Abstract
Background:
Hypertension is the most prevalent and potentially modifiable risk factor for atrial fibrillation (AF). In a previous secondary prevention study, the authors observed that the angiotensin II receptor blocker telmisartan was more effective than the calcium channel blocker amlodipine in preventing AF relapse in hypertensive patients with normal atrial size.
Hypothesis:
Telmisartan may be more effective than amlodipine in preventing AF recurrence in hypertensive patients with paroxysmal AF and normal or increased left atrial dimension (LAD).
Methods:
The authors assigned 378 mild hypertensive outpatients in sinus rhythm, but with ≥2 episodes of AF in the previous 6 months, to 1 of 2 groups. Group 1 comprised patients with LAD <40 mm in females and <45 mm in males. Group 2 comprised patients with LAD >40 mm and <45 mm in females and >45 mm and <50 mm in males. In both groups, patients were randomly treated with telmisartan or amlodipine for 1 year.
Results:
Systolic and diastolic blood pressure were similarly reduced by telmisartan and amlodipine in both groups. The AF recurrence rate was significantly lower in the telmisartan‐treated patients than in the amlodipine‐treated patients in both group 1 (12 vs 39, P < 0.01) and group 2 (40 vs 59, P < 0.05). Under telmisartan, the AF recurrence rate was significantly lower in group 1 than in group 2 (12.9% vs 42.1%, P < 0.05). Time to a first AF relapse was significantly longer with telmisartan than with amlodipine in both group 1 (176 ± 94 days vs 74 ± 61 days, P < 0.05) and group 2 (119 ± 65 days vs 38 ± 35 days, P < 0.05).
Conclusions:
Telmisartan was more effective than amlodipine in preventing AF recurrences in hypertensive patients with paroxysmal AF. Clin. Cardiol. 2012 DOI: 10.1002/clc.21994
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Hypertension is the most prevalent and potentially modifiable risk factor for atrial fibrillation (AF) on a population basis.1., 2. Hypertensive patients have a 1.5‐fold higher risk of developing AF, and the addition of AF to hypertension greatly enhances the cardiovascular risk of these patients, in particular the risk of stroke.1., 3.
Hypertension is associated with several changes in cardiac structure and physiology, such as left ventricular hypertrophy (LVH), impaired ventricular filling, interstitial fibrosis, slowing of atrial conduction velocity, and left atrial (LA) enlargement, which all favor AF development.4., 5. Atrial fibrillation itself causes electrical and structural remodeling of the heart, which in turn contribute to the recurrence or the maintenance of AF.6., 7. In particular, LA enlargement, which is associated with the progression of atrial structural remodeling, has been shown to play a key role in the occurrence of AF and to be an independent predictor of AF recurrence after successful cardioversion.8., 9., 10., 11.
Angiotensin II (Ang II) has been demonstrated to play a pivotal role in atrial remodeling by promoting atrial myocyte hypertrophy and fibrosis.12., 13. Ang II also exerts direct proarrhythmic effects in human atrial myocardium by modifying ion exchanges within atrial cells.14 Furthermore, Ang II has proinflammatory and prothrombotic properties that might contribute to a prothrombotic status in AF.15 As reviewed in a recent metanalysis,16 data from clinical trials have shown that drugs blocking the renin‐angiotensin system (RAS), such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are effective in primary prevention of AF reducing the relative risk of new‐onset AF, particularly in patients with heart failure and/or LVH. Efficacy of RAS inhibition in secondary prevention of AF is more controversial,17 with some studies showing a preventive effect18., 19., 20., 21., 22., 23. and other studies yielding neutral results.24., 25., 26. These contrasting findings might be partly related to the different study design and characteristics of the study populations at baseline, including the different degrees of structural cardiac disease, in particular different atrial size.
In a previous secondary prevention study,23 we observed that the ARB telmisartan was more effective than the calcium channel blocker amlodipine in preventing AF relapse in hypertensive patients with normal atrial size. To our knowledge, the effect of ARBs on AF recurrence in hypertensive patients with enlarged vs normal LA size has not been specifically investigated. Therefore, the present study was undertaken to evaluate the effect of antihypertensive treatment with telmisartan vs amlodipine on AF recurrence in hypertensive patients with paroxysmal AF and increased vs normal LA size.
