Abstract
Background:
Osteoprotegerin (OPG) is a member of the tumor necrosis factor superfamily and plays an important regulatory role in the skeletal, immune, and vascular systems. Intermediate coronary artery lesions that have a diameter stenosis of approximately 20%–70% might cause serious consequences. However, the prognostic value of plasma OPG levels in patients with intermediate coronary artery lesions has been less reported.
Hypothesis:
We hypothesized that OPG is a predictive marker of prognosis of intermediate coronary artery lesions.
Methods:
A prospective study was performed on 890 patients with intermediate (20%–70%) coronary lesions. The median age was 62 years (25th and 75th percentiles, 55 and 70 years, respectively) and 67.2% were male. Fasting blood was sampled at baseline. The primary clinical endpoint was a composite of readmission due to angina pectoris, nonfatal myocardial infarction, revascularization, and cardiovascular death.
Results:
During a median follow‐up of 24 months, events occurred in 11.1% of the patients. Of these patients, 7.9% were readmitted for angina pectoris, 1.5% received revascularization, 0.7% suffered nonfatal myocardial infarction, and 1.0% died. The plasma levels of OPG (median, 5304.7 pg/mL vs 2993.4 pg/mL, P<0.001) and high‐sensitivity C‐reactive protein (median, 4.8 mg/L vs 2.6 mg/L, P<0.001) were higher in patients with events than those without events. After adjusting for traditional risk factors such as age, gender, smoking, hypertension, diabetes, dyslipidemia, high‐density lipoprotein cholesterol, high‐sensitivity C‐reactive protein, percent area stenosis, and drug administration, a multivariate Cox proportional hazard analysis showed that higher OPG levels were an independent predictive factor of the composite clinical endpoint (hazard ratio: 2.49, 95% confidence interval: 1.26–4.89, fourth quartile vs first quartile).
Conclusions:
The higher level of OPG is an independent predictive factor of prognosis in patients with intermediate coronary lesions. © 2011 Wiley Periodicals, Inc.
This study was funded by Beijing Municipal Science and Technology Committee (No. D0906006000091). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Coronary artery disease (CAD) is associated with increasing cardiovascular morbidity and disability all over the world. Acute coronary syndrome (ACS) is often due to the rupture of plaques with <50% stenosis.1 Many pathological, imaging, and clinical studies show that most ACS consists of intermediate atherosclerosis plaque rupture.2 Thus, intermediate coronary lesions with a diameter stenosis of 20%–70% might cause serious consequences. Results from large prospective cohort studies suggest that the risk of future occurrence of CAD events can be estimated.3 It is necessary to find high‐risk patients with intermediate coronary stenosis; however, there is not yet a reliable method to identify these patients.
Osteoprotegerin (OPG), a key factor in bone remodeling and a member of the tumor necrosis factor receptor (TNF) superfamily,4 is classified as an osteoclastogenesis inhibitory factor. Osteoprotegerin is expressed in vivo by endothelial cells, vascular smooth cells, and osteoblasts. Over recent years, atherosclerosis has been known to be a systemic inflammatory process involving immune and vascular cells. Osteoprotegerin has been implicated in various inflammations and also has been linked to diabetes mellitus (DM), silent myocardial ischemia, acute myocardial infarction (MI), and left ventricular (LV) dysfunction.5
There seems to be a distinct relationship between OPG and severity of atherosclerosis in human studies. Some recent studies reported that elevated circulating levels of OPG were associated with cerebrovascular disease, stable angina pectoris, and the severity of CAD.6 Osteoprotegerin can be used to predict long‐term mortality in patients with ACS7 and in patients with ischemic stroke.8 In patients with stable angina pectoris, elevated serum OPG is associated with increased risk of all‐cause mortality, cardiovascular disease mortality, and MI.9
Few studies have previously assessed the associations of plasma OPG levels with long‐term risk of cardiovascular events in patients with intermediate coronary artery lesions. Further clinical studies are needed to confirm that plasma OPG levels can help to evaluate the prognosis of this patient group. We therefore hypothesized that OPG is a predictive marker of prognosis in patients with intermediate coronary artery lesions.
