Abstract
Electromagnetic interference produced by medical equipment can interact with implanted cardiac devices such as pacemakers and implantable cardioverter‐defibrillators. The most commonly observed interaction is in the operating room with electrosurgery. The risk of interactions can often be mitigated by close communication between the cardiac‐device specialist and the anesthesiology/surgical team to develop a patient‐specific strategy that accounts for factors such as type of device, type of surgery, and whether the patient is pacemaker dependent. Although magnetic resonance imaging should generally not be used in patients with implanted cardiac devices, several published guidelines provide strategies and recommendations for managing risks if magnetic resonance imaging is required with no suitable diagnostic alternatives. Other common sources of electromagnetic interference in the medical environment are ionizing radiation and left ventricular assist devices. Clin. Cardiol. 2012 DOI: 10.1002/clc.21997
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
There are multiple sources of electromagnetic interference (EMI) in the medical setting (Table 1), and recommendations for management in patients with cardiac implantable electronic devices (CIEDs) such as pacemakers and implantable cardioverter‐defibrillators (ICDs) have been published in the Heart Rhythm Society/American Society of Anesthesiology Guidelines and in other reviews.1., 2. The most common source of EMI in the medical environment is electrosurgery (Figure 1). It is also important for the clinician to understand possible interactions between CIEDs and commonly used medical sources of EMI such as magnetic resonance imaging (MRI), ventricular assist devices, therapeutic ionizing radiation, and cardioversion.
Table 1.
Recommendations to Minimize Electromagnetic Interference in Medical Settings
| Electrosurgery |
|
| MRI (see Table 2) |
| LVAD |
|
| Radiation therapy |
|
| Cardioversion |
|
| TENS |
|
| Radiofrequency ablation, lithotripsy, ECT |
|
Abbreviations: CIED, cardiovascular implantable electronic device; ECG, electrocardiogram; ECT, electroconvulsive therapy; EMI, electromagnetic interference; ICD, implantable cardioverter‐defibrillator; LVAD, left ventricular assist device; MRI, magnetic resonance imaging; TENS, transcutaneous electrical nerve stimulation.
Figure 1.
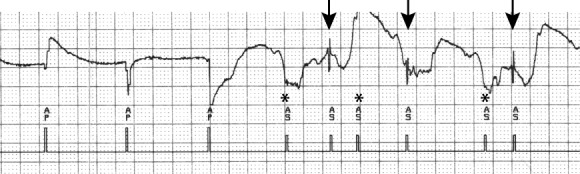
Atrial electrograms during electrosurgery used for contralateral shoulder surgery. During electrosurgery, atrial sensing of EMI (*) and intrinsic atrial activity (arrows) is observed. Abbreviations: AS, atrial sensing; EMI, electromagnetic interference.
Electrosurgery
Electrosurgery uses plasma arcs generated from alternating current in the radiofrequency (RF) range (100–5000 kHz) to cut or coagulate tissue. Most commonly the current is delivered between a cauterizing instrument and a large “return” electrode placed on the skin (monopolar configuration), but in some cases current is delivered in a bipolar configuration where electrical energy is delivered between the 2 electrodes at the tip of the surgical instrument.2 Because the EMI field is so small, bipolar electrosurgery does not interact with CIEDs and can be used without any special precautions. On the other hand, monopolar electrosurgery is the most common medical cause of EMI interaction with CIEDs.
Interaction between CIEDs and electrosurgery can be effectively managed in most circumstances by taking a few simple steps. The most critical step for elective procedures is preoperative communication between the CIED management team and anesthesiologists. A de novo preoperative evaluation is generally not required. The likelihood and risk of CIED and electrosurgery interaction depend on patient/CIED factors and surgical factors. For example, interaction is less likely as the distance between the CIED and the surgical field increases. In fact, procedures below the umbilicus are unlikely to generate EMI sensed by the CIED if the CIED is placed in the usual position in the upper chest and the return pad for electrosurgery is placed on the lower body (thigh or gluteal region). In the largest published case series to date, EMI‐CIED interaction was observed when the electrosurgery was performed within 8 cm of the CIED.3 In a more recent preliminary report of 171 patients with ICDs, EMI was identified in 9 of 22 surgical procedures performed above the umbilicus but in none of 53 procedures performed below the umbilicus.4 Often, a magnet is applied to ICDs during surgical procedures, because in most cases magnets will suspend tachycardia therapy. However, it is important to know that exceptions do exist and that in some cases the response to magnet application is a programmable option. Recently published guidelines suggest that for surgical procedures below the umbilicus, no intervention or magnet application are both reasonable options.1 For procedures above the umbilicus, where a higher likelihood of EMI and CIED interaction exists, inactivation of ICDs by either programming or magnet application are both reasonable options. For pacing systems without ICD function, the best option will often depend on whether or not the patient is pacemaker dependent. In those patients who are not pacemaker dependent, often no programming changes are required. In patients who are pacemaker dependent, where inhibition of output may lead to asystole or profound bradycardia, asynchronous pacing by mode programming or magnet application may be necessary. Other strategies to minimize risk to the patient are to use bipolar electrosurgery if possible and, if monopolar electrosurgery is required, to position the indifferent electrode so that the current path between the surgical field and the indifferent electrode is as far from the CIED as possible, and to use only short bursts of electrosurgery with the lowest clinically effective energy. Intraoperatively, plethysmographic or arterial pressure monitoring is essential, because electrosurgery will often cause artifacts on electrocardiographic (ECG) monitoring equipment, rendering surface ECG leads uninterpretable.
