Abstract
Background:
Coronary artery atherosclerosis has been associated with obstructive sleep apnea (OSA). However, the type and severity of plaque formation have not been characterized. This study evaluated the association of coronary noncalcified plaques and severity of stenosis in patients with OSA.
Hypothesis:
Methods:
This study was a retrospective analysis of 81 patients, 49 with OSA and 32 without OSA, who had undergone multidetector‐row helical computed tomography scanning. The board‐certified radiologist was blinded to the diagnosis of OSA and reviewed the scans for plaque characterization, severity of stenosis, and number of vessels involved.
Results:
Of the 81 patients reviewed, the mean apnea‐hypopnea index in the OSA group was 42.2 vs 7.5 in the non‐OSA group. The groups did not significantly differ in the distribution of comorbid conditions. We found that among the patients with OSA, 63% had noncalcified/mixed plaques, as opposed to 16% in the non‐OSA group (P < 0.0001), with unadjusted odds ratio of 9.3 (3.0, 28.4). After adjustment for other risk factors such as age, sex, race, hypercholesterolemia, and history of smoking, the association remained strong, with an odds ratio of 7.0 (1.9, 26.5; P < 0.05).
Conclusions:
Our study finds that the frequency of noncalcified/mixed plaques is much higher in patients with OSA than in non‐OSA patients. Patients with OSA also have more severe stenosis and a higher number of vessels involved. This study adds to a growing body of data regarding our understanding of the association of OSA and atherosclerosis.
Dr. Sharma, Dr. Schoepf, Mr. Parker, Mr. Abro, and Mr. Armstrong contributed to data collection and analysis, drafting of the manuscript, and final approval. Dr. Gebregziabher contributed to data analysis, drafting of the manuscript, and final approval. Dr. Sharma had access to and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors of this manuscript have certified that they comply with the principles of ethical publishing (Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1‐2.).
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
The prevalence of obstructive sleep apnea (OSA) in the general population has been reported to be 9% in females and 25% in males.1 Studies have shown that OSA may adversely affect many cardiovascular diseases, including hypertension, congestive heart failure, and stroke.2, 3, 4, 5, 6, 7 The prevalence of OSA in patients with a history of myocardial infarction (MI)/acute coronary syndrome (ACS) has been shown to be 2–3‐fold higher.8 Other studies have reported similar association between the 2 conditions, with odds ratios (ORs) generally in the range of 4.1 to 4.5 in both men and women.9, 10
Despite increasing evidence of association between OSA and coronary artery disease (CAD), it is not clear whether OSA causes or accelerates the process of atherosclerosis and plaque formations. Although there are data that presence and severity of OSA correlated with the presence and extent of calcified plaque,11, 12 no study has looked at noncalcified plaques in OSA. Calcified plaques, which are commonly found in atherosclerosis, rarely affect plaque stability.13, 14, 15 It is believed that the noncalcified plaques and mixed plaques are more prone to rupture and may play an important role in ACS.16 The traditional noninvasive imaging techniques do not identify noncalcified plaques. Multidetector‐row computed tomography (MDCT) recently has been demonstrated as a reliable noninvasive technique for detection and characterization of noncalcified atherosclerotic plaques.17 We hypothesized that patients with OSA may have a higher burden of noncalcified and mixed plaques as determined by MDCT. We also looked at severity of stenosis and number of vessels involved as markers of increased atherosclerotic burden.
Methods
This study was part of a larger ongoing prospective study performed by the department of radiology of Medical University of South Carolina evaluating MDCT and magnetic resonance imaging in a cohort of patients admitted for chest pain and suspected of having MI. The aim of this larger study was to evaluate cardiac perfusion and viability using above‐mentioned advanced radiological techniques. The major exclusion criteria for the radiology study were age <18 years and patients with asthma or impaired renal function (creatinine >1.5 mg/dL). Once the MDCT scans were completed and interpreted by the radiologist, the sleep researchers contacted the patients to get information on sleep‐apnea history. If the patient had undergone a sleep study, permission was obtained telephonically from the patient to review those records for the sleep study report or diagnosis. Only patients who underwent gold‐standard polysomnography within 3 years of the MDCT scan were included.