Methods
Study Population
Patients age 18–80 years who underwent coronary angiography from April 2007 to December 2008 in Beijing Anzhen Hospital and 10 other hospitals, and who showed ≥1 main coronary branch stenosis of 20%–70% of the luminal diameter by visual measurement, were included in this study. Exclusion criteria from the study were acute MI; cardiac shock; valvular heart disease; history of coronary revascularization or main coronary branches (diameter >2.25 mm); lumen diameter stenosis ≥70%; left ventricular ejection fraction (LVEF) <30%; systemic inflammatory diseases such as acute and chronic infection; known immune system or connective tissue disease; baseline creatinine >2.5 mg/dL (if male) or >2.0 mg/dL (if female); baseline ALT or AST 3× normal; heart transplant recipients; patients whose life expectancy is assumed to be <3 years, such as those with advanced cancer and multiple organ failure; and patients who cannot comply with research programs. The study was approved by all hospitals' ethics committees and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all patients. From April 2007 to December 2008, a total of 2025 patients met the inclusion criteria, but 926 patients were excluded for history of coronary revascularization and coronary revascularization this time. The high‐sensitivity C‐reactive protein (hs‐CRP) data for 86 patients were missed, and 17 had kidney insufficiency. The low‐density lipoprotein cholesterol (LDL‐C) data of 98 patients was missed, and finally 898 patients were recruited.
After coronary angiography, fasting blood samples were collected and the patients' demographic and clinical characteristics were recorded. The patients were given antiplatelet drugs, lipid‐lowing drugs, antihypertensive drugs, and hypoglycemic agents if necessary. As defined by the American Diabetes Association,10 DM was diagnosed as a fasting plasma glucose level ≥126 mg/dL or the use of antidiabetic medication. Patients were diagnosed as having hypertension if they were documented to have a blood pressure >140/90 mm Hg on ≥2 occasions or were already on antihypertensive therapy. Dyslipidemia was defined as total cholesterol (TC) >240 mg/dL, LDL‐C >160 mg/dL, high‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL, or triglycerides (TG) >200 mg/dL. Smokers were defined as those currently smoking any tobacco product at the beginning of the study.
Coronary Angiography and Quantitative Coronary Analysis
We used the Judkins percutaneous femoral or radial artery approach technique for coronary angiography. Multiple views of the left and the right coronary arteries were routinely recorded. Angiographic images were obtained after the administration of 200 μg of intracoronary nitroglycerine. Measurements were performed using a computer‐assistant system. Single frames demonstrating the most severe lumen narrowing without foreshortening were selected for analysis. The contrast‐filled nontapered catheter tip was used for calibration. The luminal diameter proximal or just distal to the moderate stenosis (reference lumen), and the minimum luminal diameter (MLD) at the site of the moderate lesion were determined for all lesions. Computer software automatically calculates the MLD, reference diameter, percent diameter stenosis, percent area stenosis, and lesion length.
Blood Sample Collection and Osteoprotegerin Analysis
On the morning after coronary angiography, fasting blood samples were drawn from the ulnar vein and were filled in tubes with ethylene diamine tetraacetic acid as anticoagulation. Within 20 minutes, the samples were centrifuged at 1500 rpm for 10 minutes. Plasma and serum aliquots were stored at −80°C until analysis. The concentration of plasma OPG was analyzed in a blinded manner with respect to clinical information and measured using commercially available protein arrays (RayBiotech, Norcross, GA) following the manufacturer's instructions. The sensitivity of this assay was 8 pg/mL, and the intra‐assay and the interassay coefficients of OPG variation were 4.2% and 5.1%, respectively.
