Abstract
Background
There are no sufficient data to evaluate the relationship between high‐sensitivity C‐reactive protein (hs‐CRP) and uncovered stent struts on optical coherence tomography (OCT) after drug‐eluting stent (DES) implantation.
Hypothesis
We evaluated the relationship between the preprocedural level of hs‐CRP and incomplete neointimal coverage of DES struts on OCT.
Methods
This study was conducted using 124 eligible patients (132 lesions) treated with sirolimus‐eluting stents (SES) or zotarolimus‐eluting stents (ZES). The subjects were divided into 2 groups based on the preprocedural hs‐CRP level: high‐CRP (≥3 mg/L; 58 lesions) and normal‐CRP (<3 mg/L, 74 lesions) groups. The percentage of uncovered struts, calculated as the ratio of uncovered struts to total struts in all OCT cross‐sections, was compared between the 2 groups according to initial clinical presentation (stable angina [SA] vs acute coronary syndrome) and the type of implanted DES (SES vs ZES).
Results
There was no significant correlation between hs‐CRP and the percentage of uncovered struts on OCT in all enrolled lesions. In the SA subgroup, the percentage of uncovered struts was significantly higher in the high‐CRP group than in the normal‐CRP group (8.1 ± 11.6% vs 3.8 ± 7.9%, P = 0.018). There was significant correlation between hs‐CRP level and the percentage of uncovered struts in SA patients with SES (r = 0.280, P = 0.039), but not ZES (r = − 0.063, P = 0.729).
Conclusions
Preprocedural hs‐CRP level could affect incomplete neointimal coverage of struts after DES implantation depending on the initial clinical presentation and the type of implanted DES. Copyright © 2011 Wiley Periodicals, Inc.
This study was partly supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A085012 and A102064), the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A085136), and the Cardiovascular Research Center, Seoul, Korea. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Incomplete neointimal coverage of stent struts has recently been considered one of the most important predictors of late stent thrombosis following drug‐eluting stent (DES) implantation.1., 2. Optical coherence tomography (OCT) has been in the spotlight because its higher resolution has enabled in vivo evaluation of neointimal coverage of struts.3 Although the exact mechanism of incomplete neointimal coverage of stent struts after DES implantation has not been established, inflammation might be expected to contribute.2 High‐sensitivity C‐reactive protein (hs‐CRP) has emerged as the most powerful inflammatory marker for prediction of cardiovascular events.4 However, there are no sufficient data exploring the relationship between hs‐CRP with uncovered struts on OCT. Therefore, we evaluated the relationship between the preprocedural level of hs‐CRP and incomplete neointimal coverage of DES struts on OCT. We also assessed how these associations vary according to the initial clinical presentation (stable angina [SA] vs acute coronary syndrome [ACS]) and the type of implanted DES (sirolimus‐eluting stent [SES] vs zotarolimus‐eluting stent [ZES]).
Methods
We used the Yonsei OCT registry, which was developed to study neointimal coverage in patients who underwent coronary stenting of de novo lesions.5 The study population consisted of patients treated with 2 types of DES, SES (Cypher; Cordis, Miami, FL) or ZES (Endeavor Sprint; Medtronic, Santa Rosa, CA), which were the most frequently used stents in our registry between August 2007 and November 2009. The detailed inclusion criteria were de novo lesions treated with SES or ZES, available preprocedural hs‐CRP levels, and time to follow‐up OCT study within 6–16 months after DES implantation. General exclusion criteria for the follow‐up OCT study were as follows: untreated significant left main coronary artery disease, apparent congestive heart failure, renal insufficiency (baseline creatinine ≥2.0 mg/dL), lesions unsuitable for OCT imaging (vessel size ≥3.5 mm or lesions within 10 mm of the ostium of a major epicardial artery), and poor quality of OCT images.
Blood samples obtained before coronary intervention were centrifuged and hs‐CRP was determined by immunoturbidimetric assay with an autoanalyzer (Hitachi 7600‐210; Hitachi, Tokyo, Japan). Based on the upper limit of normal value for preprocedural hs‐CRP (3 mg/L), all enrolled patients were divided into 2 groups: the high‐CRP group (hs‐CRP ≥3 mg/L) and the normal‐CRP group (hs‐CRP <3 mg/L).