Methods
This was a prospective, randomized, double‐blind, parallel‐arm study. Between October 1, 2009 and November 30, 2011, the study population was selected according to the following criteria: consecutive outpatients of either sex, age 40–80 years, with stage I hypertension (systolic blood pressure [SBP] ≥140 mm Hg and <160 mm Hg and/or diastolic blood pressure [DBP] ≥90 mm Hg and <100 mm Hg), in sinus rhythm, but with ≥2 electrocardiogram (ECG)‐documented episodes of symptomatic AF in the previous 6 months, each lasting >60 minutes but <7 days and terminating spontaneously. They were assigned to 1 of 2 groups (Table 1). Group 1 comprised patients with normal LA size, defined as LAD <40 mm in females and <45 mm in males. Group 2 comprised patients with enlarged LA size, defined as LAD >40 mm and <45 mm in females and >45 mm and <50 mm in males.27
Table 1.
Main Demographic, Clinical, and Echocardiographic Characteristics of Patients at Baseline
| Normal Atrial Size | Enlarged Atrial Size | |||
|---|---|---|---|---|
| Amlodipine (n = 97) | Telmisartan (n = 93) | Amlodipine (n = 93) | Telmisartan (n = 95) | |
| Age, y | 67 ± 7 | 68 ± 8 | 69 ± 8 | 69 ± 7 |
| Sex, M/F | 43/54 | 42/51 | 44/49 | 45/50 |
| Weight, kg | 74.3 ± 9.6 | 74.6 ± 10.2 | 75.5 ± 9.8 | 75.7 ± 10.2 |
| Smoking, % | 16 | 15 | 17 | 12 |
| SBP, mm Hg | 155.4 ± 7.9 | 154.5 ± 8.1 | 156.1 ± 8.4 | 155.9 ± 8.1 |
| DBP, mm Hg | 93.8 ± 3.6 | 94.1 ± 3.7 | 94.7 ± 4.1 | 94.9 ± 4.2 |
| Septal thickness, mm | 10.4 ± 0.25 | 10.2 ± 0.27 | 11.0 ± 0.29 | 11.1 ± 0.30 |
| Heart rate, bpm | 74.9 ± 10.6 | 75.5 ± 10.8 | 76.1 ± 10.4 | 76.4 ± 11.3 |
| LA dimension, mm | 40.6 ± 2.2 | 40.5 ± 2.1 | 45.3 ± 2.8 | 45.2 ± 2.7 |
| LVED dimension, mm | 61.9 ± 8.1 | 61.5 ± 7.9 | 60.8 ± 7.8 | 60.1 ± 8.3 |
| Previous AF episodes (last 6 months) | 1.9 ± 0.6 | 2.1 ± 0.7 | 2.3 ± 0.8 | 2.4 ± 0.8 |
Abbreviations: AF, atrial fibrillation; DBP, diastolic blood pressure; F, female; LA, left atrial; LVED, left ventricular end diastolic; M, male; SBP, systolic blood pressure. P value not significant for all the considered parameters.
The exclusion criteria were as follows: echocardiographic evidence of left ventricular hypertrophy (LVH); treatment with ARBs, ACEIs, or antiarrhythmic agents; cardioversion within the last 3 months; secondary hypertension; myocardial infarction or stroke in the preceding 6 months; congestive heart failure; coronary heart disease; valvular disease; cardiac surgery during the previous 6 months; significant thyroid, pulmonary, renal, or hepatic disease; pregnancy or fertile female; and known hypersensitivity or contraindications to the study medications.
The study protocol (Figure 1) was approved by the local ethics committee and written informed consent was obtained from each patient before enrollment.
Figure 1.