Follow‐Up
Follow‐up was obtained through telephone surveillance, clinic visits, and review of hospital records and death certificates at 1, 3, 6, 12, 18, 24, 30, and 36 months. The general state of health, incidence of the clinical endpoint, and the drug administration were recorded. The clinical endpoint includes readmission due to angina pectoris, revascularization, and nonfatal MI, and cardiovascular death. Cardiovascular death was considered as sudden unexplained death, death due to fatal MI, death after rehospitalization because of heart failure or possible acute myocardial ischemia, and death related to stroke or peripheral artery disease.
Statistical Analysis
Categorical variables are reported as percentages and continuous variables as median values (25th, 75th percentile) or mean ± SD, depending on the distribution of the variables. The association between OPG and baseline demographic variable and cardiovascular risk factors was tested using the Mann‐Whitney U test and the Spearman rank correlation statistics for categorical and continuous variables, respectively. The Mann‐Whitney U test and the χ 2 test were used to test the difference of baseline data between the event group and the event‐free group. All the patients were divided into 4 groups according to the OPG quartiles. Analysis of variance, the Kruskal‐Wallis H test, or the χ 2 test was used to determine the difference of baseline characteristics among the 4 groups. Univariate and multivariate Cox proportional hazard regression models were used to assess the association of each quartile increment of plasma OPG levels with the endpoint after unadjusting and adjusting for age, gender, smoking, hypertension, DM, and dyslipidemia. A 2‐tailed P value <0.05 was considered statistically significant. All computations were done with SPSS version 16.0 software (SPSS Inc., Chicago, IL).
Results
Baseline Characteristics and Osteoprotegerin
In total, 898 patients were recruited during the period from April 2007 to December 2008. Through April 2010, 890 patients had finished follow‐up (8 patients lost contact or refused to answer the current status). The median (25th, 75th percentile) age of the patients was 62 years (55, 70), and 598 (67.2%) were men. At baseline, according to the symptoms, the patients were classified into 3 groups: 271(30.5%) patients were diagnosed as having unstable angina pectoris, 362 (40.6%) had stable angina pectoris, and 257 (28.9%) had untypical chest pain. All the patients were divided into 4 groups according to the quartile of OPG levels. The baseline characteristics of the study population stratified by quartile are shown in Table 1. The patients having plasma OPG levels in the highest quartile were significantly older than those in the lowest quartile, and had a previous history of DM and dyslipidemia. The patients with the lowest quartile have a greater tendency to use a calcium channel blocker (CCB). We found no association of plasma OPG levels with other cardiovascular risk factors, including gender, hypertension, and current smoking. We found that the plasma OPG levels showed positive correlations only with age (r = 0.075, P = 0.025), hs‐CRP (r = 0.080, P = 0.018), HDL‐C (r = 0.108, P = 0.002), and percent area stenosis (r = 0.082, P = 0.017), as seen in Table 2.