It is important to remember that after the surgery is completed, any preoperative programming changes that have been made must be addressed. In a retrospective single‐center study, inadvertent inactivation of ICDs was found in 4 patients, 2 after surgery.5 In an evaluation of a database of ICD patients maintained by the US Food and Drug Administration (FDA) and device manufacturers, of 212 deaths documented between 1996 and 2003, 11 ICDs were deactivated, 3 after surgery.6
Magnetic Resonance Imaging
Magnetic resonance imaging offers several advantages over other available imaging techniques but has been considered to be contraindicated in patients with CIEDs. It has been estimated that patients with pacemakers or ICDs have a 50%–75% likelihood of developing a clinical indication for MRI over the lifespan of the device, so manufacturers have been interested in developing “MRI‐compatible/safe” implantable devices.7 All 3 components of the MRI—the static magnetic field, rapidly varying magnetic fields (100–200 Hz), and electromagnetic RF fields (60–70 MHz)—have the potential to affect the function of the CIED.7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24.
The static magnetic field of the MRI will usually close the reed switch of the pacemaker, resulting in a magnet mode function that results in asynchronous pacing at a manufacturer‐determined rate. Asynchronous pacing is usually tolerated well, with only rare cases of hemodynamic compromise or development of atrial or ventricular arrhythmias due to pacing stimuli being delivered in the vulnerable periods of atria and/or ventricles leading to repetitive beating.8., 9., 10., 11. Theoretically, the static magnetic field could also cause sufficient torque in CIEDs within the device pocket, but no significant physical device movement has been documented (although some patients have reported a vibrating sensation) in newer devices that use less ferromagnetic material than older ones.8
The alternating magnetic fields and the rapid RF pulses may induce inappropriate device function with rapid pacing corresponding to the frequency of pulsing due to effects of RF energy on the pacemaker output circuits.11 Electrode heating with tissue thermal injury at the electrode‐myocardial tissue interface is another potential adverse effect.11., 14., 15., 16., 17. Heating is more pronounced with abandoned leads due to resonance from the similarity in the wavelength of the RF field of a 1.5‐Tesla (T) MRI and the length of a standard lead.12 In addition, heating at the tip of an abandoned lead is higher than when the lead is connected to a pulse generator because the lead can be modeled as an “electrical open,” as the connector is usually insulated by a plastic cap.12 A substantial increase in capture threshold has been reported after MRI at 1.5 T in some patients with pacemakers, which was attributed to thermal injury in proximity to the pacemaker lead tip.8., 9., 10. If the results from 3 studies that used troponin release as a marker for myocardial injury associated with MRI are combined, an abnormal troponin elevation that correlated with increase in pacing capture threshold was observed in only 1 of 206 examinations.20., 21., 22. It is important to remember that patients with abandoned leads have generally been excluded from in vivo studies and that very little is known about the effects of MRI in these patients.
To date, approximately 2000 patients with conventional CIEDs who have undergone MRI with no significant deleterious effects on the device are described in the literature.7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23. In the largest study to date, 555 MRI studies were performed in 438 patients with CIEDs (54% with pacemakers, 46% with ICDs).24 Patients with recently implanted leads (<6 wk), epicardial leads, abandoned leads, and pacemaker dependency were excluded. The MRI studies (brain, 40%; spine, 22%; heart, 16%; abdomen or pelvis, 13%; extremity, 9%) were performed using a 1.5‐T scanner. During the MRI, 3 CIEDs went to power‐on reset state, and small, clinically insignificant decreases in ventricular signal and increases in ventricular capture threshold were observed both acutely and at 6‐month follow‐up. There have been sporadic reports of fatalities temporally related to MRI examinations in patients with CIEDs. Two deaths were described in one review: 1 patient developed asystole and another ventricular fibrillation during the MRI.16 In the most comprehensive evaluation of possible deaths associated with MRI, a query of 30 legal medicine departments in Germany identified 6 cases where patients with pacemakers died during an MRI scan. Interestingly, in all 6 the indication for pacing was sinus‐node dysfunction, and none of the patients were pacemaker dependent. In 3 of the cases ventricular fibrillation was observed, and the MRI strength was 0.5 T in 3 cases, 1.0 T in 1, and 1.5 T in 2.17 It is important to note that identification of these patients who died during an MRI examination does not provide insight into the actual cause of death, and the presence of a CIED may have been coincidental.