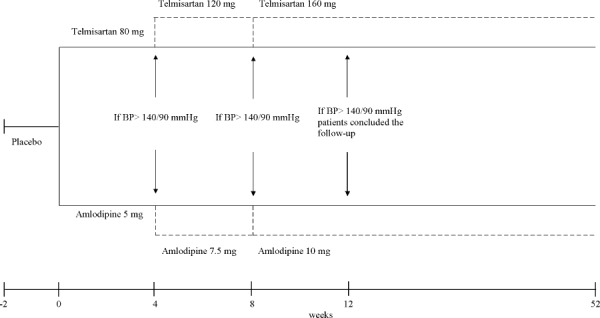
Study design. Abbreviations: BP, blood pressure.
Patients were assessed monthly for 1 year. At each clinic visit, BP values, a resting 12‐lead surface ECG, and a 24‐hour ECG were recorded. Blood pressure was measured in the seated position using a standard mercury sphygmomanometer (Korotkoff I and V) with a cuff of appropriate size. Measurements were always taken in the morning before daily drug intake (ie, 24 hours after dosing, at trough) and after the subject had rested for 10 minutes in a quiet room. Three successive BP readings were taken at 1‐minute intervals and averaged. To detect asymptomatic AF episodes, 24‐hour ambulatory ECG monitoring was performed using a Syneflash Holter recorder (ELA Medical, Paris, France). Recordings were always started after drug intake and were performed throughout a 24‐hour period, during which patients followed their normal daily routine after they left the laboratory. Patients were also asked to report any episode of palpitations, to take their pulse, and, in presence of arrhythmia, to perform an ECG as early as possible. Criteria to define AF recurrence included both nonsustained episodes of <3 minutes detected on the 24‐hour Holter and the longer‐lasting sustained episodes detected on ECGs. Palpitations were not taken into consideration, nor were patients' subjective appraisals.
The primary endpoint of the study was to assess the efficacy of telmisartan as compared with amlodipine with regard to cumulative number of patients relapsing into documented AF. The secondary endpoint was the time to a first ECG‐documented recurrence of AF.
Statistical Analysis
Data were expressed as mean ± standard deviation for continuous variables and frequencies were measured for categorical variables. The statistical analysis of the results was done using SAS software, version 6.12 (SAS Institute, Inc., Cary, NC). Continuous variables were analyzed using a 2‐way analysis of variance for repeated measurements. Analysis of variance was also used to assess comparisons within and between groups. Paired tests were also used: a 1‐sample t test was used to compare values obtained before and after treatment administration; a 2‐sample t test was used to compare the change score (final − baseline) for a given parameter between the 2 groups. The endpoints were analyzed on an intention‐to‐treat basis. The comparison of number of days to AF recurrence (median and range) among the treatment groups was performed by the nonparametric Wilcoxon test. For all statistical analyses, a P value of <0.05 was considered statistically significant.
Results
A total of 410 patients were enrolled, and 378 patients were finally randomized to participate (Figure 2).
Figure 2.
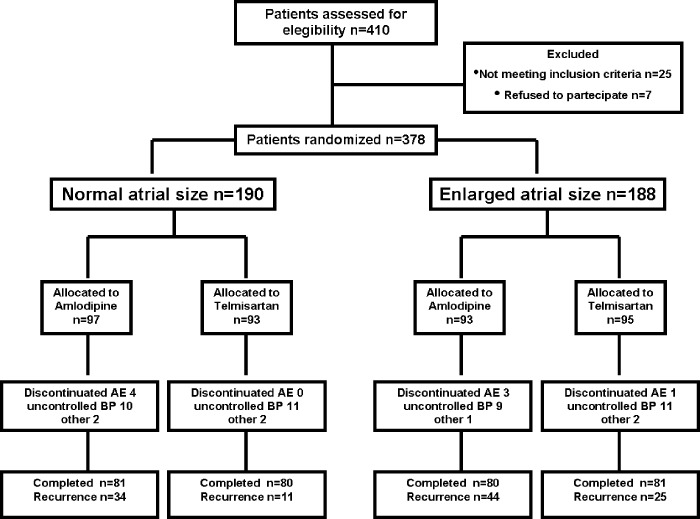
Flow diagram of the study. Abbreviations: AE, adverse event; BP, blood pressure.