Table 1.
Baseline Characteristics and Drug Administration According to Quartiles of Osteoprotegerin Levels and the Distribution of Clinical Endpoints
| Quartile 1 (n = 222) | Quartile 2 (n = 224) | Quartile 3 (n = 222) | Quartile 4 (n = 222) | P Value | |
|---|---|---|---|---|---|
| OPG (pg/mL) | <1872 | 1872–3152 | 3152–6047 | >6047 | |
| Age, y | 61 (54, 68) | 61 (54, 68) | 62 (54, 70) | 65 (56, 72) | 0.006 |
| Male, n (%) | 153 (68.9) | 161 (71.9) | 148 (66.7) | 136 (61.3) | 0.107 |
| Hypertension, n (%) | 84 (38.2) | 89 (39.7) | 78 (35.3) | 84 (38.0) | 0.850 |
| DM, n (%) | 44 (19.7) | 54 (24.3) | 51 (23.0) | 72 (32.5) | 0.023 |
| Current smoker, n (%) | 84 (38.2) | 89 (39.7) | 78 (35.3) | 84 (38.0) | 0.850 |
| Dyslipidemia, n (%) | 63 (28.4) | 73 (32.6) | 96 (43.2) | 102 (45.9) | <0.001 |
| BMI (kg/m2) | 26.2 ± 3.1 | 25.8 ± 2.9 | 25.9 ± 3.1 | 25.6 ± 3.4 | 0.191 |
| TC (mg/dL) | 170.4 (146.2, 197.7) | 167.1 (145.6, 190.2) | 169.8 (142.7, 199.2) | 174.2 (152.7, 198.1) | 0.381 |
| HDL‐C (mg/dL) | 36.2 (31.9, 43.3) | 36.9 (31.2, 42.3) | 40.8 (33.8, 48.5) | 37.7 (31.9, 44.6) | 0.001 |
| LDL‐C (mg/dL) | 111.5 (90.0, 132.7) | 105.8 (84.2, 132.7) | 109.8 (90.7, 135.2) | 109.6 (87.7, 135.4) | 0.263 |
| TG (mg/dL) | 147.3 (107.3, 213.6) | 130.9 (99.0, 196.8) | 135.0 (101.8, 193.7) | 131.8 (93.6, 204.6) | 0.251 |
| Cr (mg/dL) | 0.87 (0.77, 0.99) | 0.88 (0.76, 1.01) | 0.87 (0.77, 0.97) | 0.88 (0.74, 1.02) | 0.836 |
| WBC count (×109/L) | 6.1 (5.3, 7.3) | 6.5 (5.5, 7.7) | 6.3 (5.5, 7.5) | 6.3 (5.3, 7.3) | 0.420 |
| Hs‐CRP (mg/L) | 2.5 (0.9, 4.3) | 2.7 (1.1, 4.9) | 3.3 (1.8, 5.6) | 2.7 (1.2, 5.0) | 0.006 |
| FPG (mg/dL) | 106.3 ± 57.7 | 105.3 ± 46.8 | 101.6 ± 33.6 | 100.6 ± 54.6 | 0.540 |
| QCA | |||||
| Diameter stenosis (%) | 39.9 ± 10.7 | 40.1 ± 10.1 | 39.1 ± 11.7 | 40.4 ± 10.5 | 0.648 |
| Area stenosis (%) | 56.0 ± 12.4 | 61.5 ± 10.6 | 58.2 ± 14.1 | 60.0 ± 11.6 | 0.070 |
| MLD (mm) | 2.1 ± 0.7 | 2.0 ± 0.5 | 2.1 ± 0.6 | 2.1 ± 0.6 | 0.264 |
| Nitrate, n (%) | 116 (52.1) | 119 (53.1) | 138 (62.1) | 131 (59.0) | 0.106 |
| β‐Blocker, n (%) | 138 (62.1) | 152 (67.9) | 145 (65.5) | 148 (66.2) | 0.619 |
| CCB, n (%) | 80 (36.0) | 61 (27.4) | 51 (22.8) | 69 (31.3) | 0.019 |
| ACEI/ARB, n (%) | 117 (52.9) | 126 (56.2) | 114 (51.2) | 109 (49.0) | 0.206 |
| Aspirin, n (%) | 204 (91.8) | 210 (93.6) | 206 (92.9) | 204 (91.8) | 0.857 |
| Statins, n (%) | 196 (88.1) | 194 (86.5) | 192 (86.3) | 195 (87.8) | 0.923 |
| Clinical endpoints | |||||
| Readmission for angina pectoris (n) | 10 | 11 | 21 | 28 | |
| Nonfatal MI (n) | 1 | 0 | 2 | 4 | |
| Late revascularization (n) | 2 | 3 | 5 | 3 | |
| Cardiovascular death (n) | 1 | 2 | 2 | 4 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; Cr, creatinine; DM, diabetes mellitus; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; MLD, minimum lumen diameter; OPG, osteoprotegerin; QCA, quantitative coronary analysis; TC, total cholesterol; TG, triglycerides; WBC, white blood cell.
Table 2.