A total of 214 patients who had undergone MDCT scanning as part of the larger radiological study were identified. Of these, 134 patients responded to telephonic interview. Out of 134 patients, 83 patients had undergone formal sleep studies. Two patients had sleep studies >36 months prior to the MDCT scan, and hence were excluded. The other 51 patients had no history of OSA or sleep studies and were screened negative on the Berlin OSA screening questionnaire. Finally, 81 patients were included in the final analysis, 49 patients with OSA and 32 patients without OSA (Figure 1). Data on the use of or compliance with continuous positive airway pressure (CPAP) therapy were not available.
Figure 1.
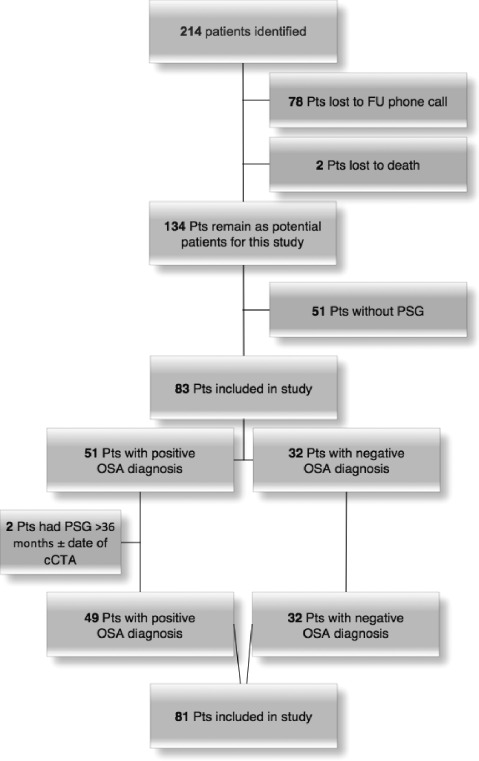
Flow diagram showing patient recruitment based on inclusion and exclusion criteria. Abbreviations: cCTA; coronary computed tomography angiography; FU, follow‐up; OSA, obstructive sleep apnea; PSG, polysomnography; Pts, patients.
Institutional review board approval was obtained from the Medical University of South Carolina and East Carolina University institutional review boards (MUSC HR#15644).
Imaging Technique
Recently, helical MDCT scanners became available that combine fast rotation of the detection device and simultaneous acquisition of multiple sections. With this type of computed tomography (CT) scanner, the patient's electrocardiogram is simultaneously recorded with the image acquisition. Based on this combined data, motion‐free images of the heart can be noninvasively generated. In contrast to cardiac catheterization, and similar to intravascular ultrasound, lesions and structures within the walls of the coronary arteries, such as calcified and noncalcified atherosclerotic lesions, can also be seen owing to the cross‐sectional nature of a CT image. A 64‐slice, multidetector‐row, dual‐source CT scanner allows for covering the entire coronary‐artery tree with a temporal resolution of 83 to 150 msec and a spatial resolution of 0.4 mm along the scan axis within a single breath‐hold. The ability of MDCT to noninvasively detect coronary‐artery stenosis and to assess the global nature (ie, calcified vs noncalcified) and extent of the atherosclerotic plaque burden has been demonstrated in several investigations.18, 19, 20, 21, 22 The scanner used in this study was a dual‐source, 64‐channel MDCT scanner (Somatom Definition; Siemens Healthcare, Forchheim, Germany).
Contrast enhancement was achieved with 60 to 120 mL of Omnipaque 350, depending on scan duration. Patients were given between 0.4 mg and 0.8 mg of nitroglycerine sublingually to decrease the risk of coronary artery spasm and to dilate the arteries to enhance visualization.