In both groups, SBP and DBP values were significantly reduced by the 2 treatments, with no significant difference between them. At the end of follow‐up, in group 1, SBP was reduced by 17.4 mm Hg in the telmisartan‐treated patients (P < 0.001 vs baseline) and by 18.2 mm Hg in the amlodipine‐treated ones (P < 0.001 vs baseline). In group 2, SBP was reduced by 17.6 mm Hg with telmisartan (P < 0.001 vs baseline) and by 18.3 mm Hg with amlodipine (P < 0.001 vs baseline), with no significant difference between treatments nor between groups. Corresponding changes for DBP were 13.5 mm Hg with telmisartan and 14.1 mm Hg with amlodipine (both P < 0.001 vs baseline) in group 1 and, respectively, 13.4 mm Hg and 13.9 mm Hg in group 2, again without any significant difference between treatments.
Data regarding AF recurrence are reported in Table 2. At the 12‐week follow‐up visit (end of titration), 19 patients in group 1 (10%) and 37 patients in group 2 (19%) had a recurrence of AF. By intention‐to‐treat analysis, in both group 1 and group 2 the number of AF relapses was lower in the telmisartan‐ than in amlodipine‐treated patients (4 vs 14 in group 1, P < 0.01; and 15 vs 22 in group 2, P = 0.054). The AF recurrence rate was significantly lower in group 1 than in group 2 under both telmisartan (4.3% vs 15.8%, P < 0.05) and amlodipine treatment (14.4% vs 23.6%, P < 0.05).
Table 2.
Results of Intention‐to‐Treat Analysis
| Normal Atrial Size | Enlarged Atrial Size | |||
|---|---|---|---|---|
| Amlodipine | Telmisartan | Amlodipine | Telmisartan | |
| Recurrence of AF at 12 weeks | 14 | 4a | 22 | 15 |
| Recurrence of AF at 1 year | 39 | 12b | 59 | 40a, a,c, a,c |
| Days to recurrence, median ± SD (range) | 74 ± 61 (33–337) | 176 ± 94 (59–348)a | 38 ± 35 (24–333) | 119 ± 65 (36–339)a |
Abbreviations: AF, atrial fibrillation; SD, standard deviation.
P < 0.05.
P < 0.01 vs amlodipine.
P < 0.005 vs normal atrial size.
At the end of the follow‐up, 51 patients in group 1 (27%) and 99 patients in group 2 (52%) had a recurrence of AF, with a significant difference between the 2 groups. Again the number of AF relapses was significantly lower in the telmisartan‐ than in the amlodipine‐treated patients in both group 1 (12 vs 39, P < 0.01) and group 2 (40 vs 59, P < 0.05). The AF recurrence rate was significantly lower in group 1 than in group 2 under both telmisartan (12.9% vs 42.1%, P < 0.05) and amlodipine treatment (40.2% vs 63.4%, P < 0.05) (Figure 3). The time to a first ECG‐confirmed AF relapse was significantly longer with telmisartan than with amlodipine in both group 1 (176 ± 94 days vs 74 ± 61 days [median ± SD], P < 0.05) and group 2 (119 ± 65 days vs 38 ± 29 days [median ± SD], P < 0.05).
Figure 3.
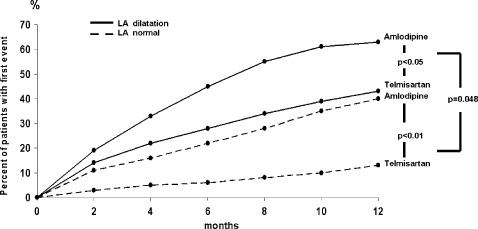
Rate of atrial fibrillation recurrence stratified by presence or absence of LA dilation. Abbreviation: LA, left atrial.
Discussion
There are 2 main results of the present study. The first is that in hypertensive patients with a history of paroxysmal AF, antihypertensive treatment with the ARB telmisartan was more effective than amlodipine therapy in reducing AF recurrence, despite a similar BP reduction. This finding, which was already evident after 3 months of therapy and persisted after 1 year, suggests that, unlike amlodipine, telmisartan might exert an antiarrhythmic action beyond its BP‐lowering effect. These results, which confirm some previous observation with this and other ARBs,18., 19., 20., 21., 22., 23. are not in agreement with those of 2 large secondary prevention studies, the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca (GISSI)‐AF study25 and the Angiotensin II‐Antagonist in Paroxysmal Atrial Fibrillation (ANTIPAF) trial,26 which failed to demonstrate the efficacy of valsartan and olmesartan, respectively, in preventing AF recurrence.