Correlation Between Osteoprotegerin and Baseline Characteristics
| Variable | Correlation Coefficient | P Value |
|---|---|---|
| Age | 0.075 | 0.025 |
| BMI | −0.048 | 0.175 |
| Cr | −0.001 | 0.968 |
| TC | 0.055 | 0.108 |
| HDL‐C | 0.108 | 0.002 |
| LDL‐C | 0.004 | 0.910 |
| TG | −0.033 | 0.340 |
| FPG | 0.060 | 0.083 |
| WBC count | 0.030 | 0.383 |
| Hs‐CRP | 0.080 | 0.018 |
| Diameter stenosis, % | 0.064 | 0.063 |
| Area stenosis, % | 0.082 | 0.017 |
| MLD | 0.060 | 0.080 |
| Systolic BP | 0.012 | 0.722 |
Abbreviations: BMI, body mass index; BP, blood pressure; Cr, creatinine; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; MLD, minimum lumen diameter; TC, total cholesterol; TG, triglycerides; WBC, white blood cell.
Follow‐Up and Endpoint
The median follow‐up time was 24 months (minimum 1 mo, maximum 36 mo). The clinical endpoint occurred in 99 (11.1%) of patients in total. Seventy patients (7.9% of total patients in this study, the same description thereafter) were readmitted for recurrent angina pectoris. Of those 70 patients, 55 (6.2%) underwent coronary angiography or computed tomography angiography, and there was no evidence of severe coronary artery stenosis in more than 70%. The others did not undergo coronary angiography or computed tomography angiography because no equipment was available in local hospitals. Thirteen patients (1.5%) received revascularization for the ischemia evidence of stress test and the local doctors' decisions. Only 1 (0.1%) patient died of stroke and the other 8 (0.9%) died of heart attack. Seven (0.7%) were defined as nonfatal MIs. We found that the patients in the event group had significantly higher OPG levels than those in the event‐free group (5304.7 vs 2993.4 pg/mL, respectively, P<0.001). The Mann‐Whitney U test showed that the patients in the event group were older, and had more dyslipidemia history and a higher hs‐CRP level than those in the event‐free group (Table 3). The distribution of the clinical endpoint among the OPG quartiles is shown in Table 1.
Table 3.
Difference Between Event Group and Event‐Free Group
| Event Group (n = 99) | Event‐Free (n = 791) | P Value | |
|---|---|---|---|
| Age, y | 64.0 ± 10.7 | 61.6 ± 9.9 | 0.023 |
| Male, n (%) | 63 (63.6) | 535 (89.5) | 0.424 |
| Current smoker, n (%) | 35 (35.9) | 301 (38.1) | 0.608 |
| DM, n (%) | 30 (30.3) | 191 (24.1) | 0.181 |
| Hypertension, n (%) | 71 (71.7) | 501 (63.3) | 0.101 |
| Dyslipidemia, n (%) | 50 (50.5) | 283 (35.8) | 0.004 |
| BMI (kg/m2) | 25.2 ± 3.1 | 25.8 ± 3.1 | 0.079 |
| hs‐CRP (mg/L) | 4.8 (3.3, 6.4) | 2.6 (1.1, 4.7) | <0.001 |
| OPG (pg/mL) | 5304.7 (2815.4, 8643.0) | 2993.4 (1821.8, 5702.0) | <0.001 |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; hs‐CRP, high‐sensitivity C‐reactive protein; OPG, osteoprotegerin.
High Plasma OPG Levels and the Composite Endpoint
Univariate Cox hazard proportion analysis showed age (hazard ratio [HR]: 1.02, 95% confidence interval [CI]: 1.01–1.04), dyslipidemia (HR: 1.55, 95% CI: 1.03–2.32), hs‐CRP (HR: 1.15, 95% CI: 1.10–1.21), and higher plasma OPG levels (HR: 1.94, 95% CI: 1.03–3.65, third quartile vs first quartile; HR: 2.98, 95% CI: 1.62–5.49, fourth quartile vs first quartile) were prognostic in these patients. After adjusting for traditional risk factors such as age, gender, smoking, hypertension, DM, dyslipidemia, HDL‐C, hs‐CRP, percent area stenosis, and the use of CCB, a multivariate Cox proportional hazard analysis was performed to predict the associations between the OPG levels and the endpoint. The higher OPG levels can still forecast the outcome of the intermediate coronary lesions (HR: 2.49, 95% CI: 1.26–4.89, fourth quartile vs first quartile) (Table 4, Figure 1), but the value of HR has become a little attenuated.
