Abstract
Background:
People over the age of 85 years have a high incidence of cardiovascular disease and chronic kidney disease.
Hypothesis:
There is an association between renal function and cardiac structure and function in subjects 85 years of age.
Methods:
Subjects born in the years 1920 and 1921 were recruited from the Jerusalem Longitudinal Cohort Study. Echocardiography was performed at the subject's home with assessment of cardiac structure and function. Glomerular filtration rate (GFR) was assessed by the Cockroft‐Gault formula, with abnormal GFR defined as ≤60 mL/min/1.73 m2.
Results:
There were 310 subjects who were enrolled. When GFR was examined as a continuous variable, linear regression showed a small although statistically significant relationship between GFR and left atrial volume (r = 0.15, P < 0.014), left ventricular mass index (r = 0.12, P < 0.04), and ejection fraction (r = 0.19, P < 0.03) but not with indices of diastolic function (r = 0.02, P < 0.72). However, using the accepted clinical cutoff of 60 mL/min/1.73 m2, there were no significant differences between subjects with normal and abnormal GFR in indices of cardiac structure. Ejection fraction (57.0 ± 10.4% vs 54.4 ± 10.3%; P = 0.08) and indices of diastolic function (E/e′ 12.4 ± 5.0 vs 12.3 ± 4.6; P = 0.89) were not significantly different between the 2 groups.
Conclusions:
A weak and clinically insignificant association was found between GFR as a continuous variable and indices of cardiac function. However, using the clinically accepted cutoff, no association between abnormal GFR and cardiac structure or function was observed.
David Leibowitz, MD, and Yoram Maaravi, MD, contributed equally to this report.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
People over the age of 85 (the oldest old) are the world's most rapidly growing age group.1 Aging is known to be associated with decreased renal function and with chronic kidney disease (CKD), the latter appearing as a major global public health problem over the last decade. CKD is especially prevalent in the elderly population, and there is extensive evidence that mild and moderate CKD caries a significant risk for all‐cause and cardiovascular mortality.2., 3. The mortality risk is so high that the majority of afflicted subjects do not reach the stage of advanced kidney disease.3 The high incidence of hypertension in this population may also contribute to both cardiac and renal disease.4 Current clinical guidelines published by the National Kidney Foundation's Kidney Disease Quality Outcome Initiative define evaluation, classification, and risk stratification in chronic kidney disease.5 These guidelines recommend using the Abbreviated Modification of Diet in Renal Disease (MDRD) or the Cockcroft‐Gault (CG) equations to estimate glomerular filtration rate (GFR) and are being gradually implemented in different health care systems.
Despite the rapid growth of this population, its high incidence of cardiac and renal disease and the significant risk of cardiovascular complications in CKD patients, the mechanisms relating them are still largely unknown. Elucidating these mechanisms is crucial to design preventive interventions to reduce vascular morbidity in this population.
Echocardiographic studies examining the relationship of abnormal cardiac structure and function to renal insufficiency have been limited and performed mainly in younger populations or those with end‐stage renal disease.6., 7., 8. A better understanding of this relationship is important for the design of interventions to reduce vascular morbidity in this population. Studies of echocardiography in the oldest old have generally been performed in the hospital or clinic setting, possibly contributing to a biased study population in this elderly age group as subjects may have difficulty in leaving their homes.9 The recent introduction of portable echocardiography machines has made it possible to study patients in the home and therefore is a more representative population of the oldest old. The objective of this study was to examine the association between echocardiographic measures of cardiac structure and function performed at the subjects home and renal function in an age‐homogenous, community‐dwelling population born in 1920 and 1921.