Actually, substantial differences exist in the characteristics of the study populations between our study and these 2 trials. Whereas our study included only patients with hypertension, the GISSI‐AF study also included patients with heart failure or left ventricular dysfunction, history of stroke, coronary or peripheral artery disease, and diabetes mellitus. Moreover, unlike our patients, who did not undergo cardioversion for AF within the last 8 weeks and were not taking antiarrhythmic or RAS‐blocking drugs at the time of enrollment, the majority of the GISSI‐AF patients had undergone cardioversion for AF within 2 weeks prior to randomization and were taking an antiarrhythmic drug (78% of patients), an ACEI (58%), a statin (27%), and a β‐blocker (30%), which are all likely to attenuate the potential beneficial effect of valsartan on AF. Furthermore, the proportion of patients with coronary heart disease was higher in the valsartan group than in the placebo group, as was the proportion of patients with pathologic Q‐wave and with peripheral artery disease, so that patients in the valsartan group were more complicated and prone to AF development. As regards the ANTIPAF study, this trial included patients with a very low risk of developing AF recurrence: the majority were normotensive, and none underwent cardioversion for AF within the prior 3 months. In addition, 71% were taking a β‐blocker (the percentage of patients receiving sotalol is not known). Furthermore, in our study, patients were excluded from follow‐up after the first AF recurrence, whereas in the ANTIPAF study patients with AF relapse were not withdrawn from the study, but were treated with amiodarone and all the recurrences were counted. Finally, the different pharmacologic characteristics of the ARBs used also might have played a role; telmisartan has been shown to have the strongest binding affinity to Ang II type 1 receptors among various ARBs, the rank order of affinity being telmisartan > olmesartan > candesartan > valsartan > losartan.27 Due to the greater Ang II type 1 receptor–blocking ability and longer plasma half‐life, telmisartan might be superior to other ARBs in preventing AF recurrence. Also, the selective peroxisome proliferator‐activated receptor‐gamma–modulating activity, which is peculiar to telmisartan,28., 29., 30., 31. might contribute to its AF‐preventive effect, through the insulin‐sensitizing effect and the attenuation of AF‐promoting atrial remodeling related to peroxisome proliferator‐activated receptor‐gamma stimulation.32
The second main result of the study is represented by the observation that the preventive effect of telmisartan on AF relapse was significantly greater in hypertensive patients with normal atrial size than in those with enlarged atrial size. From this finding, which represents the main element of novelty of the present study, it follows that in hypertensive patients with a history of paroxysmal AF, atrial enlargement may promote AF recurrence; this was also demonstrated by the greater AF relapse rate in our group 2 than in group 1 in both treatment arms and confirmed the observations of numerous previous studies conducted in a variety of populations.4., 8., 9., 10., 11. Possible mechanisms for the AF‐promoting effect of atrial enlargement, which is associated with the progression of atrial structural remodeling, include triggering premature atrial beats, slowing atrial conduction velocity, leading to persistent electrophysiological inhomogeneities and providing a greater area for re‐entry.33., 34.
The previous observations suggest that in hypertensive patients with paroxysmal AF, antihypertensive treatment with an ARB should be started as early as possible, before the structural atrial remodeling was established. Atrial remodeling is considered to be a time‐dependent adaptive modification of atrial myocytes aiming to maintain homeostasis against external stressors, including pressure/ volume overload.7., 11. The extent and reversibility of atrial ultrastructural changes, including myocyte hypertrophy and degeneration, inflammatory infiltrates, and fibrosis, depend on the strength and the duration of exposure to the stressors. The probability of eliminating AF completely is likely to be related to a point of no return of structural atrial remodeling. Although a cutoff value has not yet been provided, it has been suggested that some grade of irreversibility begins in the range of moderate LA enlargement, probably because of the higher grade of fibrosis.11., 35. Thus, the potential ability of ARBs to prevent AF by reversing LA remodeling is likely to be limited by some degree of atrial enlargement, an expression of irreversible structural changes. Additional studies are needed to provide definitive threshold values of LA size for identifying patients with a high likelihood of AF recurrence during antihypertensive therapy.