Table 4.
Analysis Clinical Endpoint by Osteoprotegerin Levels
| Model | HR (95% CI)a | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| No. of events (n) | 14 | 16 | 30 | 39 |
| Univariate analysis | 1.00 | 1.05 (0.50, 2.16) | 1.94 (1.03, 3.65) | 2.98 (1.62, 5.49) |
| Multivariate analysisb | 1.00 | 0.94 (0.44, 1.99) | 1.34 (0.67, 2.70) | 2.49 (1.26, 4.89) |
Abbreviations: CCB, calcium channel blocker; CI, confidence interval; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein.
HR per quartile.
Cox proportional hazard model adjusted for age, gender, hypertension, DM, dyslipidemia, smoking, hs‐CRP, percent area stenosis, HDL‐C, and use of CCB.
Figure 1.
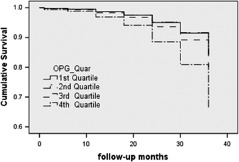
Cumulative survival curve for the clinical composite endpoint by OPG quartiles. Abbreviations: OPG, osteoprotegerin.
Discussion
Principal Findings
Atherosclerosis is a chronic inflammatory disease; a variety of inflammatory biomarkers participate in the atherosclerotic plaque formation during various stages of atherosclerosis development. Inflammatory biomarkers may have prognostic value for future cardiovascular risk among those at high risk or with documented cardiovascular disease.11 The present study demonstrates the association between the elevated levels of the soluble decoy receptor OPG and the adverse prognosis in patients with intermediate coronary artery lesions. We found significant associations between baseline levels of OPG and other risk factors for cardiovascular events such as age, HDL‐C, hs‐CRP, and the percent area stenosis. After adjusting for traditional risk factors, HDL‐C, and hs‐CRP, the data demonstrated that a high level of OPG raised the risk between 2‐ and 3‐fold. Therefore, we conclude that OPG is an independent predictive factor of the clinical endpoints, including readmission due to recurrent angina pectoris, nonfatal MI, revascularization, and death.
The clinical significance of intermediate lesions has attracted increasingly more attention. Usually, stents are not recommended to treat these lesions. Legalery et al12 reported that the clinical events (death, ACS, and revascularization) happened in almost 15% of patients with intermediate coronary lesions, similar to this study (11.1%). Therefore, it is important to identify these high‐risk patients.
Numerous clinical studies have now been performed demonstrating a link between OPG and vascular disease, in particular the association of the OPG levels and the severity of CAD. Sandberg et al13 demonstrated that OPG was significantly higher in patients with unstable angina pectoris than in those with stable angina pectoris. We also found that circulating elevated OPG has a correlation with the severity of coronary artery stenosis. Until now, a few studies have been performed to study prognostic value of OPG and CAD, and the present study's results are still consistent with previous studies where OPG is a risk marker of cardiovascular disease. In patients with acute MI, OPG was a novel marker for cardiovascular mortality and clinical events.14 Omland et al15 reported higher baseline OPG levels were associated with mortality and heart failure (HF) hospitalization independent of CRP and troponin in patients presenting with ACS during an 89‐month follow‐up. Serum OPG is strongly predictive of long‐term mortality and HF development in patients with ACS, independent of conventional risk markers. In patients with stable angina pectoris, elevated serum OPG is associated with increased risk of cardiovascular events.3., 6. A prior prospective large population‐based survey study has shown that the relative risk of cardiovascular mortality is increased by 3‐fold in patients with high serum OPG levels.16 To the authors' knowledge, the present study is the first on the prognostic significance of plasma OPG in patients with intermediate coronary artery lesions, suggesting that OPG measurements may be used to screen patients at risk and help identify subgroups that may benefit from aggressive intervention.