Three professional societies, the American Heart Association (AHA), the European Society of Cardiology (ESC), and the American College of Radiology (ACR), have published position statements, guideline‐type documents, or scientific statements on performing MRI in patients with CIEDs (Table 2).18., 19., 20. Despite some differences in their recommendations, all emphasize that the decision to perform an MRI must be made on a patient‐specific basis with careful and thorough assessment of the potential risks and benefits, and all stress the importance of performing the MRI at an experienced center with close coordination between cardiology and radiology services. The AHA and ESC documents provide more specific recommendations based on the type of CIED and whether or not the patient is pacemaker dependent (Table 2).
Table 2.
Summary of Different Guidelines for the Use of Magnetic Resonance Imaging in Patients With Cardiovascular Implantable Electronic Devices
| AHA Scientific Statement | ESC Position Paper | ACR Guidance Document | |
| Patient selection | Should not be performed in pacemaker‐dependent patients or patients with ICDs unless there are “highly compelling circumstances”; discouraged in non–pacemaker‐dependent patients unless there is a “strong clinical indication” | Pacemaker‐dependent patients (very high risk), ICD patients (high risk), non–pacemaker‐dependent patients (low risk) | CIEDs are a relative contraindication to MRI; MRI should be performed on a “case‐by‐case and site‐by‐site basis.” |
| MRI considerations | Lowest RF power levels, weakest/ slowest necessary gradient magnetic fields | Field strength <1.5 T; limit SAR—no SAR >2 W/kg; minimize number/ length of sequences; send/receive coils preferred to surface coils | None given |
| Preoperative CIED evaluation | Interrogate the CIED; program to asynchronous pacing for pacemaker‐dependent patients; disable tachycardia therapy in ICD patients | Interrogate the CIED; program to asynchronous pacing for pacemaker‐dependent patients; disable tachycardia therapy in ICD patients; program to bipolar sensing; disable special algorithms (eg, rate adaptation) | No specific recommendations |
| Intraoperative | Monitor heart rhythm and vital signs; audio and visual contact; crash cart available; appropriate personnel available | ECG and pulse oximetry; audio and visual contact; crash cart available; ACLS‐certified personnel available; CIED programmer available | ECG and pulse oximetry; crash cart available; radiology and cardiology personnel available |
| Postoperative CIED evaluation | For any ICDs and pacemaker‐dependent patients, interrogate the CIED and reprogram to original parameters; for non–pacemaker‐dependent patients, reprogram as needed | Reinterrogate the CIED and reprogram to original parameters if required; interrogate the CIED at 1 week and 3 months | Reinterrogate the CIED; interrogate the CIED again 1–6 weeks after the MRI |
Abbreviations: ACLS, advanced cardiac life support; ACR, American College of Radiology; AHA, American Heart Association; CIED, cardiovascular implantable electronic device; ECG, electrocardiography; ESC, European Society of Cardiology; ICD, implantable cardioverter‐defibrillator; MRI, magnetic resonance imaging; RF, radiofrequency; SAR: specific absorption rate.
This is a field that is rapidly evolving. Recently, results from a multicenter trial that evaluated the use of a pacing system (both pacemaker and leads) designed specifically to reduce the likelihood of MRI‐CIED interaction have been published.25 This pacing system was implanted in 464 patients, who were then randomized to MRI (head and lumbar sequences with a 1.5‐T scanner) or no MRI at 9–12 weeks. At the 1‐week and 1‐month follow‐up, there were no differences between the 2 groups in pacing parameters or development of complications associated with the MRI. Based on the results of this study, the FDA approved the use of this pacing system in early 2011. It is important to keep several caveats in mind. First, in this study MRI of the chest area was not performed, and a larger postmarket surveillance study should be considered to identify infrequent adverse events. Second, these pacing systems are more expensive than standard systems. Finally, and most important, although some authors have estimated that 50%–75% of patients with CIEDs will need an MRI, this number is probably a significant overestimate, as often the clinical question can be answered by using alternative imaging modalities.
Left Ventricular Assist Devices
Placement of a left ventricular assist device (LVAD) has become an important option for the treatment of heart failure, either as a bridge to transplant or as destination therapy.26., 27. Not surprisingly, there have been recent reports of interactions between CIEDs and LVADs.28., 29., 30., 31., 32., 33., 34., 35.