A single board‐certified radiologist reviewed and interpreted all CT scans for the study patients. Overnight polysomnography was performed at nationally accredited sleep centers, and final diagnosis with apnea‐hypopnea index (AHI; measure of the severity of disease) was available in all patients included in this study. Obstructive sleep apnea was further classified as no OSA (AHI <10), mild OSA (AHI 10–20), and severe OSA (AHI> 20). The number of vessels involved was defined as 0 if there was no stenosis and 1 if any stenosis or plaque was present. Severe stenosis was defined as ≥70% occlusion of the coronary vessels.
Statistical Analysis
All analyses were performed with SAS/STAT software, version 9.2 (SAS Institute Inc., Cary, NC). Categorical variables were presented as percentages, and continuous variables were presented as means along with their corresponding 2‐sided 95% confidence intervals (CIs). The Student t test and Wilcoxon rank‐sum test were used to assess the differences in sample characteristics between OSA‐positive and OSA‐negative patients, as well as among the AHI severity. Due to small sample size with AHI information (n = 51), AHI groups were collapsed to categories (category 0, AHI ≤10; category 1, AHI 10–20; category 2, AHI ≥20). Unadjusted association between coronary measures such as stenosis severity and number of vessels involved with patient level measurements such as volume, Agatston score, age, race, sex, and history of diabetes and hypertension were assessed using the χ 2 test. We also used the χ 2 test to examine the relationship between these coronary measures and OSA measures (OSA status and AHI score).
To study the adjusted association (adjusted for patient characteristics and comorbidities) between the main coronary outcomes—severity of stenosis (1 = normal, 2 = mild, 3 = severe) and number of vessels involved (0, 1, or >1)—with OSA status and AHI severity (1, AHI <10; 2, ahi 10–20; 3, ahi >20), we used polytomous logistic regression. First, we tested whether the odds are proportional using the score test for proportionality of odds in SAS (Hosmer‐Lemeshow, 2000), and when the proportionality assumption did not hold true (P > 0.05), we used the generalized logit model. We used the Hosmer‐Lemeshow test of goodness of fit, which is based on the deciles of predicted probabilities, to test whether the model fits the data or not. Model diagnostics was performed using SAS tools that detect outliers and influential data points. We used residual deviance, the hat matrix diagonal, and the residual χ 2 deviance and the difference between χ 2 goodness of fit when an observation is deleted. Plots of these against the predicted values were used to investigate the influence of each data point on the model.
Results
Baseline characteristics between the 2 groups revealed a mean age of 59.6 ± 11.8 years in the OSA group vs 54.1 ± 12.7 years in the non‐OSA group (P = 0.05). The mean BMI was 34.4 kg/m2 in the OSA group vs 34.4 kg/m2 in non‐OSA group (P = 0.98). The mean AHI in the OSA group was 42.2 vs 7.5 in the non‐OSA group (P = 0.0001). Baseline characteristics of the 2 groups are compared in the Table 1.
Table 1.
Patient Characteristics by OSA Status (N = 81)
| Variables | OSA (n = 49) | Non‐OSA (n = 32) | P Value (Chisp) |
|---|---|---|---|
| Age, y (mean ± SD) | 59.6 ± 11.8 | 54.1 ± 12.7 | 0.05 |
| Male sex, n (%) | 31 (63) | 14 (43) | 0.08 |
| DM, n (%) | 14 (31) | 8 (26) | 0.62 |
| Hypercholesterolemia, n (%) | 28 (62) | 13 (42) | 0.08 |
| Hypertension, n (%) | 38 (84) | 24 (77) | 0.44 |
| Caucasian, n (%) | 37 (75) | 17 (53) | 0.04 |
| Past smoking, n (%) | 28 (62) | 12 (39) | 0.04 |
| Mean BMI (kg/m2) | 34.4 | 34.4 | 0.98 |
| Mean AHI | 42.2 | 7.5 | 0.0001 |
Abbreviations: AHI, apnea‐hypopnea index; BMI, body mass index; DM, diabetes mellitus; OSA, obstructive sleep apnea; SD, standard deviation.
