Abstract
Background
We evaluated the effect of atenolol vs metoprolol succinate on vascular function in patients with essential hypertension.
Hypothesis
Given intrinsic differences between these agents, we hypothesized that atenolol and metoprolol succinate would have disparate effects on vascular function.
Methods
This study included 24 patients with hypertension (age 56 ± 2 years, 8 female, body mass index 28 ± 1) and featured a randomized, double‐blind, crossover design. Each β‐blocker (atenolol or metoprolol succinate) was taken by patients once daily for a 4‐week period. Measures of vascular function included peripheral augmentation index (AIx) and pulse wave amplitude reactive hyperemia index from peripheral arterial tonometry, and brachial artery flow‐mediated dilation from ultrasound.
Results
There were similar reductions in mean arterial pressure following treatment with atenolol and metoprolol succinate. Compared with metoprolol succinate, there was a significant increase in peripheral AIx following atenolol therapy (P < 0.05). There were no changes in brachial artery flow‐mediated dilation or pulse wave amplitude reactive hyperemia index following either drug treatment.
Conclusions
Although atenolol and metoprolol succinate have similar effects on blood‐pressure reduction, they have different effects on vascular function. Compared with metoprolol succinate, atenolol increases peripheral AIx. Neither drug has an effect on vascular endothelial function. These findings may have clinical implications, depending on the indication for treatment in an individual patient. Copyright © 2011 Wiley Periodicals, Inc.
These data are derived from a parent study which was funded, in part, by AstraZeneca, Inc. K. Heffernan was supported by funding from NIH T32 HL069770‐06. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Hypertension is a risk factor for future cardiovascular (CV) morbidity and mortality, and antihypertensive therapy remains a cornerstone for reducing CV risk associated with hypertension.1 Recent clinical trials suggest that the beneficial effect of these agents on CV risk extends beyond their ability to reduce blood pressure (BP) and may reside in their ancillary abilities to improve vascular function.2
Atenolol and metoprolol (available as an immediate‐release tartrate salt and a slow‐release succinate) are among the most widely used β 1‐selective agents for BP reduction. Although they are in the same antihypertensive class, there are important pharmacokinetic and pharmacodynamic differences between these agents.3, 4, 5 For example, metoprolol is lipophilic, which may affect tissue penetration, including penetration into the vasculature.6 As such, it has been shown that these drugs have disparate effects on important correlates of vascular function including inflammation,7, 8, 9 oxidative stress,10, 11 and autonomic function.12, 13 Whether these differences translate into agent‐specific differences in vascular function or whether there is a uniform class effect remains unclear.
The purpose of this study was to compare the effect of atenolol vs metoprolol succinate on vascular function in patients with hypertension. Several vascular measures were employed to provide a comprehensive appraisal of vascular responsiveness to β‐blocker therapy; these included digital augmentation index (AIx; a measure of ventricular‐vascular coupling),14 index of digital pulse wave amplitude during reactive hyperemia (PWA‐RHI; a measure of resistance vessel endothelial function),15, 16 and brachial artery flow‐mediated dilation (FMD; a measure of conduit vessel endothelial function).15 Given intrinsic pharmacokinetic and pharmacodynamic differences between these agents, we hypothesized that atenolol and metoprolol succinate would have disparate effects on vascular function.
Methods
Men and women with documented essential hypertension (defined as systolic BP ≥140 mm Hg or diastolic BP ≥ 90 mm Hg, or as the use of antihypertensive medication) participated in this investigation. Clinically stable patients with previously diagnosed hypertension who were age > 21 years and had stage 1 or stage 2 hypertension controlled with ≤ 2 drugs were invited to participate in this study. Exclusion criteria included patients with low‐density lipoprotein cholesterol > 100 mg/dL, severe valvular heart disease, recent myocardial infarction or stroke (within 3 months) or unstable cardiac symptoms, congestive heart failure or left ventricular ejection fraction < 40%, renal insufficiency (serum creatinine > 2 mg/dL), active liver disease, Raynaud's disease, chronic obstructive pulmonary disease, bronchial asthma, and uncontrolled hypertension defined as baseline BP > 190/100 mm Hg during screening or washout. Coronary artery disease (CAD) was defined as the presence of ischemia or infarction on single‐photon emission computed tomographic nuclear myocardial perfusion imaging or > 50% stenosis of an epicardial coronary artery by angiography.