Methods
Participants
Subjects were recruited from the Jerusalem Longitudinal Cohort Study that was initiated in 1990 and has followed an age‐homogenous representative cohort of West Jerusalem residents born between June 1920 and May 1921. The methodology has been described elsewhere in detail.10., 11. The present study examines data from the third most recent phase of data collection, which took place during 2005 and 2006. Subjects were interviewed and examined in their homes on 2 separate occasions, each session requiring the completion of a structured interview that lasted about an hour and a half. Information was collected from socioeconomic, demographic, medical, functional, cultural, and cognitive domains. The institutional ethics committee of the Hadassah Hebrew University Medical Center approved the study design, and written informed consent was obtained from all participants.
Sampling
Subjects identified from the electoral register were randomly chosen from the total sample of people born in 1920 and 1921 and living in Jerusalem in 2005. As reported previously, we performed an examination of death certificates and hospital admission records 3 years following the initiation of the study. We compared the study group to other subjects of the sample frame in Jerusalem (also born in 1920 and 1921) who either refused or were not invited to enroll in the cohort study.10., 11. Subjects of the study group, those who declined to participate, and those baseline cohort members not enrolled had near identical mortality and disease‐specific hospital morbidity, thus demonstrating the representative nature of the initial study group in comparison to the total same‐age stratum of the Jerusalem population. Echocardiography was performed in randomly selected subjects, evenly distributed between new recruits and subjects participating from previous phases.
Echocardiography
Subjects had standard 2‐dimensional and Doppler echocardiography at their place of residence with a portable echocardiograph (Vivid I; GE Healthcare, Haifa, Israel). All subjects underwent 2‐dimensional and Doppler echocardiography with m‐mode measurements of the interventricular septum, posterior wall, and left ventricular (LV) end‐systolic and end‐diastolic diameters according to the recommendations of the European Association of Echocardiography/American Society of Echocardiography.12 Measurements were performed for 3 consecutive cardiac cycles and averaged. Subject height and weight at the time of the study were recorded and body surface area calculated. LV mass index (LVMI) was calculated according to a necropsy validated formula of LV mass (grams) = 0.8 × (1.04 × [(septal thickness + LV internal diameter + posterior wall thickness)3 − (LV internal diameter)3 ) + 0.6 and indexed to body surface area.13 Left atrial volumes (LAV) were calculated at end‐systole from the apical 4‐chamber view using the area‐length method and indexed to body surface area.14 Measurement of tricuspid regurgitation velocity was performed in standard fashion and converted to right ventricular to right atrial pressure gradient using the modified Bernoulli equation.
Ejection fraction (EF) was calculated by averaging measurements of end‐diastolic and end‐systolic volumes from the apical 4‐chamber view using the area‐length method for 3 consecutive beats. In patients with atrial fibrillation (n = 25), measurements were averaged for 5 consecutive beats. Subjects with inadequate imaging of the endocardial surface in apical views were excluded. Normal systolic function was defined as ejection fraction ≥55%. In addition, peak systolic mitral annular function (s wave) was measured as an additional index of systolic function.
Diastolic parameters were measured from the apical 4‐chamber view using pulsed‐wave Doppler at the level of the mitral annulus and tissue Doppler imaging of the septal and lateral myocardial walls and included early (E) and late transmitral flow velocities, the ratio of early to late velocities, deceleration time of E velocity, and isovolumic relaxation time. Early (e′) and late diastolic mitral annular tissue velocities at both the septum and lateral walls were obtained, and the ratio of E/e′ using the average of septal and lateral tissue velocities obtained was calculated as an index of diastolic function.15 Normal E/e′ was defined as ≤13.15 Patients with atrial fibrillation were excluded from analyses of wave velocities.
Assessment of Renal Function
Subjects who had undergone echocardiography and blood sampling with measurement of serum creatinine at the time of assessment in 2005 formed the study population. Serum creatinine levels were all assayed using the rate‐Jaffe reaction on a Hitachi 747 autoanalyzer (Roche Diagnostics Corp., Indianapolis, IN) calibrated with the compensated method. GFR was estimated based on the Cockcroft‐Gault equation: [CrCl = ((140‐age) × weight)/(72 × serum creatinine) × (0.85, if female)].