Two recent small retrospective studies of patients with ICDs at the time of LVAD placement reported significant changes in ventricular‐lead function after LVAD implantation, which appeared to persist over time. These changes included a decrease in the amplitude of the sensed ventricular electrogram, increase in capture threshold, and decreased lead impedance thought to be due to lead insulation breach during surgery.28., 29. Because the changes are almost always observed in patients with right ventricular leads, it is likely that placement of the LVAD cannula in the left ventricular apex causes changes in ventricular geometry or actual mechanical damage to the ventricular lead. As a result of alterations in lead parameters, in one of the above‐cited series 4 patients (13%) required lead revision and 6 patients (20%) required ICD testing because of decreases in the sensed R wave identified after LVAD implant.29
There is one case in the medical literature of EMI between the LVAD and an ICD that resulted in an inappropriate shock due to oversensing of noise generated by the LVAD battery system.30 A unique interaction between LVADs and a manufacturer‐specific (St. Jude Medical) CIED is loss of telemetry.31., 32., 33., 34., 35. The problem arises because the HeartMate II LVAD pulse‐width modulators, which serve to regulate voltage input to the LVAD motor, operate at the 7.2‐kHz frequency and interfere with the 8‐KHz ICD telemetry operating frequency used by St. Jude Medical. To resume communication between the programmer and ICD, some patients have required use of metal shielding or ICD replacement with a device that communicates on a different frequency. Despite the potential for interactions between LVADs and CIEDs, generally these devices can coexist if a few simple guidelines are followed (Table 1).
Radiotherapy (Diagnostic and Therapeutic)
Diagnostic radiation generally does not have any significant adverse effect on CIEDs. Transient effects due to oversensing have been reported when the CIEDs were exposed to multislice computed tomography, but clinical symptoms are rarely observed.36., 37.
There are 2 documented effects of therapeutic radiation therapy on CIEDs.1., 2., 38., 39., 40., 41., 42., 43. First, the EMI can result in output inhibition, inappropriate tracking, activation of noise algorithms, and, in ICDs, inappropriate antitachycardia therapy due to device oversensing. Second, a problem unique to therapeutic radiation is direct effects to CIED circuitry that can lead to temporary or permanent changes. The current widespread use of complementary metal oxide semiconductors (CMOS) in modern programmable devices has made them more vulnerable to ionizing effects via damage to the silicon and the silicon oxide insulators within the semiconductors.1., 2., 38., 39., 40., 41., 42., 43. The effect of radiation on implanted devices is cumulative and depends on the type of radiation, and the location and type of CIED.1., 2., 38., 39., 40., 41., 42., 43. The mode of device failure is somewhat unpredictable, due in part to the random nature of the specific site of the CIED circuitry that is affected. The dose at the point of failure can vary dramatically. In a study of 11 patients with ICDs, the dose at point of failure ranged from 0.5 to 120 Gy even for the same device.41 Recent reports include 8 patients with pacemakers who underwent radiation therapy to the neck or chest area with no untoward effects observed during the treatment sessions or after a median follow‐up of 5 months, and another case report of an ICD that had a power‐on reset even though the device was located outside the radiation field.42., 43.
Device manufacturers have provided guidelines for patients undergoing radiation therapy. All of the major manufacturers do not recommend radiation therapy if the CIED is within the treatment field. The maximal recommended dose a CIED generator can tolerate varies from manufacturer to manufacturer: St. Jude Medical lists 20–30 Gy for pacemakers and does not make recommendations for ICDs; Medtronic lists 5 Gy for pacemakers and 1–5 Gy for ICDs; Boston Scientific states that there is no safe dose for CIEDs; and Biotronik lists 10 Gy for pacemakers and does not make recommendations for ICDs. Professional societies provide some guidance. Almost 2 decades ago, the American Association of Physicists in Medicine published guidelines for radiation therapy in patients with pacemakers that emphasized the importance of excluding the pacemaker from the radiation field and recommended a maximal dose of 2 Gy. More recently, the Heart Rhythm Society/American Society of Anesthesiologists Expert Consensus Statement provides several broad recommendations.3 In some high‐risk cases in which a direct beam to the chest or high‐energy photon radiation is used, CIEDs should be evaluated within 24 hours of each treatment; whereas in lower‐risk cases (for example, non–pacemaker‐dependent patients), weekly evaluation may be appropriate. Remote monitoring, if available, can be used for this purpose. One practical solution provided in the Consensus Statement is to program the device to a relatively high rate that exceeds the patient's intrinsic rate, and have the radiation therapy staff check the heart rate after each treatment. If the heart rate is lower than the programmed rate, the patient should be referred for a formal CIED check, as a power‐on reset may have occurred (power‐on reset rates for most manufacturers are <80 bpm).
In all situations, close collaboration between members of the CIED and radiation therapy teams is required because, for optimum patient safety and therapy yield, an individualized treatment plan is required.