We found that among patients who had OSA, 63% had noncalcified/mixed plaques, as opposed to 16% in the non‐OSA group (P < 0.0001) (figure 2). After adjustment for other risk factors such as age, sex, race, smoking history, and hypercholesterolemia (which showed a statistically significant trend [Table]), the association remained strong (OR: 7.0, 95% CI: 1.9‐26.5, P < 0.05). On further stratifying OSA based on AHI score, the odds of having noncalcified/mixed plaques in mild OSA (AHI 10–20) was 3.2 (95% CI: 0.2‐43.7), and in moderate‐to‐severe OSA (AHI >20) it was 14.2 (95% CI: 1.3‐158.5). These odds were adjusted for age, sex, race, smoking history, and hypercholesterolemia.
Figure 2.
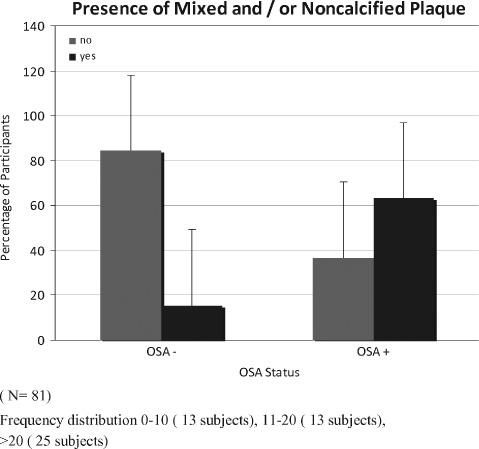
Comparison of percentage of patients who had mixed/noncalcified plaques in obstructive sleep apnea (OSA) vs non‐OSA. This dataset contained 81 participants, 49 with OSA and 32 without OSA (P < 0.0001). Abbreviations: OSA, obstructive sleep apnea.
We looked at the number of vessels involved in both groups. The OSA group had significantly more vessel involvement (85.7% having multiple vessels involved) than did the non‐OSA group (34.5% having multiple vessels involved), with an adjusted OR for having multiple vessels of 8.5 (95% CI: 2.3‐30.8).
When patients were divided by OSA severity into normal (AHI <10), mild (AHI 10–20), and severe (AHI >20) groups, the odds for any vessel involvement were 9.6 (95% CI: 1.1‐85.3) for mild OSA and 42.1 (95% CI: 4.1‐428.4) for moderate to severe OSA. These odds were adjusted for age, sex, race, hypercholesterolemia, and smoking history.
Similarly, patients with OSA had significantly higher severity of stenosis as compared with those in the non‐OSA group. Whereas 22.5% of patients with OSA had ≥70% stenosis, only 6% of those without OSA had ≥70% stenosis (P < 0.05).
When the patients were grouped into 3 categories by severity of OSA (AHI <10, ahi 10–20, and ahi >20), we found that with increasing severity of OSA the severity of stenosis also increases. The percentage of patients with ≥70% stenosis in the 3 groups were 0%, 7.7%, and 24%, respectively (P < 0.05).
Discussion
The most important finding of our study is that the frequency of noncalcified and mixed plaques was much higher in patients with OSA as compared with non‐OSA patients. This relationship remained robust even after adjusting for other common confounding factors (adjusted OR: 7). Whereas a few studies have evaluated the relationship of calcified plaques and OSA,11, 12, 23 we are not aware of any study that has explored the relationship of noncalcified plaques with OSA. Identification of these plaques with increased frequency on MDCT scans in patients with OSA is especially relevant, as prevalence of OSA is high in patients with ACS.24 We also found that patients with OSA have significantly higher severity of stenosis and have significantly greater number of vessels involved as compared with the non‐OSA group (P < 0.05).
It has been established that the majority of MI and ACS events are a result not of slowly progressing luminal narrowing, but instead of acute disruption of unstable (vulnerable) atherosclerotic plaques.25 Although it is still controversial as to what constitutes a vulnerable plaque, most agree that presence of lipid‐rich core or noncalcified plaque is an important component.26 Hence, exploring the relationship of noncalcified plaques to OSA adds a novel dimension to our understanding of this complex interplay between the 2 diseases.