Data Analysis
Descriptive statistics were performed, and as the cardiac parameter data were normally distributed, results are described as means and standard deviations. Percentages were calculated as appropriate. For continuous variables differences between means were calculated using t tests. Linear regression unadjusted and adjusted models were performed. Models were adjusted for sex, diabetes, congestive heart failure, ischemic heart disease, hypertension, health perception, physical activity, and body mass index. All P values were 2‐tailed, and P < 0.05 was considered significant. The data storage and analysis was performed using SAS version 9.1e (SAS Institute, Inc., Cary, NC).
Results
Three hundred ten subjects of whom 164 were female and 146 male were entered into the study. Clinical characteristics are demonstrated in Table 1. As expected there was a high prevalence of hypertension and vascular disease in this elderly cohort, and 77% of the subjects had impaired renal function as evidenced by a GFR ≤60 mL/min/1.73m2. However, only 5.8% had a GFR ≤30 mL/min/1.73m2. There were no significant differences in clinical characteristics between subjects with and without CKD, except for a higher body mass index in the normal GFR group. The cohort in general had elevated LVMI (122 ± 36 gm/m2) and LAV (64.6 ± 26 mL), with normal mean EF (55.3 ± 10.2%) and elevated E/e′ (12.3 ± 4.9) suggesting significantly impaired diastolic function.
Table 1.
Baseline Characteristics
| Total | GFR >60 | GFR ≤60 | P Value | |
|---|---|---|---|---|
| Total population | 100 (310) | 22.6 (70) | 77.4 (240) | |
| Male | 47.1 (146) | 54.3 (38) | 45 (108) | NS |
| Education ≤12 years | 50.3 (156) | 61.4 (43) | 47.1 (113) | 0.04 |
| Smoking (pack years) | 7.7 ± 18.8 | 7.8 ± 19.9 | 7.6 ± 18.6 | NS |
| Diabetes | 19 (59) | 24.3 (17) | 17.5 (42) | NS |
| Ischemic heart disease | 39 (121) | 34.3 (24) | 40.4 (97) | NS |
| Hypertension | 71.6 (222) | 67.1 (47) | 72.9 (175) | NS |
| Cerebrovascular disease | 14.9 (44) | 10 (7) | 15.4 (37) | NS |
| Peripheral artery disease | 11 (34) | 8.6 (6) | 11.7 (28) | NS |
| Chronic obstructive pulmonary disease | 6.1 (19) | 5 (7.1) | 5.8 (14) | NS |
| Depression | 26.7 (81) | 26.5 (18) | 26.8 (63) | NS |
| Dementia | 17 (52) | 14.5 (10) | 17.8 (42) | NS |
| Poor self‐rated health | 31.3 (96) | 32.9 (23) | 30.8 (73) | NS |
| Comorbidity index | 20.3 (63) | 15.7 (11) | 21.7 (52) | NS |
| Body mass index (kg/m2) | 27.1 ± 4.3 | 29.3 ± 4.6 | 26.4 ± 4 | <0.0001 |
| Anemia | 27.7 (86) | 28.6 (20) | 27.5 (66) | NS |
| Diuretics | 40.7 (126) | 47.1 (33) | 38.8 (93) | NS |
| Peripheral vasodilators | 10 (3.2) | 5.7 (4) | 2.5 (6) | NS |
| β‐blockers | 42.9 (133) | 41.4 (29) | 43.3 (104) | NS |
| Calcium channel blocker | 31.6 (98) | 29.6 (71) | 38.6 (27) | NS |
| ACE/ARB | 50.7 (157) | 48.6 (34) | 51.3 (123) | NS |
| Mean systolic blood pressure (mm Hg) | 147.2 ± 21.6 | 145 ± 19.5 | 147 ± 22.2 | NS |
| Mean diastolic blood pressure (mm Hg) | 73.8 ± 10.8 | 75.3 ± 10.1 | 73.3 ± 11 | NS |
| Mean pulse (bpm) | 65.4 ± 10.3 | 67.3 ± 9.5 | 64.7 ± 10.4 | NS |
| Mean GFR | 50.3 ± 14.9 | 71.5 ± 10.4 | 44.1 ± 9.3 | <0.0001 |
| New York Heart Association Class I | 90.6% | 92.9% | 90% | |
| New York Heart Association Class II | 8.1% | 7.1% | 8.4% | |
| New York Heart Association Class III | 0 | 0 | 1.7% | |
| New York Heart Association Class IV | 0 | 0 | 0 | NS |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; GFR, glomerular filtration rate; NS, not significant.