Implantation of DES was performed using current conventional techniques. The interventional strategy was left to the discretion of the operator. After DES implantation, all patients received dual antiplatelet therapy with aspirin and clopidogrel until follow‐up OCT. The OCT was performed using a conventional OCT system (Model M2 Cardiology Imaging System; LightLab Imaging, Inc., Westford, MA) with a motorized pull‐back system at 1 mm/s. The occlusion catheter was positioned proximal to the stent and a 0.014‐inch wire‐type imaging catheter (ImageWire; LightLab Imaging) was positioned distal to the stent. During image acquisition, the occlusion balloon (Helios; Avantec Vascular Corp., Sunnyvale, CA) was inflated to 0.4–0.6 atm, and lactated Ringer's solution was infused at 0.5–1.0 mL/s. The imaging wire was pulled from distal to proximal, and continuous images were acquired and stored digitally for subsequent analysis. Motorized pullback was performed at a rate of 1.0 mm/s with images at 15 frames/s.6., 7.
The OCT analysis was performed by independent staff blinded to patient and procedural information. Cross‐sectional OCT images were analyzed at 1‐mm intervals (every 15 frames). Stent and luminal cross‐sectional areas (CSAs) were measured at 1‐mm intervals and neointimal hyperplasia (NIH) CSA was calculated as stent CSA − luminal CSA. Mean values are reported in this study. Neointimal hyperplasia thickness, defined as the distance between the endoluminal surface of neointima and the strut, was measured inside all struts along a line as perpendicular as possible to the neointima and strut.3 An uncovered strut was defined as having an NIH thickness of 0 μm.3., 8. The percentage of uncovered struts in each stented lesion was calculated as (number of uncovered struts) × 100/(total number of struts in all cross‐sections of the lesion). Thrombi were defined as signal‐rich, low‐backscattering protrusions or high‐backscattering protrusions inside the artery lumen with signal‐free shadowing on OCT images.3
Quantitative coronary angiography was performed using an offline system (CAAS System II; Pie Medical Imaging, Maastricht, The Netherlands) before and after stent implantation, and on follow‐up angiogram. The minimal luminal diameter of the treated coronary segments and the reference segment diameter were measured each time. The reference vessel diameter was the average of the proximal and distal reference lumen diameters.
This study was approved by the institutional review board of Yonsei University College of Medicine, and written informed consent was obtained from each patient.
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL). Categorical data are presented as numbers and percentages, and were compared using χ 2 or Fisher exact tests. Continuous data are presented as mean ± SD, and were compared using the Student t test. If the distributions were skewed, a nonparametric test was used. The correlation of hs‐CRP levels with the percentage of uncovered stents was examined by Pearson's correlation. A P value <0.05 was considered statistically significant.
Results
A total of 124 patients with 132 lesions were eligible for analysis in this OCT study. The high‐CRP group (hs‐CRP ≥3 mg/L; 17.3 ± 20.3 mg/L) was composed of 54 patients with 58 lesions, and the normal‐CRP group (hs‐CRP <3 mg/L; 1.0 ± 0.7 mg/L) had 70 patients with 74 lesions. The baseline clinical, angiographic, and procedural characteristics of the 2 groups are shown in Tables 1 and 2. Follow‐up OCT measurements are shown in Table 3.
Table 1.
Baseline Clinical Characteristics
| Variable | High‐CRP Groupa (n = 54) | Normal‐CRP Groupb (n = 70) | P Value |
|---|---|---|---|
| Age, y | 62 ± 9 | 58 ± 9 | 0.017 |
| Male sex, n (%) | 30 (56) | 53 (76) | 0.022 |
| HT, n (%) | 24 (44) | 41 (59) | 0.118 |
| DM, n (%) | 17 (32) | 23 (33) | 0.871 |
| Dyslipidemia, n (%) | 17 (32) | 27 (39) | 0.413 |
| Current smoker, n (%) | 18 (33) | 17 (24) | 0.275 |
| Previous MI, n (%) | 1 (2) | 4 (6) | 0.381 |
| Clinical presentation, n (%) | 0.015 | ||
| SA | 29 (54) | 49 (70) | |
| ACS | 25 (46) | 21 (30) | |
| UA | 4 (7) | 4 (6) | |
| NSTEMI | 6 (11) | 7 (10) | |
| STEMI | 15 (28) | 10 (14) | |
| Medications at index procedure, n (%) | |||
| Aspirin | 54 (100) | 69 (99) | 0.825 |
| Clopidogrel | 56 (100) | 70 (100) | 1.000 |
| β‐Blocker | 46 (87) | 55 (79) | 0.239 |
| ACEI or ARB | 38 (70) | 49 (71) | 0.938 |
| Statin | 52 (96) | 67 (96) | 0.813 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; DM, diabetes mellitus; hs‐CRP, high‐sensitivity C‐reactive protein; HT, hypertension; MI, myocardial infarction; NA, not applicable; NSTEMI, non–ST‐elevation myocardial infarction; SA, stable angina; STEMI, ST‐elevation myocardial infarction; UA, unstable angina.