There is significant literature showing association of obesity with CAD, though none of them looked for OSA in those studies. It is possible that many of those patients may have had OSA.12, 27, 28, 29 Several studies have looked at the mechanism of endothelial damage secondary to OSA.25, 26, 30 These studies suggest that endothelial damage occurs due to increased endothelial oxidative stress, inflammation, and reduced endothelial‐repair capacity. Furthermore, there is evidence that treatment of OSA with nasal CPAP therapy improves endothelial function and reduces the risk for fatal and nonfatal cardiovascular events.31 The possibility of OSA causing or accelerating atherosclerosis has been supported by data from several studies.32, 33, 34, 35 Obstructive sleep apnea being an independent risk factor for atherosclerosis is further supported by a randomized controlled trial in which intervention by CPAP resulted in significant reduction in carotid intima‐media thickness.36 The mechanisms by which OSA may lead to atherosclerosis are multiple and complex37; however, OSA is believed to cause atherosclerosis by both indirect mechanism (causing hypertension)38 or direct mechanism (intermittent hypoxia leads to plaque formation, dyslipidemia, and endothelial damage).32, 33, 34, 35
Study Limitations
The strengths of the study are (1) gold‐standard polysomnography on all patients and (2) MDCT scan evaluated by a single board‐certified radiologist with no interobserver variability. There are several limitations of this study, including the retrospective design of the study, which suggests independent association but does not establish causal relationship between the OSA and noncalcified plaques. All the patients in the study were recruited from a chest pain center and a potential referral bias is possible, though the idea was to look at patients with underlying coronary disease. The time between some of the polysomnography and MDCT was up to 36 months. This may have resulted in some changes that may not reflect the real magnitude of OSA on coronary plaques. However, atherosclerosis is a slow process and unlikely to exhibit major variation in 36 months. A similar timeframe has been used by a prior study on calcified plaques.11 The patients in the OSA group were older and noted to have higher prevalence of male sex, smoking history, and hypercholesterolemia, all independent risk factors for CAD. However, multiple regression analysis revealed that OSA was independently associated with noncalcified lesions in coronary vessels as measured by MDCT scan.
The broad CI was most likely due to small sample size, but we cannot rule out the effect of unobserved confounding factors. We also do not have actual data on low‐density lipoprotein cholesterol at the time of OSA diagnosis. Similarly, the study did not collect data on blood pressure control in these patients prior to diagnosis of OSA. We also did not have data on use or compliance with CPAP therapy, which may have impacted the outcome. Assuming that at least a certain percentage of patients were compliant with therapy, which would have had a salutary effect,36 our results are even more significant.
Conclusion
The study suggests an independent association of OSA with noncalcified and mixed lesions in coronary vessels by MDCT. Patients with OSA also demonstrate higher atherosclerotic burden, as noted by increased number of vessels involved and severity of stenosis. Due to the above‐mentioned limitations, larger prospective studies are needed to firmly conclude an unequivocal relationship between noncalcified plaques and OSA.
Clinicians need to be aware of the higher burden of CAD in patients with OSA. Conversely, because OSA is an established risk factor for cardiac diseases and has effective therapy, screening for OSA in patients with CAD/ACS is recommended.