Patients were evaluated at a screening visit, and those who met all inclusion/exclusion criteria were enrolled. Patients already taking a β‐blocker underwent a 2‐week washout period during which the medication was halted. This time period also served as a monitoring period for all patients to ensure that consecutive BP readings did not exceed 190/100 mm Hg. Patients were then randomized in a double‐blind, crossover fashion to two 4‐week active treatment periods with either metoprolol succinate or atenolol (Figure 1). Following the 2‐week monitoring/washout period, patients were given 50 mg per day of either metoprolol succinate or atenolol. These dosages were selected as they are both commonly prescribed initial clinical doses. Patients were instructed to take the medication at the same time every morning. There was a 2‐week washout period between the 2 active treatment periods. At baseline (prior to initiation of the first drug randomization) and at the end of each active treatment period (Week 4 and Week 10; Figure 1), subjects underwent noninvasive assessment of vascular function. Other vasoactive medications were not withheld during the study period. Blood pressure and heart rate were assessed at the aforementioned time points, as well as prior to the initiation of the second drug therapy intervention (ie, prior to Week 7; Figure 1) to establish that BP and heart rate had returned to baseline values.
Figure 1.
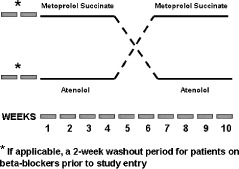
Study design. AIx, PWA‐RHI, and FMD were measured before Week 1 (after a 2‐week washout), after Week 4, and after Week 10. Abbreviations: AIx, digital augmentation index; FMD, flow‐mediated dilation; PWA‐RHI, pulse wave amplitude reactive hyperemia index.
Subjects were instructed to fast overnight and refrain from caffeine or alcohol intake and smoking on the day of testing. All vascular measures were made with the subject in the supine position in a dimly lit, temperature‐controlled room following a 10‐minute acclimatization period. All subjects gave written informed consent, and this study was approved by the institutional review board at Tufts Medical Center.
Blood Pressure
Blood pressure was assessed by a trained nurse (P. Mooney) using auscultation and sphygmomanometry following standard guidelines. Measurements were made with patients in a seated position following 5 to 10 minutes of quiet rest.
Finger Pulse Wave Amplitude
Beat‐by‐beat PWA was captured using peripheral arterial tonometry (PAT) with the EndoPAT 2000 (Itamar Medical Ltd., Israel) as previously described in detail.15 The PWA‐RHI was calculated as the ratio of the average PWA over a 1‐minute epoch starting after 5 minutes of ischemia induced by brachial cuff inflation to a suprasystolic BP, divided by the average PWA of a 3.5‐minute baseline epoch. The PWA obtained from the finger of the nonoccluded arm was also measured continuously and served as a control signal. Final values were normalized to the contralateral hand to account for any drift in the magnitude of the signal due to systemic factors. Peripheral AIx was calculated from PWA waveforms obtained during the baseline epoch and expressed as a percentage according to the EndoPAT integral software as (P2−P1/P1 × 100).
Brachial Artery Endothelial‐Dependent Vasodilation
Endothelial‐dependent vasodilation of the brachial artery was assessed using high‐resolution ultrasonography as previously described.17 Briefly, the brachial artery was longitudinally imaged 2 cm above the antecubital fossa using a 10‐MHz linear array vascular ultrasound transducer. Diameters were measured during end‐diastole (gated with electrocardiographic R‐waves) using ultrasonic calipers. The average of 5 evenly spaced measures (distance between the anterior and posterior intima‐blood interfaces) obtained within a 5‐cm segment of the vessel was used for subsequent analysis. Following baseline diameter measurement, reactive hyperemia was induced by an ischemic stimulus (rapid inflation of a blood pressure cuff around the upper arm to a suprasystolic pressure for 5 minutes). Immediately post cuff release, reactive hyperemia was confirmed by qualitatively assessing blood velocity for 10 seconds using spectral Doppler. Sixty seconds following release of the occlusion cuff, brachial diameter was once again measured as aforementioned. Responses were calculated as percentage change in brachial artery diameter from baseline (FMD).