Hs‐CRP ≥3 mg/L.
Hs‐CRP <3 mg/L.
Table 2.
Angiographic and Procedural Characteristics
| Variable | High‐CRP Groupa (n = 58) | Normal‐CRP Groupb (n = 74) | P Value |
|---|---|---|---|
| Lesion type, B2 or C, n (%) | 53 (91) | 71 (96) | 0.363 |
| Lesion length (mm) | 23.5 ± 5.5 | 21.7 ± 6.0 | 0.076 |
| Stent diameter (mm) | 2.94 ± 0.31 | 3.04 ± 0.38 | 0.127 |
| Stent length (mm) | 25.9 ± 6.1 | 24.3 ± 6.2 | 0.111 |
| Type of DES, n (%) | 0.604 | ||
| SES | 34 (59) | 40 (54) | |
| ZES | 24 (41) | 34 (46) | |
| Quantitative coronary angiography analysis | |||
| Reference vessel diameter (mm) | 2.67 ± 0.37 | 2.67 ± 0.37 | 0.876 |
| Preintervention MLD (mm) | 0.76 ± 0.53 | 0.76 ± 0.49 | 0.983 |
| Postintervention MLD (mm) | 2.66 ± 0.30 | 2.70 ± 0.41 | 0.242 |
| Follow‐up MLD (mm) | 2.30 ± 0.42 | 2.30 ± 0.42 | 0.959 |
Abbreviations: DES, drug‐eluting stent; hs‐CRP, high‐sensitivity C‐reactive protein; MLD, minimal lumen diameter; NA, not applicable; SES, sirolimus‐eluting stent; ZES, zotarolimus‐eluting stent.
Hs‐CRP ≥3 mg/L.
Hs‐CRP <3 mg/L.
Table 3.
Follow‐Up OCT Measurements
| Variable | High‐CRP Groupa (n = 58) | Normal‐CRP Groupb (n = 74) | P Value |
|---|---|---|---|
| No. of cross‐sections | 1600 | 1773 | NA |
| Total no. of analyzable struts | 14 717 | 17 618 | NA |
| Time to follow‐up OCT (d) | 284 ± 85 | 284 ± 59 | 0.952 |
| Uncovered struts (%) | 6.9 ± 10.4 | 5.4 ± 11.4 | 0.080 |
| Mean stent CSA (mm2) | 7.3 ± 1.8 | 7.4 ± 1.9 | 0.925 |
| Mean lumen CSA (mm2) | 6.1 ± 1.7 | 5.9 ± 1.6 | 0.410 |
| Mean NIH CSA (mm2) | 1.2 ± 1.0 | 1.4 ± 1.0 | 0.193 |
| Mean NIH thickness (μm) | 139 ± 119 | 182 ± 141 | 0.060 |
| Presence of thrombi, n (%) | 15 (25) | 11 (15) | 0.151 |
Abbreviations: CSA, cross‐sectional area; hs‐CRP, high‐sensitivity C‐reactive protein; NA, not applicable; NIH, neointimal hyperplasia; OCT, optical coherence tomography.
Hs‐CRP ≥3 mg/L.
Hs‐CRP <3 mg/L.
Although statistical significance was not achieved for the difference in percentage of uncovered struts between the high‐CRP and normal‐CRP groups in the all enrolled patients (6.9 ± 10.4% vs 5.4 ± 11.4%, respectively, P = 0.080), the percentage of uncovered struts was significantly higher in the high‐CRP group compared with the normal CRP group in the subgroup of patients with SA (8.1 ± 11.6% vs 3.8 ± 7.9%, respectively, P = 0.018). There was no significant correlation between preprocedural hs‐CRP level and the percentage of uncovered struts in the all enrolled patients (r = 0.030, P = 0.729). However, in the SA subgroup, there was significant correlation of the preprocedural hs‐CRP level with the percentage of uncovered struts (r = 0.302, P = 0.005).