One possible limitation of the present study is that, due to the lack of a placebo group, we cannot rule out that the preventive effect of telmisartan with amlodipine as a comparator might be, at least in theory, related to a hypothetical proarrhythmic effect of amlodipine.
Conclusion
Despite a similar BP‐lowering effect, antihypertensive treatment with telmisartan was more effective than amlodipine in preventing AF recurrences in hypertensive patients with paroxysmal AF, but such a preventive effect was significantly more evident in patients with normal atrial size than in those with enlarged atrial size. This emphasizes the advantage of starting antihypertensive treatment with an ARB as early as possible, before the structural atrial remodeling has reached a point of no return.
References
- 1. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 2. European Heart Rhythm Association , European Association for Cardio‐Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed]
- 3. Fang MC, Go AS, Chang Y, et al; ATRIA Study Group. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verdecchia P, Reboldi G, Gattobigio R, et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension. 2003;41:218–223. [DOI] [PubMed] [Google Scholar]
- 5. Okin PM, Gerdts E, Wachtell K, et al. Relationship of left atrial enlargement to persistence or development of ECG left ventricular hypertrophy in hypertensive patients: implications for the development of new atrial fibrillation. J Hypertens. 2010;28:1534–1540. [DOI] [PubMed] [Google Scholar]
- 6. Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol. 1998;9:1378–1393. [DOI] [PubMed] [Google Scholar]
- 7. Casaclang‐Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. [DOI] [PubMed] [Google Scholar]
- 8. Raitt MH, Volgman AS, Zoble RG, et al; AFFIRM Investigators. Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2006;151:390–396. [DOI] [PubMed] [Google Scholar]
- 9. Shin SH, Park MY, Oh WJ, et al. Left atrial volume is a predictor of atrial fibrillation recurrence after catheter ablation. J Am Soc Echocardiogr. 2008;21:697–702. [DOI] [PubMed] [Google Scholar]
- 10. Toh N, Kanzaki H, Nakatani S, et al. Left atrial volume combined with atrial pump function identifies hypertensive patients with a history of paroxysmal atrial fibrillation. Hypertension. 2010;55:1150–1156. [DOI] [PubMed] [Google Scholar]
- 11. Marchese P, Bursi F, Delle Donne G, et al. Indexed left atrial volume predicts the recurrence of non‐valvular atrial fibrillation after successful cardioversion. Eur J Echocardiogr. 2011;12:214–221. [DOI] [PubMed] [Google Scholar]
- 12. McEwan PE, Gray GA, Sherry L, et al. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation. 1998;98:2765–2773. [DOI] [PubMed] [Google Scholar]
- 13. Serra JL, Bendersky M. Atrial fibrillation and the renin‐angiotensin system. Ther Adv Cardiovasc Dis. 2008;2:215–223. [DOI] [PubMed] [Google Scholar]
- 14. Von Lewinski D, Kockskämper J, Rübertus SU, et al. Direct pro‐arrhythmogenic effects of angiotensin II can be suppressed by AT1 receptor blockade in human atrial myocardium. Eur J Heart Fail. 2008;10:1172–1176. [DOI] [PubMed] [Google Scholar]
- 15. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. [DOI] [PubMed] [Google Scholar]
- 16. Schneider MP, Hua TA, Böhm M, et al. Prevention of atrial fibrillation by renin‐angiotensin system inhibition: a meta‐analysis. J Am Coll Cardiol. 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 17. Durin O, Pedrinazzi C, Inama G. Focus on renin‐angiotensin system modulation and atrial fibrillation control after GISSI AF results. J Cardiovasc Med (Hagerstown). 2010;11:912–918. [DOI] [PubMed] [Google Scholar]