Statistical Analysis
An analysis of variance with repeated measures was used to assess variables over 3 time points (baseline, post–metoprolol succinate, post‐atenolol). When a significant main effect was detected at a significance level of P < 0.05, t tests were used for post hoc comparisons. Adjustment for multiple comparisons was made with the Bonferroni adjustment. All results are presented as mean ± standard error of the mean (SEM). Data analyses were carried out using SPSS version 12.0.1 (SPSS Inc., Chicago, IL).
Results
Twenty‐four men and women participated in this study. Patients had an average age of 56 ± 2 years and body mass index of 28 ± 1 kg/m2. Eight of the study participants were female. Additional patient characteristics are presented in Table 1. There were no significant differences in office systolic BP (SBP) measured before initiation of each intervention (Pre1 139.7 ± 2.1 vs Pre2 138.9 ± 2.4 mm Hg, P > 0.05; intraclass correlation coefficient 0.72, P < 0.05). There were no significant differences in office diastolic BP (DBP) measured before initiation of each intervention (Pre1 80.7 ± 1.8 vs Pre2 78.7 ± 2.1 mm Hg, P > 0.05; intraclass correlation coefficient 0.77, P < 0.05). There were no significant differences in office heart rate (HR) measured before initiation of each intervention (Pre1 74.3 ± 2.5 vs Pre2 76.1 ± 1.3 beats per minute, P > 0.05; intraclass correlation coefficient 0.58, P < 0.05).
Table 1.
Patient Characteristics
| Variable | N = 24 |
|---|---|
| CAD (%) | 46 |
| Family history of CVD (%) | 33 |
| Smoking (%) | 33 |
| Total cholesterol (mg/dL) | 164 ± 8 |
| HDL‐C (mg/dL) | 46 ± 3 |
| LDL‐C (mg/dL) | 90 ± 7 |
| TG (mg/dL) | 124 ± 21 |
| Medications (%) | |
| β‐Blocker | 58 |
| ACEI | 42 |
| ASA | 75 |
| Statin | 71 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ASA, aspirin; CAD, coronary artery disease; CVD, cardiovascular disease; HDL‐C , high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TG, triglycerides.
Values are mean ± SEM unless otherwise indicated.
Changes in hemodynamics are presented in Table 2. There were similar reductions in SBP, DBP, mean arterial pressure, and HR following both atenolol and metoprolol succinate therapy (P < 0.05). Changes in vascular function are presented in Table 3. There was a significant increase in AIx following atenolol treatment (Figure 2; P < 0.05), whereas there was no significant change following metoprolol succinate treatment. Co‐varying for HR abolished the change in AIx with atenolol treatment (P > 0.05). Co‐varying for HR had no effect on the lack of change in AIx with metoprolol treatment (P > 0.05). There was no change in brachial FMD or digital PWA‐RHI following either treatment (Table 2). Current drug therapy (angiotensin‐converting enzyme inhibitors, aspirin, or statins) had no effect on the hemodynamic or vascular response to β‐blocker therapy (P > 0.05). Presence or absence of CAD also had no effect on the hemodynamic or vascular response to therapy (P > 0.05).
Table 2.