Cardioversion
External direct cardioversion or defibrillation may result in CIED malfunction. All permanent pacing systems and ICDs utilize a special circuit composed of several zener diodes that is designed to protect the CIED electronics from high voltage by shunting away any excess energy. However, this may result in delivery of sufficient energy at the electrode‐myocardial interface to cause changes in pacing and sensing function. Potential adverse interactions between cardiac pacemakers and electrical cardioversion or defibrillation include activation of the backup function at the manufacturer‐specific backup rate, pacing‐mode change (reported with some older unipolar pacemakers), and increases in myocardial stimulation thresholds (Figure 2).44., 45., 46., 47. These capture threshold increases may be transient, with an acute loss of capture and complete recovery of function some time later, but in rare cases the increase in threshold is permanent.47 In an older study utilizing unipolar leads and an anterior‐apical patch configuration for cardioversion, transient loss of capture occurred in 50% of patients, with a time range of 5 seconds to 30 minutes.46 Conversely, cardioversion appears to have very few adverse effects in pacing systems using bipolar leads if an anterior‐posterior pad position is chosen, with the pads as far away from the CIED as possible (>8 cm).48 In a randomized controlled study of 44 patients with pacemakers with no preprocedure programming, no evidence of device or clinically significant lead malfunction was detected at interrogations performed 1 hour and 1 week after cardioversion. In general, using simple risk‐mitigation steps, both cardioversion and defibrillation can be performed without problems (Table 1).
Figure 2.
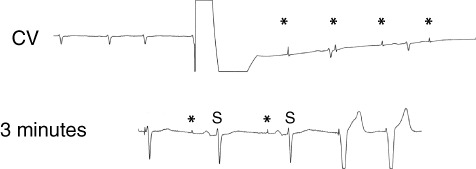
Immediately after external cardioversion, loss of capture (*) and sensing is present. After 3 minutes, sensing returns first, followed by ventricular capture. Abbreviations: CV, cardioversion; S, sensing.
Other Medical Equipment, Radiofrequency Ablation, Transcutaneous Electrical Nerve Stimulation, Lithotripsy, Electroconvulsive Therapy
Interactions between CIEDs and a variety of medical equipment, such as dental equipment, have been described, generally in older generations of devices that used unipolar leads.2 Interestingly, more recent reports of potential interactions between dental equipment and CIEDs probably represent EMI with the ECG telemetry rather than with the CIED. Although capsule endoscopy devices carry warnings about use in patients with CIEDs, clinical studies have failed to show any interactions.3., 49. One unique interaction is between pacemakers that use minute ventilation as a rate‐adaptive sensor and some cardiac telemetry monitors that monitor respiratory rate using changes in impedance observed by sending very‐small‐amplitude, high‐frequency currents through the ECG leads.50., 51. Several case reports have been published describing unexpected pacing at high rates due to activation of the minute ventilation sensor within the CIED.
Radiofrequency catheter ablation has become a common procedure in management of various arrhythmias. Radiofrequency generators produce signals with strengths of 5–50 W and frequencies of 400–500 kHz, delivered in a unipolar manner between the RF catheter tip and a large indifferent electrode usually placed on the patient's thigh. Potential interactions between CIEDs and RF ablations are similar to those of electrosurgery and include asynchronous pacing, reset to the backup mode and rate, induction of runaway pacing at extremely rapid rates (rare today), inappropriate stimulus output inhibition, and transient loss of capture.1., 2. The newer generation of pacemakers, which incorporate protection elements to avoid EMI, did not show any of these interactions in a study of 86 patients with CIEDs who underwent RF ablation of atrial fibrillation, although atrial‐lead dislodgement was noted in 2 cases in which the leads had been recently implanted (<6 mo).52
Transcutaneous electrical nerve stimulation (TENS) is commonly used for the relief of acute and chronic musculoskeletal pain. The TENS output can interfere with CIED function, causing inhibition of output, reversion to noise mode in pacemakers, or triggering of inappropriate shocks from an ICD.1., 2., 53., 54., 55., 56. However, in many if not most pacemaker patients, TENS can be used safely. In one study of 51 patients with 20 different pacemaker models, there were no cases of interference, inhibition, or reprogramming reported.55 In pacemaker‐dependent patients, TENS should be used carefully and should be reserved for those in whom it will be important for improving quality of life and in whom prior monitored testing is performed and safety confirmed. Continued assessment is important, as there has been a published case report of inhibition of pacing due to TENS‐CIED interaction occurring 6 months after initial monitored assessment had demonstrated no interaction.56
Lithotripsy is an important tool for the treatment of renal calculi. Initially, the presence of a pacemaker was believed to be an absolute contraindication for lithotripsy. In addition, early ICD generators placed in the abdomen were located relatively near the focal point of energy delivery by the lithotriptor. However, with the newest generation of pectorally implanted CIEDs that employ specialized feedthrough filters, and lithotriptors that do not require hydroimmersion and allow more focal delivery of energy, the likelihood of EMI interaction is extremely small, estimated by some to be <1% even without special precautions.1., 57.