The medication used by CAD patients may influence plasma level of OPG; however, there was no association between the concentration of OPG and the use of the actual medication, except for CCB. Also, there is no linear relationship between the concentration of OPG and the use of CCB. Other studies showed the association between the concentration of OPG and the use of loop diuretics and angiotensin‐converting enzyme inhibitors (ACEI),8 and the association between the concentration of OPG and use of diuretics, β‐blockers, and statins.13 The difference is because each study had different inclusion criteria. The patients were treated with an ACEI or angiotensin II receptor blocker (ARB), statins, and aspirin before inclusion and during follow‐up. These medications might improve the prognosis of CAD, and ARBs can decrease the OPG expression in vascular smooth muscle cells.17
Consistent with previous reports, hs‐CRP can predict near‐term cardiovascular events in this study. This may be because they reflect different pathologic processes, whereas percent area stenosis and percent diameter stenosis are the index of plaque burden, and hs‐CRP seems to provide an assessment of atherosclerotic plaque activity. It is possible that inflammation has a permissive role in determining coronary artery plaque volume or stability but that other factors are responsible for driving these processes.
Possible Mechanisms
Osteoprotegerin produced by vascular smooth muscle cells and endothelial cells following stimulation by several cytokines has been implicated in inflammation.7 Osteoprotegerin is a secretory protein that belongs to the TNF superfamily and acts by binding to the receptor activator of nuclear factor‐κ B ligand (RANKL) and TNF‐related apoptosis‐inducing ligand (TRAIL), thus preventing the specific binding of these mediators with their putative receptors, and thereby inhibiting osteoclastogenesis.4 CD40 ligand, TNF‐α, and interleukin‐1‐β have been shown to enhance OPG expression in various cells.18
Atherosclerosis has recently been classified as an inflammatory disease characterized by persistent inflammation in the vascular wall. Mineral deposition and arterial calcification are other features of the atherosclerotic process. Accordingly, some bone matrix–associated regulatory proteins that include OPG and RANKL have been shown to be localized within human atherosclerotic plaques and at sites of arterial calcifications.12 Intermediate lesions are at a particular stage during atherosclerosis process and their expression is ACS, stable angina pectoris, and untypical chest pain. Osteoprotegerin is a promising prognostic marker in the setting of ACS because it mirrors the extent of acute tissue damage. In the nonacute setting, OPG may be prognostic because it reflects factors that influence the progression of atherothrombotic plaques. Osteoprotegerin represents an interesting putative pathway in vascular remodeling and atherosclerotic disease.19 The marked rise in circulating OPG levels observed in intermediate lesions may reflect a counter‐regulatory mechanism aimed at balancing plaque instability in an attempt to regulate acute coronary events that might contribute to the progression and aggravation of the disease, leading to enhanced destabilization of the coronary plaque. Data suggest the direct relationship between increased OPG plasma levels and plaque destabilization. This may imply that increased OPG levels could be a compensatory self‐defensive response rather than causal in the atherosclerotic process.
Study Strengths and Limitations
Strengths of this study include the larger population‐based size, long‐term follow‐up, and detailed information about clinical characteristics and OPG on follow‐up. Routine angiographic follow‐up should be arranged to investigate the progression of the lesions.
There are a few limitations in this study. First, because of the comparatively short follow‐up period, both hard and soft endpoints included in the analysis limited the rate of cardiovascular events, which decreased statistical efficiency. Second, the occurrence of angina may not be caused by stenotic lesions, for example coronary spasm and microvascular disease. Third, analysis does not include correction of electrocardiogram findings, heart rate, and LVEF of response for LV systolic function, which will affect the prognostic significance. Finally, OPG was stored at −80°C until analysis, and the possibility of protein degradation cannot be excluded.