Data are presented as % (no.) or mean ± standard deviation.
Echocardiographic measurements as continuous variables in groups with and without CKD are depicted in Table 2. Indices of cardiac structure including LAV and LVMI were not significantly different between groups. EF (57.0 ± 10.4% vs 54.4 ± 10.3%; P < 0.08) tissue Doppler s wave measurements and indices of diastolic function (E/e′ 12.4 ± 5.0 vs 12.3 ± 4.6; P = 0.89) were not significantly different between the 2 groups as well.
Table 2.
Cardiac Function by Glomerular Filtration Rate Level
| >60 mL/min/1.73 m2, Mean ± STD | ≤60 mL/min/1.73m2, Mean ± STD | |
|---|---|---|
| Measurements of cardiac morphology | ||
| LA volume index (mL/m2) | 37 ± 10.8 | 38 ± 15 |
| LV end diastolic volume index (mL/m2) | 66.9 ± 14.3 | 69.6 ± 19.2 |
| LV end systolic volume index (mL/m2) | 29.3 ± 12.3 | 32.7 ± 14.8 |
| LV mass index (g/m2) | 118 ± 28.2 | 122.5 ± 34.6 |
| LV ejection fraction (%) | 57 ± 10.4 | 54.4 ± 10.3 |
| Tissue Doppler lateral s wave (cm/s) | 7.8 ± 2 | 7.7 ± 2.2 |
| Tissue Doppler septal s wave (cm/s) | 6.6 ± 1.8 | 6.6 ± 1.9 |
| Mitral valve E wave (cm/s) | 78.4 ± 21.4 | 76.7 ± 22 |
| Mitral valve A wave (cm/s) | 90.7 ± 26.4 | 88 ± 24.6 |
| E/A ratio | 1 ± 0.8 | 1.1 ± 1.3 |
| Deceleration time (m/sec) | 208 ± 67.6 | 201.5 ± 64.6 |
| Tissue Doppler lateral e′ wave (cm/s) | 7.5 ± 2.3 | 7.1 ± 2.2 |
| Tissue Doppler lateral a wave (cm/s) | 10 ± 3.2 | 9.6 ± 3.5 |
| Tissue Doppler septal e′ wave (cm/s) | 6 ± 2.2 | 5.9 ± 1.9 |
| Tissue Doppler septal a wave (cm/s) | 7.7 ± 2.3 | 8.1 ± 2.7 |
| E/e′ | 12.4 ± 5 | 12.3 ± 4.6 |
Abbreviations: E, early transmitral flow velocities; A, late transmitral flow velocities; e′, early diastolic mitral annular tissue velocities; LA, left atrial; LV, left ventricular; STD, standard deviation.
When parameters were dichotomized into normal and abnormal EF, E/e′, and LVMI, the rate of subjects with and without CKD was not different between the 2 groups (Table 3). There were no significant differences in mean GFR between these groups as well (Table 4). Similarly, when GFR was examined as a dichotomous variable in multivariate logistic regression analysis, no association was found for any of the cardiac parameters (whether as dichotomous or continuous variables).
Table 3.