- 18. Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long‐lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106:331–336. [DOI] [PubMed] [Google Scholar]
- 19. Ueng KC, Tsai TP, Yu WC, et al. Use of enalapril to facilitate sinus rhythm maintenance after external cardioversion of long‐standing persistent atrial fibrillation: results of a prospective and controlled study. Eur Heart J. 2003;24:2090–2098. [DOI] [PubMed] [Google Scholar]
- 20. Yin Y, Dalal D, Liu Z, et al. Prospective randomized study comparing amiodarone vs. amiodarone plus losartan vs. amiodarone plus perindopril for the prevention of atrial fibrillation recurrence in patients with lone paroxysmal atrial fibrillation. Eur Heart J. 2006;27:1841–1846. [DOI] [PubMed] [Google Scholar]
- 21. Fogari R, Mugellini A, Destro M, et al. Losartan and prevention of atrial fibrillation recurrence in hypertensive patients. J Cardiovasc Pharmacol. 2006;47:46–50. [DOI] [PubMed] [Google Scholar]
- 22. Fogari R, Derosa G, Ferrari I, et al. Effect of valsartan and ramipril on atrial fibrillation recurrence and P‐wave dispersion in hypertensive patients with recurrent symptomatic lone atrial fibrillation. Am J Hypertens. 2008;21:1034–1039. [DOI] [PubMed] [Google Scholar]
- 23. Fogari R, Mugellini A, Zoppi A, et al. Effect of telmisartan and ramipril on atrial fibrillation recurrence and severity in hypertensive patients with metabolic syndrome and recurrent symptomatic paroxysmal and persistent atrial fibrillation. J Cardiovasc Pharmacol Ther. 2012;17:34–43. [DOI] [PubMed] [Google Scholar]
- 24. Tveit A, Grundvold I, Olufsen M, et al. Candesartan in the prevention of relapsing atrial fibrillation. Int J Cardiol. 2007;120:85–91. [DOI] [PubMed] [Google Scholar]
- 25. Disertori M, Latini R, Barlera S, et al; GISSI‐AF Investigators. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. [DOI] [PubMed] [Google Scholar]
- 26. Goette A, Schön N, Kirchhof P, et al. Angiotensin II antagonist in paroxysmal atrial fibrillation: results from the ANTIPAF trial. Circ Arrhythm Electrophysiol. 2012;5:43–51. [DOI] [PubMed] [Google Scholar]
- 27. Kakuta H, Sudoh K, Sasamata M, et al. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25:41–46. [PubMed] [Google Scholar]
- 28. Fogari R, Zoppi A, Ferrari I, et al. Comparative effects of telmisartan and eprosartan on insulin sensitivity in the treatment of overweight hypertensive patients. Horm Metab Res. 2009;41: 893–898. [DOI] [PubMed] [Google Scholar]
- 29. Derosa G, Cicero AF, D'Angelo A, et al. Telmisartan and irbesartan therapy in type 2 diabetic patients treated with rosiglitazone: effects on insulin‐resistance, leptin and tumor necrosis factor‐alpha. Hypertens Res. 2006;29:849–856. [DOI] [PubMed] [Google Scholar]
- 30. Derosa G, Ragonesi PD, Mugellini A, et al. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: a randomized, double‐blind, placebo‐controlled 12‐month study. Hypertens Res. 2004;27:457–464. [DOI] [PubMed] [Google Scholar]
- 31. Benson SC, Pershadsingh HA, Ho CI, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR‐gamma–modulating activity. Hypertension. 2004;43:993–1002. [DOI] [PubMed] [Google Scholar]
- 32. Yamagishi SI, Matsui T, Nakamura K. Possible molecular mechanisms by which angiotensin II type 1 receptor blockers (ARBs) prevent the development of atrial fibrillation in insulin‐resistant patients. Horm Metab Res. 2008;40:640–644. [DOI] [PubMed] [Google Scholar]
- 33. Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff‐perfused rabbit heart. Circulation. 1997;96:1686–1695. [DOI] [PubMed] [Google Scholar]
- 34. Kalifa J, Jalife J, Zaitsev AV, et al. Intra‐atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. [DOI] [PubMed] [Google Scholar]
- 35. Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]