Electroconvulsive therapy (ECT) may be used in patients with severe depression. In the medical literature there have been approximately 60 patients with CIEDs who have safely undergone ECT.58 Generally, patients with pacemakers require no specific reprogramming, and although it is reasonable to disable antitachycardia therapies in patients with ICDs during their ECT session, no study has evaluated different strategies in a randomized manner. The recent HRS/ASA Guidelines document suggests that in general the risk of EMI with ECT is low, and simply having a magnet available is a reasonable option for most patients with pacemakers or ICDs.1
Conclusion
The number of patients with CIEDs has increased dramatically over the past decade, and medical sources for EMI have also increased substantially. Fortunately, device manufacturers have responded by developing sophisticated noise‐response algorithms, improved circuitry that is intended to minimize interactions from EMI sources, and device components that use less ferromagnetic material. However, the possibility of interactions between CIEDs and EMI sources remains, particularly from medical sources such as electrosurgery, cardioversion, and MRI. A thorough understanding of techniques for risk mitigation of adverse clinical outcomes from known EMI sources is essential for all cardiologists.
References
- 1. Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management: executive summary. This document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm. 2011;8:e1–e18. [DOI] [PubMed] [Google Scholar]
- 2. Cohan L, Kusumoto FM, Goldschlager NF. Environmental effects on cardiac pacing systems. In: Kusumoto FM, Goldschlager NF, eds. Cardiac Pacing for the Clinician. New York, NY: Springer; 2008;595–618. [Google Scholar]
- 3. Cheng A, Nazarian S, Spragg DD, et al. Effects of surgical and endoscopic electrocautery on modern‐day permanent pacemaker and implantable cardioverter‐defibrillator systems. Pacing Clin Electrophysiol. 2008;31:344–350. [DOI] [PubMed] [Google Scholar]
- 4. Hoyt R, Johnson W, Lieserwitz A. Monopolar electrosurgery interactions with the implantable cardioverter‐defibrillator. Heart Rhythm. 2010;7(suppl 2):33. 20129283 [Google Scholar]
- 5. Rasmussen MJ, Friedman PA, Hammill SC, et al. Unintentional deactivation of implantable cardioverter‐defibrillators in health care settings. Mayo Clin Proc. 2002;77:855–859. [DOI] [PubMed] [Google Scholar]
- 6. Hauser RG, Kallinen L. Deaths associated with implantable cardioverter defibrillator failure and deactivation reported in the United States Food and Drug Administration Manufacturer and User Facility Device Experience Database. Heart Rhythm. 2004;1:399–405. [DOI] [PubMed] [Google Scholar]
- 7. Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28:326–328. [DOI] [PubMed] [Google Scholar]
- 8. Roguin A, Zviman MM, Meininger GR, et al. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable‐cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roguin A, Donahue JK, Bomma CS, et al. Cardiac magnetic resonance imaging in a patient with implantable cardioverter‐defibrillator. Pacing Clin Electrophysiol. 2005;28:336–338. [DOI] [PubMed] [Google Scholar]
- 11. Hayes DL, Holmes DR Jr, Gray JE. Effect of 1.5 tesla nuclear magnetic resonance imaging scanner on implanted permanent pacemakers. J Am Coll Cardiol. 1987;10:782–786. [DOI] [PubMed] [Google Scholar]
- 12. Langman DA, Finn JP, Ennis DB. Abandoned pacemaker leads are a potential risk for patients undergoing MRI. Pacing Clin Electrophysiol. 2011;34:1051–1053. [DOI] [PubMed] [Google Scholar]
- 13. Naehle CP, Strach K, Thomas D, et al. Magnetic resonance imaging at 1.5‐T in patients with implantable cardioverter‐defibrillators. J Am Coll Cardiol. 2009;54:549–555. [DOI] [PubMed] [Google Scholar]
- 14. Zikria JF, Machnicki S, Rhim E, et al. MRI of patients with cardiac pacemakers: a review of the medical literature. AJR Am J Roentgenol. 2011;196:390–401. [DOI] [PubMed] [Google Scholar]
- 15. Naehle CP, Kreuz J, Strach K, et al. Safety, feasibility, and diagnostic value of cardiac magnetic resonance imaging in patients with cardiac pacemakers and implantable cardioverters/defibrillators at 1.5 T. Am Heart J. 2011;161:1096–1105. [DOI] [PubMed] [Google Scholar]