Hemodynamic Variables at Baseline and Following Drug Therapy
| Variable | Baseline | Post‐Therapy |
|---|---|---|
| SBP (mm Hg) | ||
| Atenolol | 141 ± 2 | 128 ± 2a |
| Metoprolol succinate | 138 ± 2 | 129 ± 2a |
| DBP (mm Hg) | ||
| Atenolol | 79 ± 2 | 76 ± 4 |
| Metoprolol succinate | 80 ± 2 | 74 ± 2a |
| MAP (mm Hg) | ||
| Atenolol | 100 ± 2 | 93 ± 2a |
| Metoprolol succinate | 99 ± 2 | 92 ± 2a |
| HR (bpm) | ||
| Atenolol | 73 ± 2 | 60 ± 2a |
| Metoprolol succinate | 77 ± 3 | 63 ± 3a |
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure.
Values are mean ± SEM.
Significantly different from baseline (P < 0.05).
Table 3.
Vascular Function Following Drug Therapy
| Variable | Baseline | Atenolol | Metoprolol Succinate |
|---|---|---|---|
| AIx (%) | 24.0 ± 4.8 | 33.2 ± 4.1a | 19.5 ± 3.0b |
| Brachial FMD (%) | 8.4 ± 1.1 | 9.1 ± 1.4 | 10.3 ± 1.4 |
| PWA‐RHI | 1.90 ± 0.2 | 1.80 ± 0.2 | 1.60 ± 0.2 |
Abbreviations: AIx, augmentation index; FMD, flow mediated dilation; PWA‐RHI, pulse wave amplitude reactive hyperemia index.
Values are mean ± SEM.
Significantly different from baseline (P < 0.05).
Significantly different from atenolol (P < 0.05).
Figure 2.
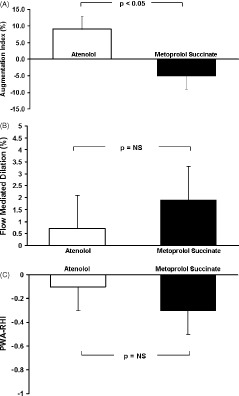
Absolute change in (A) AIx, (B) brachial artery FMD, and (C) PWA‐RHI following atenolol (□) vs metoprolol succinate (▪). Abbreviations: AIx, augmentation index; FMD, flow‐mediated dilation; NS, not significant; PWA‐RHI, pulse wave amplitude reactive hyperemia index.
Discussion
The novel finding of the present study was that atenolol produced HR‐mediated increases in peripheral AIx and metoprolol succinate did not. Thus, although having a similar effect on reductions in mean distension pressure, atenolol and metoprolol have diverging effects on peripheral vascular function.
Peripheral Augmentation Index
Following atenolol therapy, we noted an increase in AIx, measured from a digital volume pulse by PAT. This is similar to other studies that have noted increases in AIx measured from central and peripheral pressure waveforms.18, 19, 20, 21, 22 Increases in AIx were likely due to reductions in HR, as adjusting for HR abolished the significant change in AIx following atenolol treatment. With a reduction in HR, systolic ejection duration is increased. This alters pressure wave temporal associations, allowing greater time for the reflected pressure wave to arrive during systole than during diastolic decay, increasing AIx.23, 24 As such, there is an inverse association between HR and AIx. A change in HR of approximately 10 beats produces a change in AIx of approximately 4%–6%.23, 24 Increased arterial stiffness, as occurs with hypertension, may exacerbate the influence of HR on AIx. Recently, Papaioannou et al showed that the correlation of AIx with HR is higher in subjects with higher levels of aortic stiffness.25 That is, the same reduction in HR induces a greater increase in AIx in persons with stiffer vessels compared with those with more compliant vessels.25 Thus, in the present study, the approximately 13‐beat reduction of HR with atenolol in patients with hypertension resulted in an increase in AIx of approximately 9%.