When we compared the percentage of uncovered struts between the high‐ and normal‐CRP groups according to the type of implanted DES, a significant difference was found only in SA patients treated with a SES (12.7 ± 12.5% vs 6.7 ± 9.7%, respectively, P = 0.029; Figure 1A). In the patients with ZES implantation, there were no significant differences in the percentage of uncovered struts, irrespective of initial clinical presentation (Figure 1B). In the subgroup of SA patients, the preprocedural hs‐CRP level in the lesions receiving SES correlated significantly with the percentage of uncovered struts on OCT (r = 0.280, P = 0.039; Fig. 2A). No correlation was observed in the patients treated with ZES (r = − 0.063, P = 0.729; Figure 2B). In the subgroup of ACS, there were no significant correlations between hs‐CRP levels and the percentage of uncovered struts on OCT in the both SES (r = − 0.216, P = 0.311; Fig. 2C) and ZES (r = − 0.119, P = 0.601; Fig. 2D).
Figure 1.
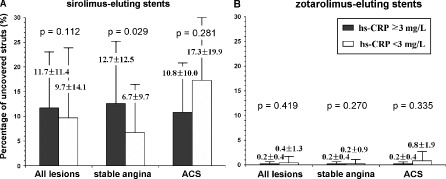
Comparisons of the percentage of uncovered struts on OCT between patients with hs‐CRP ≥3 mg/L vs hs‐CRP <3 mg/L according to the type of implanted drug‐eluting stents in all enrolled patients, the patients with SA, and the patients with ACS. (A) sirolimus‐eluting stents and (B) zotarolimus‐eluting stents. Abbreviations: ACS, acute coronary syndrome; hs‐CRP, high‐sensitivity C‐reactive protein; OCT, optical coherence tomography.
Figure 2.
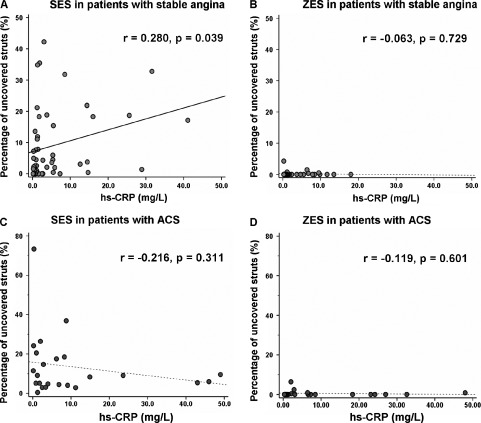
Association between preprocedural level of hs‐CRP and the percentage of uncovered struts on OCT in patients with SA (A) and (B) or ACS (C) and (D) according to the type of implanted DES: (A) and (C), SES and (B) and (D), ZES. Abbreviations: ACS, acute coronary syndrome; DES, drug‐eluting stent; hs‐CRP, high‐sensitivity C‐reactive protein; OCT, optical coherence tomography; SA, stable angina; SES, sirolimus‐eluting stent; ZES, zotarolimus‐eluting stent.
Discussion
This study suggested that the preprocedural hs‐CRP level could conditionally influence incomplete neointimal coverage of stent struts on OCT after DES implantation depending on the initial clinical presentation or the type of implanted DES. In the subgroup of patients who presented with SA and were treated with SES, the preprocedural hs‐CRP level significantly correlated with a higher percentage of uncovered struts. However, there was no significant correlation between the preprocedural hs‐CRP level and the percentage of uncovered struts on OCT in the lesions treated with ZES.
The exact mechanisms contributing to uncovered struts or incomplete endothelialization after DES implantation have not been clearly elucidated. Clinical factors such as ACS and diabetes mellitus, and type of implanted DES have been suggested as possible predictors of uncovered struts in several OCT studies.9., 10., 11., 12. However, little is known about the role played by the degree of inflammation, which may be pivotal in the pathological process of incomplete neointimal coverage after DES implantation.4
Although clinical presentation and DES type have been regarded as the most important predictors of uncovered struts, there have been no sufficient data on the effects of these 2 predictors on neointimal coverage according to the level of preprocedural hs‐CRP.3., 9., 10., 12. In this study, the relationship between preprocedural hs‐CRP and the proportion of uncovered struts on OCT varied depending on the initial clinical presentation and the type of implanted DES. Compared with the ACS patients, a higher inflammatory activity may predominantly affect neointimal coverage in the SA patients. The effects of CRP in ACS patients have been well established.13., 14. However, poor relations between CRP and neointimal coverage in the ACS patients could partly be explained by other factors such as thrombotic milieu, higher incidence of stent malapposition, and resolution of compressed thrombus by stent struts rather than inflammatory activity.
