a prospective study with careful screening and follow‐up of consecutive patients to avoid any selection or reporting bias. Thus, the reported data provide the most accurate picture of clinical outcomes across the current practice possible within these constraints.
The Tp‐e and QT intervals were measured manually using a conventional 12‐lead electrocardiogram recorded at 25 mm/s. This method of measurement may have reduced the accuracy of these measurements and affected the Tp‐e/QT ratio. To compensate, Tp‐e intervals were also measured using different ECG leads depending on the leads with ST elevation. Measurements of Tp‐e in different leads may have complicated the results. To reduce the effects of this variance, 3 complexes were measured, and the mean was adopted, thereby decreasing the differences between leads.
Furthermore, the indexes in patients with STEMI were only measured or calculated, and no measurement of healthy subjects was performed. Thus, no information for comparison of results between these groups exists, a baseline that would be a useful comparison to explore in future studies. In addition, the relatively small observational sample size and the limited number of events may have reduced the statistical power of the analysis; however, the clear statistical results suggest the validity of this approach. Finally, the current study was a single‐center study, with findings that necessitate confirmation in broader and larger multicenter trials.
Conclusion
In the STEMI, Tp‐e/QT ratio is a simple and useful tool in predicting the patients at high risk of suffering adverse events both in hospital and after discharge. Tp‐e/QT ratio may serve as a prognostic predictor of adverse outcomes after successful pPCI in STEMI patients, and more studies should be carried to further evaluate its clinical value.
Acknowledgements
The authors gratefully acknowledge the important contributions of both the staff and study participants for their assistance with data collection and analysis.
References
- 1. Cannon CP, Greenberg BH. Risk stratification and prognostic factors in the post‐myocardial infarction patient. Am J Cardiol. 2008;102:13G–20G. [DOI] [PubMed] [Google Scholar]
- 2. Huikuri HV, Raatikainen MJ, Moerch‐Joergensen R, et al. Prediction of fatal or near‐fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction. Eur Heart J. 2009;30:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuriachan V, Exner DV. Role of risk stratification after myocardial infarction. Curr Treat Options Cardiovasc Med. 2009;11:10–21. [DOI] [PubMed] [Google Scholar]
- 4. Dekker JM, Crow RS, Hannan PJ, et al. Heart rate‐corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle‐aged men and women: the ARIC study. J Am Coll Cardiol. 2004;43:565–571. [DOI] [PubMed] [Google Scholar]
- 5. Goldenberg I, Mathew J, Moss AJ, et al. Corrected QT variability in serial electrocardiograms in long QT syndrome: the importance of the maximum corrected QT for risk stratification. J Am Coll Cardiol. 2006;48:1047–1052. [DOI] [PubMed] [Google Scholar]
- 6. Jimenez‐Candil J, Gonzalez IC, Gonzalez Matas JM, et al. Short‐ and long‐term prognostic value of the corrected QT interval in the non‐ST‐elevation acute coronary syndrome. J Electrocardiol. 2007;40:180–187. [DOI] [PubMed] [Google Scholar]
- 7. Castro Hevia J, Antzelevitch C, Tornes Barzaga F, et al. Tpeak‐Tend and Tpeak‐Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanters JK, Haarmark C, Vedel‐Larsen E, et al. Tpeak Tend interval in long QT syndrome. J Electrocardiol. 2008;41:603–608. [DOI] [PubMed] [Google Scholar]
- 9. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol. 2008;41:575–580. [DOI] [PubMed] [Google Scholar]
- 10. Yan GX, Lankipalli RS, Burke JF, et al. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401–409. [DOI] [PubMed] [Google Scholar]
- 11. Yan GX, Martin J. Electrocardiographic T wave: a symbol of transmural dispersion of repolarization in the ventricles. J Cardiovasc Electrophysiol. 2003;14:639–640. [DOI] [PubMed] [Google Scholar]
- 12. Antzelevitch C, Sicouri S, Di Diego JM, et al. Does Tpeak‐Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta P, Patel C, Patel H, et al. Tp‐e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–574. [DOI] [PubMed] [Google Scholar]
- 14. Shu J, Li H, Yan G, Cui C. Tp‐e/QT ratio as a predictive index of sudden cardiac death in patients with ST‐segment elevation myocardial infarction. J Xi′an Jiaotong Univ Med Sci. 2010;31: 441–443. [Google Scholar]
- 15. Radke PW. Acute myocardial infarction: diagnosis and treatment [in German]. Med Monatsschr Pharm. 2011;34:78–84. [PubMed] [Google Scholar]
- 16. Porthan K, Viitasalo M, Jula A, et al. Predictive value of electrocardiographic QT interval and T‐wave morphology parameters for all‐cause and cardiovascular mortality in a general population sample. Heart Rhythm. 2009;6:1202–1208. [DOI] [PubMed] [Google Scholar]
- 17. Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut‐point estimated from observations affected by a lower limit of detection. Biom J. 2008;50: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamaguchi M, Shimizu M, Ino H, et al. T wave peak‐to‐end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond). 2003;105:671–676. [DOI] [PubMed] [Google Scholar]
- 19. Shimizu M, Ino H, Okeie K, et al. T‐peak to T‐end interval may be a better predictor of high‐risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Letsas KP, Weber R, Astheimer K, et al. Tpeak‐Tend interval and Tpeak‐Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12:271–274. [DOI] [PubMed] [Google Scholar]
- 21. Yan GX, Joshi A, Guo D, et al. Phase 2 reentry as a trigger to initiate ventricular fibrillation during early acute myocardial ischemia. Circulation. 2004;110:1036–1041. [DOI] [PubMed] [Google Scholar]