Metoprolol succinate also lowered HR; yet there was a slight decrease in AIx following therapy, and adjusting for HR had no effect on the change in AIx with therapy. Thus, similar to what has been reported with vasodilating β‐blockers such as nebivolol,19, 20 metoprolol succinate may have pleiotropic properties that improve wave‐reflection dynamics, offsetting the increase in AIx that normally occurs with a reduction in HR. Metoprolol has been shown to cause direct arteriolar smooth‐muscle relaxation, with atenolol having no effect.26, 27, 28 This may reduce peripheral reflection coefficients (ie, alter impedance matching) and reduce discrete wave‐reflection magnitude.29
Conduit and Resistance Vessel Endothelial Function
Endothelial dysfunction, as evaluated by FMD of the brachial artery, identifies hypertensive patients at increased risk of nonfatal and fatal CV events.30 Improving FMD with select antihypertensive therapy translates to improvement in prognosis.31 Moreover, inability to improve FMD with standard therapy has an adverse impact on clinical outcomes.32 In accordance with previous findings, we noted that neither atenolol nor metoprolol succinate altered vascular endothelial function of conduit or resistance vessels.11, 33, 34, 35 Thus, atenolol and metoprolol succinate may not be viable therapeutic options to improve vascular endothelial function in patients with hypertension.
Clinical Implications
It has been suggested that antihypertensive agents that reduce pressure from wave reflections will have the most advantageous effect on clinical outcomes.2 Recent findings from the Conduit Artery Function Evaluation (CAFÉ) study have concluded that HR reduction with β‐blocker therapy contributes to less‐effective reductions in pressure from wave reflections and thus central BP,36 and this may contribute to less‐effective CV risk reduction. To date, no study had examined the effect of metoprolol succinate on AIx. Our findings suggest that although metoprolol succinate reduces HR, it does not have a concomitant deleterious effect on AIx. Future research is needed to examine the clinical significance of this finding. Moreover, studies note that metoprolol succinate reverses left ventricular (LV) remodeling and reduces LV mass,37 and this is superior to modulation seen with atenolol.38, 39, 40 Our findings suggest that the favorable effect of metoprolol succinate on LV morphology may be related to its ability to abrogate potentially detrimental changes in wave reflections concomitant with reductions in HR.
Study Limitations
We did not obtain vascular measures following the 2‐week washout period, prior to initiation of the second drug intervention. Therefore, it is possible that vascular adaptations following the first drug intervention did not return to baseline. Given the half‐life of these agents, and the documentation that HR and BP returned to baseline following the washout period in the present study, it is unlikely that there were residual vascular effects prior to initiation of the second drug intervention. Moreover, the crossover nature of the study design ensures that any possible residual vascular effect would affect outcome equitably and not skew results in favor or disfavor of any one drug. The sample size was small, preventing adequate subgroup analyses. The lack of change in endothelial function may be related to the relatively short duration of the study intervention. However, as aforementioned, our findings are consistent with numerous reports in the literature noting an inability of these agents to modulate peripheral conduit artery and resistance artery endothelial function. While the validity of AIx measured from PAT in adults is still being established, PAT‐AIx has been shown to correlate with AIx derived from tonometric pressure waves in children41 and pregnant women.42 Moreover, our finding of an increased AIx following atenolol therapy is consistent with numerous studies noting similar increases in AIx derived from pressure waves.18, 19, 20, 21, 22 This would suggest that change in AIx detected following antihypertensive therapy with PAT accurately reflects change in AIx previously established with other reputed techniques. Finally, we do not have data pertaining to clinical endpoints. Whether the disparate effect of atenolol and metoprolol succinate on the vasculature translates into disparate effects on central BP and clinical outcome will require further investigation.
Conclusion
Although having similar effects on mean arterial pressure reduction, atenolol and metoprolol succinate have diverging effects on vascular function. This may have clinical implications, depending on the indication for treatment in an individual patient.