This study also showed that the type of implanted DES affected the relationship between preprocedural hs‐CRP and the percentage of uncovered struts on OCT in selected patients. Especially in patients with SA treated with SES, preprocedural hs‐CRP level was significantly correlated with the percentage of uncovered struts on OCT. Use of SES is well known to be a risk factor for the occurrence of uncovered stent struts.3., 9., 15. The relatively higher suppression of NIH by sirolimus might be aggravated by high inflammatory states, and this could cause less‐complete coverage of stent struts, compared with ZES.9., 15. Unlike SES, ZES have been reported to be associated with higher rates of neointimal coverage than SES in previous imaging studies.9., 12. The superior capacity for neointimal coverage by ZES might be due to the rapid drug diffusion and different polymer type. However, these results would need to be verified in a large, controlled, long‐term clinical study for definitive conclusions.
This study has several limitations. Being a retrospective analysis, this small observational study could be subject to selection bias. The number of study patients was not large enough to perform separate analyses according to initial clinical presentation and the type of implanted DES. In addition, the study was limited to only 2 DES types (SES and ZES) among many currently available DES types. Therefore, these results might not be applicable to other types of DES. Finally, the percentage of uncovered struts on OCT was used as a parameter for delayed healing, but the clinical implication of this parameter has not been clearly established.
Conclusion
Preprocedural hs‐CRP level could affect incomplete neointimal coverage of struts after DES implantation, depending on the initial clinical presentation and the type of implanted DES.
References
- 1. Farb A, Burke AP, Kolodgie FD, et al. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003;108:1701–1706. [DOI] [PubMed] [Google Scholar]
- 2. Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug‐eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. [DOI] [PubMed] [Google Scholar]
- 3. Takano M, Inami S, Jang IK, et al. Evaluation by optical coherence tomography of neointimal coverage of sirolimus‐eluting stent three months after implantation. Am J Cardiol. 2007;99:1033–1038. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM. High‐sensitivity C‐reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. [DOI] [PubMed] [Google Scholar]
- 5. Kim JS, Hong MK, Fan C, et al. Intracoronary thrombus formation after drug‐eluting stents implantation: optical coherence tomographic study. Am Heart J. 2010;159:278–283. [DOI] [PubMed] [Google Scholar]
- 6. Kim U, Kim JS, Kim JS, et al. The initial extent of malapposition in ST‐elevation myocardial infarction treated with drug‐eluting stent: the usefulness of optical coherence tomography. Yonsei Med J. 2010;51:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prati F, Regar E, Mintz GS, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31: 401–415. [DOI] [PubMed] [Google Scholar]
- 8. Barlis P, Dimopoulos K, Tanigawa J, et al. Quantitative analysis of intracoronary optical coherence tomography measurements of stent strut apposition and tissue coverage. Int J Cardiol. 2010;141:151–156. [DOI] [PubMed] [Google Scholar]
- 9. Kim JS, Jang IK, Kim JS, et al. Optical coherence tomography evaluation of zotarolimus‐eluting stents at 9‐month follow‐up: comparison with sirolimus‐eluting stents. Heart. 2009;95: 1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kubo T, Imanishi T, Kitabata H, et al. Comparison of vascular response after sirolimus‐eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2008;1:475–484. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalo N, Barlis P, Serruys PW, et al. Incomplete stent apposition and delayed tissue coverage are more frequent in drug‐eluting stents implanted during primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction than in drug‐eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2:445–452. [DOI] [PubMed] [Google Scholar]
- 12. Kim JS, Fan C, Choi D, et al. Different patterns of neointimal coverage between acute coronary syndrome and stable angina after various types of drug‐eluting stents implantation: 9‐month follow‐up optical coherence tomography study. Int J Cardiol. In press. doi:10.1016/j.ijcard.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 13. Park DW, Lee CW, Yun SC, et al. Prognostic impact of preprocedural C reactive protein levels on 6‐month angiographic and 1‐year clinical outcomes after drug‐eluting stent implantation. Heart. 2007;93:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller C, Buettner HJ, Hodgson JM, et al. Inflammation and long‐term mortality after non‐ST elevation acute coronary syndrome treated with a very early invasive strategy in 1042 consecutive patients. Circulation. 2002;105:1412–1415. [DOI] [PubMed] [Google Scholar]
- 15. Takano M, Yamamoto M, Inami S, et al. Long‐term follow‐up evaluation after sirolimus‐eluting stent implantation by optical coherence tomography: do uncovered struts persist? J Am Coll Cardiol. 2008;51:968–969. [DOI] [PubMed] [Google Scholar]