Conclusions
This prospective study followed up 890 patients with luminal stenosis of 20%–70% for a median of 24 months, and found that higher levels of OPG are an independent predictive factor of prognosis of the intermediate coronary lesions.
Acknowledgements
The authors sincerely express their gratitude to professors Zhizhong Li, Quanming Zhao, Jinghua Liu, and Hongbing Yan in Beijing Anzhen Hospital; Shuyang Zhang in Peking Union Medical College Hospital; Lijun Guo in Peking University Third Hospital; Weimin Wang in Peking University People's Hospital; Xinchun Yang in Beijing Chao‐Yang Hospital; Buxing Chen in Beijing Electric Power Hospital; Hongwei Li in Beijing Friendship Hospital; Yuannan Ke in China‐Japan Friendship Hospital; and Keji Chen and Dazhuo Shi in Xiyuan Hospital of China Academy of Chinese Medical Sciences for suggestions and for providing the research samples.
References
- 1. Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. [DOI] [PubMed] [Google Scholar]
- 2. Gotlieb AI. Atherosclerosis and acute coronary syndromes. Cardiovasc Pathol. 2005;14:181–184. [DOI] [PubMed] [Google Scholar]
- 3. Anderson KM, Odell PM, Wilson PW, et al. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 part 2):293–298. [DOI] [PubMed] [Google Scholar]
- 4. Simonet WS, Lancy DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. [DOI] [PubMed] [Google Scholar]
- 5. Venuraju SM, Yerramasu A, Corder R, et al. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. [DOI] [PubMed] [Google Scholar]
- 6. Jono S, Ikari Y, Shioi A, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. [DOI] [PubMed] [Google Scholar]
- 7. Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. [DOI] [PubMed] [Google Scholar]
- 8. Jensen JK, Ueland T, Atar D, et al. Osteoprotegerin concentrations and prognosis in acute ischaemic stroke. J Intern Med. 2010;267: 410–417. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen ER, Ueland T, Seifert R, et al. Serum osteoprotegerin levels and long‐term prognosis in patients with stable angina pectoris. Atherosclerosis. 2010;212:644–649. [DOI] [PubMed] [Google Scholar]
- 10. Genuth S, Alberti KG, Bennett P, et al. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 11. Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53:317–333. [DOI] [PubMed] [Google Scholar]
- 12. Legalery P, Schiele F, Seronde MF, et al. One‐year outcome of patients submitted to routine fractional flow reserve assessment to determine the need for angioplasty. Eur Heart J. 2005;26: 2623–2629. [DOI] [PubMed] [Google Scholar]
- 13. Sandberg WJ, Yndestad A, Oie E, et al. Enhanced T‐cell expression of RANK ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2006;26:857–863. [DOI] [PubMed] [Google Scholar]
- 14. Ueland T, Jemtland R, Godang K, et al. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–1976. [DOI] [PubMed] [Google Scholar]
- 15. Omland T, Ueland T, Jansson AM, et al. Circulating osteoprotegerin levels and long‐term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51:627–633. [DOI] [PubMed] [Google Scholar]
- 16. Kiechl S, Schett G, Wenning G, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Fu M, Myles D, et al. PDGF induces osteoprotegerin expression in vascular smooth muscle cells by multiple signal pathways. FEBS Lett. 2002;521:180–184. [DOI] [PubMed] [Google Scholar]
- 18. Brändström H, Jonsson KB, Vidal O, et al. Tumor necrosis factor‐alpha and ‐beta upregulate the levels of osteoprotegerin mRNA in human osteosarcoma MG‐63 cells. Biochem Biophys Res Commun. 1998;248:454–457. [DOI] [PubMed] [Google Scholar]
- 19. Kiechl S, Werner P, Knoflach M, et al. The osteoprotegerin/RANK‐RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006;4:801–811. [DOI] [PubMed] [Google Scholar]