References
- 1. Bangalore S, Messerli FH, Kostis JB, et al. Cardiovascular protection using beta‐blockers: a critical review of the evidence. J Am Coll Cardiol. 2007;50:563–572. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 3. Blomqvist I, Westergren G, Sandberg A, et al. Pharmacokinetics and pharmacodynamics of controlled‐release metoprolol: a comparison with atenolol. Eur J Clin Pharmacol. 1988;33(suppl): S19–S24. [DOI] [PubMed] [Google Scholar]
- 4. Darmansjah I, Wong E, Setiawati A, et al. Pharmacokinetic and pharmacodynamic properties of controlled release (CR/ZOK) metoprolol in healthy Oriental subjects: a comparison with conventional formulations of metoprolol and atenolol. J Clin Pharmacol. 1990;30(2 suppl): S39–S45. [DOI] [PubMed] [Google Scholar]
- 5. Karagiannis A, Mikhailidis DP, Kakafika AI, et al. Atenolol: differences in mode of action compared with other antihypertensives. An opportunity to identify features that influence outcome? Curr Pharm Des. 2007;13:229–239. [DOI] [PubMed] [Google Scholar]
- 6. Hwang CW, Edelman ER. Arterial ultrastructure influences transport of locally delivered drugs. Circ Res. 2002;90:826–832. [DOI] [PubMed] [Google Scholar]
- 7. Pöyhönen‐Alho MK, Manhem K, Katzman P, et al. Central sympatholytic therapy has anti‐inflammatory properties in hypertensive postmenopausal women. J Hypertens. 2008;26:2445–2449. [DOI] [PubMed] [Google Scholar]
- 8. Lanza GA, Pitocco D, Navarese EP, et al. Association between cardiac autonomic dysfunction and inflammation in type 1 diabetic patients: effect of beta‐blockade. Eur Heart J. 2007;28:814–820. [DOI] [PubMed] [Google Scholar]
- 9. King DE, Egan BM, Mainous AG III, et al. The effect of extended‐release metoprolol succinate on C‐reactive protein levels in persons with hypertension. J Clin Hypertens (Greenwich). 2006;8:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomes A, Costa D, Lima JL, et al. Antioxidant activity of beta‐blockers: an effect mediated by scavenging reactive oxygen and nitrogen species? Bioorg Med Chem. 2006;14:4568–4577. [DOI] [PubMed] [Google Scholar]
- 11. Bank AJ, Kelly AS, Thelen AM, et al. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Hypertens. 2007;20:777–783. [DOI] [PubMed] [Google Scholar]
- 12. Burns J, Mary DA, Mackintosh AF, et al. Arterial pressure lowering effect of chronic atenolol therapy in hypertension and vasoconstrictor sympathetic drive. Hypertension. 2004;44:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallin BG, Sundlöf G, Strömgren E, et al. Sympathetic outflow to muscles during treatment of hypertension with metoprolol. Hypertension. 1984;6:557–562. [DOI] [PubMed] [Google Scholar]
- 14. Heffernan KS, Patvardhan EA, Hession M, et al. Elevated augmentation index derived from peripheral arterial tonometry is associated with abnormal ventricular‐vascular coupling. Clin Physiol Funct Imaging. 2010;30:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 16. Heffernan KS, Karas RH, Patvardhan EA, et al. Peripheral arterial tonometry for risk stratification in men with coronary artery disease. Clin Cardiol. 2010;33:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuvin JT, Patel AR, Sliney KA, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38:1843–1849. [DOI] [PubMed] [Google Scholar]
- 18. Dhakam Z, McEniery CM, Yasmin, et al. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–219. [DOI] [PubMed] [Google Scholar]
- 19. Dhakam Z, Yasmin , McEniery CM, et al. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–356. [DOI] [PubMed] [Google Scholar]
- 20. Mahmud A, Feely J. Beta‐blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–667. [DOI] [PubMed] [Google Scholar]
- 21. Ting CT, Chen CH, Chang MS, et al. Short‐ and long‐term effects of antihypertensive drugs on arterial reflections, compliance, and impedance. Hypertension. 1995;26:524–530. [DOI] [PubMed] [Google Scholar]
- 22. Mackenzie IS, McEniery CM, Dhakam Z, et al. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413. [DOI] [PubMed] [Google Scholar]