Categorical Cardiac Function by Glomerular Filtration Rate Level
| EF (N = 251) | >60, % (n), n = 64 | ≤60, % (n), n = 187 | P Value | |
|---|---|---|---|---|
| EF | <55 | 43.8 (28) | 49.7 (93) | 0.4 |
| 0.45–0.55 | 32.8 (21) | 33.7 (63) | ||
| 0.35–0.45 | 6.3 (4) | 11.8 (22) | ||
| 0–0.35 | 4.7 (3) | 4.3 (8) | 0.62 | |
| E/e′ (N = 274) | (n = 59) | (n = 215) | ||
| ≥13 | 35.6 (21) | 35.8 (77) | 0.97 | |
| 8–14 | 64.4 (38) | 63.3 (136) | ||
| ≥15 | 20.3 (12) | 22.3 (48) | 0.94 | |
| LV mass index (N = 227) | (n = 64) | (n = 213) | ||
| High | 46.9 (30) | 53.5 (114) | 0.35 | |
Abbreviations: E, early transmitral flow velocities; E′, early diastolic mitral annular tissue velocities; EF, ejection fraction; LV, left ventricular.
Table 4.
Mean GFR and Dichotomized Cardiac Function
| Normal, Mean GFR ± STD (n) | Abnormal, Mean GFR ± STD (n) | P Value | |
|---|---|---|---|
| E:e′ | 50.9 ± 14.9 (176) | 49.2 ± 13.8 (98) | 0.33 |
| EF | 52.6 ± 15.1 (130) | 49.1 ± 15.1 (121) | 0.07 |
| LV mass index | 51.2 ± 13.6 (133) | 49.3 ± 15.4 (144) | 0.29 |
Abbreviations: E, early transmitral flow velocities; e′ early diastolic mitral annular tissue velocities; EF, ejection fraction; GFR, glomerular filtration rate; LV, left ventricular; STD, standard deviation.
When GFR was analyzed as a continuous variable by linear regression, a clinically small yet statistically significant relationship between GFR and LAV (r = 0.15, P < 0.014), LVMI (r = 0.12, P < 0.04), and EF (r = 0.19, P < 0.03) was noted. GFR did not correlate with indices of diastolic function (r = 0.02, P < 0.72) (Figure 1). After multivariate analysis, the association between GFR and LVMI was no longer significant, whereas the association with LAV and EF remained significant (Table 5).
Figure 1.
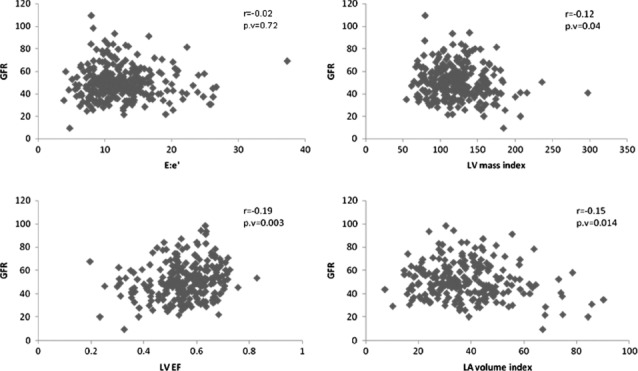
Linear regression analysis of eGFR with measures of cardiac structure and function. Abbreviations: E, early transmitral flow velocities; e′, early diastolic mitral annular tissue velocities; GFR, glomerular filtration rate; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction.
Table 5.
Linear Regression for Kidney Function With Selected Cardiac Functions
| Unadjusted P Valuea | Adjusted P Valueb | |
|---|---|---|
| LA volume | 0.026 | 0.048 |
| LV mass index | 0.048 | 0.16 |
| LVEF | 0.0014 | 0.0092 |
| E/e′ | 0.84 | 0.77 |
Abbreviations: E, early transmitral flow velocities; e′ early diastolic mitral annular tissue velocities; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction.
Adjusted for sex.
Adjusted for sex, diabetes, congestive heart failure, ischemic heart disease, hypertension, health perception, physical activity, and body mass index.