References
- 1. Young T, Palta M, Dempsey J, et al. The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2. Hla KM, Skatrud JB, Finn L, et al. The effect of correction of sleep‐disordered breathing on BP in untreated hypertension. Chest. 2002;122:1125–1132. [DOI] [PubMed] [Google Scholar]
- 3. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. [DOI] [PubMed] [Google Scholar]
- 4. Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. [DOI] [PubMed] [Google Scholar]
- 5. Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671–1678. [DOI] [PubMed] [Google Scholar]
- 6. Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–1437. [DOI] [PubMed] [Google Scholar]
- 7. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 8. Mooe T, Rabben T, Wiklund U, et al. Sleep‐disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. [DOI] [PubMed] [Google Scholar]
- 9. D'Alessandro R, Magelli C, Gamberini G, et al. Snoring every night as a risk factor for myocardial infarction: a case‐control study. BMJ. 1990;300:1557–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mooe T, Rabben T, Wiklund U, et al. Sleep‐disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–256. [DOI] [PubMed] [Google Scholar]
- 11. Sorajja D, Gami AS, Somers VK, et al. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SH, Cho GY, Baik I, et al. Association of coronary artery calcification with obstructive sleep apnea and obesity in middle‐aged men. Nutr Metab Cardiovasc Dis. 2010;20:575–582. [DOI] [PubMed] [Google Scholar]
- 13. Virmani R, Burke AP, Kolodgie FD, et al. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. [DOI] [PubMed] [Google Scholar]
- 14. Schmermund A, Erbel R. Unstable coronary plaque and its relation to coronary calcium. Circulation. 2001;104:1682–1687. [DOI] [PubMed] [Google Scholar]
- 15. Huang H, Virmani R, Younis H, et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. [DOI] [PubMed] [Google Scholar]
- 16. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. [DOI] [PubMed] [Google Scholar]
- 17. Viles‐Gonzalez JF, Poon M, Sanz J, et al. In vivo 16‐slice, multidetector‐row computed tomography for the assessment of experimental atherosclerosis: comparison with magnetic resonance imaging and histopathology. Circulation. 2004;110:1467–1472. [DOI] [PubMed] [Google Scholar]
- 18. Becker CR, Nikolaou K, Muders M, et al. Ex vivo coronary atherosclerotic plaque characterization with multi‐detector‐row CT. Eur Radiol. 2003;13:2094–2098. [DOI] [PubMed] [Google Scholar]
- 19. Leber AW, Knez A, White CW, et al. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast‐enhanced multislice computed tomography. Am J Cardiol. 2003;91:714–718. [DOI] [PubMed] [Google Scholar]
- 20. Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques. J Am Coll Cardiol. 2004;43:1241–1247. [DOI] [PubMed] [Google Scholar]
- 21. Leber AW, Knez A, Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64‐slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–154. [DOI] [PubMed] [Google Scholar]
- 22. Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified plaque by contrast‐enhanced, submillimeter multidetector spiral computed tomography: a segment‐based comparison with intravascular ultrasound. Circulation. 2004; 109:14–17. [DOI] [PubMed] [Google Scholar]
- 23. Matthews KA, Strolo PJ Jr, Hall M, et al. Association of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle‐aged men and women: Pittsburgh SleepSCORE study. Sleep. 2011;34:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C‐reactive protein and interleukin‐6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. [DOI] [PubMed] [Google Scholar]
- 25. Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Atkeson A, Yeh SY, Malhotra A, et al. Endothelial function in obstructive sleep apnea. Prog Cardiovasc Dis. 2009;51:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lakka HM, Lakka TA, Tuomilehto J, et al. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur Heart J. 2002;23:706–713. [DOI] [PubMed] [Google Scholar]
- 28. Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280: 1843–1848. [DOI] [PubMed] [Google Scholar]
- 29. Arad Y, Newstein D, Cadet F, et al. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron‐beam computed tomographic study. Arterioscler Thromb Vasc Biol. 2001;21:2051–2058. [DOI] [PubMed] [Google Scholar]
- 30. Dean RT, Wilcox I. Possible atherogenic effects of hypoxia during obstructive sleep apnea. Sleep. 1993;16:S15–S23. [DOI] [PubMed] [Google Scholar]
- 31. Ip MS, Tse HF, Lam B, et al. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. [DOI] [PubMed] [Google Scholar]
- 32. Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E‐deficient mice. Atherosclerosis. 2010;209:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savransky V, Nanayakkara A, Li, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dematteis M, Julien C, Guillermet C, et al. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177:227–235. [DOI] [PubMed] [Google Scholar]
- 35. Jun J, Polotsky VY. Metabolic consequences of sleep disordered breathing. ILAR J. 2009;50:289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drager LF, Bortolotto LA, Figueiredo AC, et al. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. [DOI] [PubMed] [Google Scholar]
- 37. Drager LF, Polotsky VY, Lorenzi‐Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42: 1206–1252. [DOI] [PubMed] [Google Scholar]