- 23. Wilkinson IB, MacCallum H, Flint L, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(part 1):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson IB, Mohammad NH, Tyrrell S, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15(part 1):24–30. [DOI] [PubMed] [Google Scholar]
- 25. Papaioannou TG, Vlachopoulos CV, Alexopoulos NA, et al. The effect of heart rate on wave reflections may be determined by the level of aortic stiffness: clinical and technical implications. Am J Hypertens. 2008;21:334–340. [DOI] [PubMed] [Google Scholar]
- 26. Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey [published correction appears in J Hypertens. 2001;19:1697]. J Hypertens. 2001;19:1001–1006. [DOI] [PubMed] [Google Scholar]
- 27. Mathiassen ON, Buus NH, Larsen ML, et al. Small artery structure adapts to vasodilatation rather than to blood pressure during antihypertensive treatment. J Hypertens. 2007;25:1027–1034. [DOI] [PubMed] [Google Scholar]
- 28. Priviero FB, Teixeira CE, Toque HA, et al. Vasorelaxing effects of propranolol in rat aorta and mesenteric artery: a role for nitric oxide and calcium entry blockade. Clin Exp Pharmacol Physiol. 2006;33:448–455. [DOI] [PubMed] [Google Scholar]
- 29. Kelly R, Daley J, Avolio A, et al. Arterial dilation and reduced wave reflection: benefit of dilevalol in hypertension. Hypertension. 1989;14:14–21. [DOI] [PubMed] [Google Scholar]
- 30. Muiesan ML, Salvetti M, Paini A, et al. Prognostic role of flow‐mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008;26:1612–1618. [DOI] [PubMed] [Google Scholar]
- 31. Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. [DOI] [PubMed] [Google Scholar]
- 32. Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. [DOI] [PubMed] [Google Scholar]
- 33. Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–1286. [DOI] [PubMed] [Google Scholar]
- 34. Higashi Y, Sasaki S, Nakagawa K, et al. A comparison of angiotensin‐converting enzyme inhibitors, calcium antagonists, beta‐blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol. 2000;35:284–291. [DOI] [PubMed] [Google Scholar]
- 35. Gokce N, Holbrook M, Hunter LM, et al. Acute effects of vasoactive drug treatment on brachial artery reactivity. J Am Coll Cardiol. 2002;40:761–765. [DOI] [PubMed] [Google Scholar]
- 36. Williams B, Lacy PS; for the CAFE and ASCOT (Anglo‐Scandinavian Cardiac Outcomes Trial) Investigators . Impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE‐Heart Rate. J Am Coll Cardiol. 2009;54: 705–713. [DOI] [PubMed] [Google Scholar]
- 37. Colucci WS, Kolias TJ, Adams KF, et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the Reversal of Ventricular Remodeling with Toprol‐XL (REVERT) trial. Circulation. 2007;116:49–56. [DOI] [PubMed] [Google Scholar]
- 38. Zacà V, Rastogi S, Mishra S, et al. Atenolol is inferior to metoprolol in improving left ventricular function and preventing ventricular remodeling in dogs with heart failure. Cardiology. 2009;112:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barrios V, Escobar C, Tomás JP, et al. Comparison of the effects of doxazosin and atenolol on target organ damage in adults with type 2 diabetes mellitus and hypertension in the CARDHIAC study: A 9‐month , prospective, randomized, open‐label, blinded‐evaluation trial. Clin Ther. 2008;30:98–107. [DOI] [PubMed] [Google Scholar]
- 40. Ariff B, Zambanini A, Vamadeva S, et al. Candesartan‐ and atenolol‐based treatments induce different patterns of carotid artery and left ventricular remodeling in hypertension. Stroke. 2006;37:2381–2384. [DOI] [PubMed] [Google Scholar]
- 41. Haller MJ, Silverstein JH, Shuster JJ. Correlation between radial artery tonometry‐ and fingertip tonometry‐derived augmentation index in children with type 1 diabetes. Diab Vasc Dis Res. 2007; 4:66. [DOI] [PubMed] [Google Scholar]
- 42. Carty DM, Brennand JE, Delles C, Dominiczak AF. Peripheral arterial tone technology to assess endothelial function in pregnancy. J Hypertens. 2010;28:e85–e86. [Google Scholar]