Discussion
Our study is the first to our knowledge to examine associations between cardiac structure and function and renal function in a community‐dwelling population of the oldest old. This study did not demonstrate an association between renal insufficiency (when using the clinically accepted cutoff of an estimated GFR (eGFR) <60 mL/min/1.73m2) and cardiac structure and function. We did demonstrate a weak although significant association between GFR and LAV as well as LV EF; however, the clinical magnitude of this association as well as the strength of the association are likely to be of little clinical relevance.
Several previous studies using echocardiography or magnetic resonance imaging have examined the association between renal insufficiency and cardiac structure and function, mainly focusing on left ventricular hypertrophy (LVH) in hypertensive populations. These studies have demonstrated conflicting results, with some, but not others, demonstrating a relationship between eGFR and LVH.16., 17., 18. Shah et al recently examined hypertensive patients with a mean age of 60 years with echocardiography and did not demonstrate an association between eGFR and cardiac structure, systolic or diastolic function, a finding consistent with our results in an older general, community‐dwelling population with a high prevalence of hypertension.7 In a study examining the effect of renal insufficiency on diastolic heart failure in hypertensive patients with a mean age of 64 years, Nishio et al also failed to demonstrate differences in EF or E:e′ between subjects with eGFR above or below 60 mL/min/1.73 m2.19
Although there is a clear association between renal insufficiency and cardiovascular morbidity and mortality, our findings of only a small association between LV EF, LAV, and GFR suggest that this may be primarily mediated by extracardiac factors such as the diffuse vascular stiffening seen with aging to which the kidneys are particularly susceptible.20 This finding suggests that strategies to improve cardiorenal syndromes should not be solely designed at improving cardiac output. It is also possible that an association with cardiac structure and function would be demonstrated in very elderly subjects with more pronounced renal insufficiency than seen in our cohort.21 We have recently demonstrated that physical activity in the same elderly population, although conferring survival benefits, is not associated with changes in cardiac structure and function on echocardiography,22 further suggesting that extracardiac factors may be involved in the related morbidity and mortality in this population. However, further study is needed to clarify these mechanisms.
In the present study we used the CG formula to define CKD, whereas other studies have used the MDRD formula. There is controversy over which of the equations is more accurate in detecting reduced GFR, but there is extensive evidence supporting the superiority of CG in old age for CKD classification, medication dosing, and predicting mortality.23., 24., 25. As relatively few subjects had GFR <30, we could not do a separate analysis of this subgroup.
The major strengths of our study are the use of an age‐homogenous cohort to minimize variability of the clinical findings and the use of home echocardiography. Previous studies using echocardiography in the oldest old population examined subjects in the hospital setting introducing significant bias. The major limitation is the availability of assessment of renal function in a subset of the total cohort; however, there is no reason to suppose that this represents a selected subpopulation. Prevalence of CKD was in accordance with that reported in the United Kingdom in subjects over 80 years living in residential care homes.26 Another limitation is the cross‐sectional nature of the data, which prevents conclusions regarding changes over time in the variables studied.
Conclusion
Our study did not demonstrate a clear clinically significant association between reduced GFR and echocardiographic indices of systolic and diastolic function in a community‐dwelling population of the oldest old. Further study is necessary to elucidate the mechanisms of cardiorenal interactions in the oldest old.