- 16. Pohost GM, Blackwell GG, Shellock FG. Safety of patients with medical devices during application of medical resonance methods. Ann NY Acad Sci. 1992;649:302–312. [DOI] [PubMed] [Google Scholar]
- 17. Irnich W, Irnich B, Bartsch C, et al. Do we need pacemakers resistant to magnetic resonance imaging? Europace. 2005;7:353–365. [DOI] [PubMed] [Google Scholar]
- 18. Levine GN, Gomes AS, Arai AE, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Radiology and Intervention. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–2891. [DOI] [PubMed] [Google Scholar]
- 19. Roguin A, Schwitter J, Vahlhaus C, et al. Position paper: magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace. 2008;10:336–346. [DOI] [PubMed] [Google Scholar]
- 20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447–1474. [DOI] [PubMed] [Google Scholar]
- 21. Naehle CP, Meyer C, Thomas D, et al. Safety of brain 3‐T MR imaging with transmit‐receive head coil in patients with cardiac pacemakers: pilot prospective study with 51 examinations. Radiology. 2008;249:991–1001. [DOI] [PubMed] [Google Scholar]
- 22. Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non‐pacemaker‐dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285–1292. [DOI] [PubMed] [Google Scholar]
- 23. Mollerus M, Albin G, Lipinski M, et al. Cardiac biomarkers in patients with permanent pacemakers and implantable cardioverter‐defibrillators undergoing an MRI scan. Pacing Clin Electrophysiol. 2008;31:1241–1245. [DOI] [PubMed] [Google Scholar]
- 24. Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkoff BL, Bello D, Taborsky M, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm. 2011;8: 65–73. [DOI] [PubMed] [Google Scholar]
- 26. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–1533. [DOI] [PubMed] [Google Scholar]
- 28. Ambardekar AV, Lowery CM, Allen LA, et al. Effect of left ventricular assist device placement on preexisting implantable cardioverter‐defibrillator leads. J Card Fail. 2010;16:327–331. [DOI] [PubMed] [Google Scholar]
- 29. Foo D, Walker BD, Kuchar DL, et al. Left ventricular mechanical assist devices and cardiac device interactions: an observational case series. Pacing Clin Electrophysiol. 2009;32:879–887. [DOI] [PubMed] [Google Scholar]
- 30. Matthews JC, Betley D, Morady F, et al. Adverse interaction between a left ventricular assist device and an implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2007;18: 1107–1108. [DOI] [PubMed] [Google Scholar]
- 31. Andersen M, Videbaek R, Boesgaard S, et al. Incidence of ventricular arrhythmias in patients on long‐term support with a continuous‐flow assist device (HeartMate II). J Heart Lung Transplant. 2009;28:733–735. [DOI] [PubMed] [Google Scholar]
- 32. Oswald H, Klein G, Strüber M, et al. Implantable defibrillator with left ventricular assist device compatibility. Interact Cardiovasc Thorac Surg. 2009;8:579–580. [DOI] [PubMed] [Google Scholar]
- 33. Kühne M, Sakumura M, Reich SS, et al. Simultaneous use of implantable cardioverter‐defibrillators and left ventricular assist devices in patients with severe heart failure. Am J Cardiol. 2010;105:378–382. [DOI] [PubMed] [Google Scholar]
- 34. Biviano A, Mancini D, Naka Y, et al. Overcoming electromagnetic interference by LVADs on ICD function by shielding the ICD programmer wand and extension cable. Pacing Clin Electrophysiol. 2009;32:945–948. [DOI] [PubMed] [Google Scholar]
- 35. Jacob S, Cherian PK, Ghumman WS, et al. “Pseudo” Faraday cage: a solution for telemetry link interaction between a left ventricular assist device and an implantable cardioverter defibrillator. J Interv Card Electrophysiol. 2010;28:221–225. [DOI] [PubMed] [Google Scholar]
- 36. McCollough CH, Zhang J, Primak AN, et al. Effects of CT irradiation on implantable cardiac rhythm management devices. Radiology. 2007;243:766–774. [DOI] [PubMed] [Google Scholar]
- 37. Yamaji S, Imai S, Saito F, et al. Does high‐power computed tomography scanning equipment affect the operation of pacemakers? Circ J. 2006;70:190–197. [DOI] [PubMed] [Google Scholar]
- 38. Adamec R, Haefliger JM, Killisch JP, et al. Damaging effect of therapeutic radiation on programmable pacemakers. Pacing Clin Electrophysiol. 1982;5:146–150. [DOI] [PubMed] [Google Scholar]