References
- 1. He W, Sengupta M, Velkoff VA, et al. 65+ in the United States: 2005. US Census Bureau, Current Population Reports; 2005. www.census.gov/prod/2006pubs/p23‐209.pdf. Accessed 2010
- 2. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risk of death, cardiovascular events and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 4. Burt VL, Whelton P, Roccella P, et al. Prevalence of hypertension in the US adult population. Results from the Third national Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. [DOI] [PubMed] [Google Scholar]
- 5. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 6. Lee Y‐T, Chiu H‐C, Su H‐M, et al. Presence of chronic kidney disease and subsequent changes of left ventricular geometry over 4 years in an apparently healthy population aged 60 and older. Hypertens Res. 2008;31:913–920. [DOI] [PubMed] [Google Scholar]
- 7. Shah AM, Lam CSP, Cheng S, et al. The relationship between renal impairment and left ventricular structure, function and ventricular‐arterial interaction in hypertension. J Hypertens. 2011;29:1829–1836. [DOI] [PubMed] [Google Scholar]
- 8. Masugata H, Senda S, Goda F, et al. Echocardiographic assessment of the cardio‐renal connection: is left ventricular hypertrophy or diastolic function more closely correlated with estimated glomerular filtration rate in patients with cardiovascular risk factors? Clin Exp Hypertens. 2010;32:113–120. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Safar ME, Iaria P, et al. Prevalence and prognosis of left ventricular diastolic dysfunction in the elderly: the Proteger Study. Am Heart J. 2010;160:471–478. [DOI] [PubMed] [Google Scholar]
- 10. Stessman J, Cohen A, Ginsberg GM, et al. The Jerusalem 70‐year‐old longitudinal study. I: description of the initial cross sectional survey. Eur J Epidemiol. 1995;11:675–684. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs JM, Cohen A, Bursztyn M, et al. Cohort profile: the Jerusalem Longitudinal Cohort Study. Int J Epidemiol. 2009;39: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereaux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 13. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 14. Lester SJ, Ryan EW, Schiller NB, et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 16. Leib W, Mayer B, Stritzke J, et al. Association of low‐grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg echocardiographic substudy. Nephrol Dial Transplant. 2006;21:2780–2787. [DOI] [PubMed] [Google Scholar]
- 17. Afshinnia F, Spitalewitz S, Chou S‐Y, et al. Left ventricular geometry and renal function in hypertensive patients with diastolic heart failure. Am J Kidney Dis. 2007;49:227–236. [DOI] [PubMed] [Google Scholar]
- 18. Moran A, Katz R, Jenny NS, et al. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2008;52:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishio M, Sakata Y, Mano T, et al. Difference of clinical characteristics between hypertensive patients with and without diastolic heart failure: the roles of diastolic dysfunction and renal insufficiency. Hypertens Res. 2008;31:1865–1872. [DOI] [PubMed] [Google Scholar]
- 20. El Nahas M. Cardio‐kidney damage: a unifying concept. Kidney Int. 2010;78:14–18. [DOI] [PubMed] [Google Scholar]
- 21. Hung M‐J, Yang N‐I, Wu I‐W, et al. Echocardiographic assessment of structural and functional cardiac remodeling in patients with predialysis chronic kidney disease. Echocardiography. 2010;27:621–629. [DOI] [PubMed] [Google Scholar]
- 22. Stessman‐Lande I, Jacobs JM, Gilon D, et al. Physical activity and cardiac function in the oldest old. Rejuvenation Res. 2012;15: 32–40. [DOI] [PubMed] [Google Scholar]
- 23. Verhave JC, Fesler P, Ribstein J, et al. Estimation of renal function in subjects with normal serum creatinine levels: Influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. [DOI] [PubMed] [Google Scholar]
- 24. Szummer K, Lundman P, Jacobson SH, et al. Cockcroft‐Gault is better than the modification of diet in renal disease study formula at predicting outcome after a myocardial infarction: Data from the Swedish Web‐system for Enhancement and Development of Evidence‐based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Am Heart J. 2010;159:979–986. [DOI] [PubMed] [Google Scholar]
- 25. Pizzarelli F, Laurentani F, Bandinelli S, et al. Predictivity of survival according to different equations for estimating renal function in community‐dwelling elderly subjects. Nephrol Dial Transplant. 2009;24:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carter JL, O'Riordan SE, Eaglestone GL, et al. Chronic kidney disease prevalence in a UK residential care home population. Nephrol Dial Transplant. 2008;23:1257–1264. [DOI] [PubMed] [Google Scholar]