- 39. Wadasadawala T, Pandey A, Agarwal JP, et al. Radiation therapy with implanted cardiac pacemaker devices: a clinical and dosimetric analysis of patients and proposed precautions. Clin Oncol (R Coll Radiol). 2011;23:79–85. [DOI] [PubMed] [Google Scholar]
- 40. Maxted KJ. The effect of therapeutic x‐radiation on a sample of pacemaker generators. Phys Med Biol. 1984;29:1143–1146. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez F, Filimonov A, Henning A, et al. Radiation‐induced effects in multiprogrammable pacemakers and implantable defibrillators. Pacing Clin Electrophysiol. 1991;14:2143–2153. [DOI] [PubMed] [Google Scholar]
- 42. Hurkmans CW, Scheepers E, Springorum BG, et al. Influence of radiotherapy on the latest generation of implantable cardioverter‐defibrillators. Int J Radiat Oncol Biol Phys. 2005;63:282–289. [DOI] [PubMed] [Google Scholar]
- 43. Thomas D, Becker R, Katus HA, et al. Radiation therapy–induced electrical reset of an implantable cardioverter defibrillator device located outside the irradiation field. J Electrocardiol. 2004;37: 73–74. [DOI] [PubMed] [Google Scholar]
- 44. Barold SS, Ong LS, Scovil J, et al. Reprogramming of implanted pacemaker following external defibrillation. Pacing Clin Electrophysiol. 1978;1:514–520. [DOI] [PubMed] [Google Scholar]
- 45. Levine PA, Barold SS, Fletcher RD, et al. Adverse acute and chronic effects of electrical defibrillation and cardioversion on implanted unipolar cardiac pacing systems. J Am Coll Cardiol. 1983;1:1413–1422. [DOI] [PubMed] [Google Scholar]
- 46. Altamura G, Bianconi L, Lo Bianco F, et al. Transthoracic DC shock may represent a serious hazard in pacemaker dependent patients. Pacing Clin Electrophysiol. 1995;18:194–198. [DOI] [PubMed] [Google Scholar]
- 47. Das G, Staffanson DB. Selective dysfunction of ventricular electrode‐endocardial junction following CD cardioversion in a patient with a dual‐chamber pacemaker. Pacing Clin Electrophysiol. 1997; 20(2 part 1):346–365. [DOI] [PubMed] [Google Scholar]
- 48. Waller C, Callies F, Langenfeld H. Adverse effects of direct current cardioversion on cardiac pacemakers and electrodes: is external cardioversion contraindicated in patients with permanent pacing systems? Europace. 2004;6:165–168. [DOI] [PubMed] [Google Scholar]
- 49. Bandorski D, Lotterer E, Hartmann D, et al. Capsule endoscopy in patients with cardiac pacemakers and implantable cardioverter‐defibrillators—a retrospective multicenter investigation. J Gastrointestin Liver Dis. 2011;20:33–37. [PubMed] [Google Scholar]
- 50. Houtman S, Rinia M, Kalkman C. Monitor‐induced tachycardia in a patient with a rate responsive pacemaker. Anaesthesia. 2006; 61:399–401. [DOI] [PubMed] [Google Scholar]
- 51. Southorn PA, Kamath GS, Vasdev GM, et al. Monitoring equipment induced tachycardia in patients with minute ventilation pacemakers. Br J Anaesth. 2000;84:508–509. [DOI] [PubMed] [Google Scholar]
- 52. Lakkireddy D, Patel D, Ryschon K, et al. Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators. Heart Rhythm. 2005;2:1309–1316. [DOI] [PubMed] [Google Scholar]
- 53. Philbin DM, Marieb MA, Aithal KH, et al. Inappropriate shocks delivered by an ICD as a result of sensed potentials from a transcutaneous electronic nerve stimulation unit. Pacing Clin Electrophysiol. 1998;21:2010–2011. [DOI] [PubMed] [Google Scholar]
- 54. Rasmussen MJ, Hayes DL, Vlietstra RE, et al. Can transcutaneous electrical nerve stimulation be safely used in patients with permanent cardiac pacemakers? Mayo Clin Proc. 1988;63:443–445. [DOI] [PubMed] [Google Scholar]
- 55. Pyatt JR, Trenbath D, Chester M, et al. The simultaneous use of a biventricular implantable cardioverter defibrillator (ICD) and transcutaneous electrical nerve stimulation (TENS) unit: implications for device interaction. Europace. 2003;5:91–93. [DOI] [PubMed] [Google Scholar]
- 56. Holmgren C, Carlsson T, Mannheimer C, et al. Risk of interference from transcutaneous electrical nerve stimulation on the sensing function of implantable defibrillators. Pacing Clin Electrophysiol. 2008;31:151–158. [DOI] [PubMed] [Google Scholar]
- 57. Platonov MA, Gillis AM, Kavanagh KM. Pacemakers, implantable cardioverter/defibrillators, and extracorporeal shockwave lithotripsy: evidence‐based guidelines for the modern era. J Endourol. 2008;22:243–247. [DOI] [PubMed] [Google Scholar]
- 58. Kokras N, Politis AM, Zervas IM, et al. Cardiac rhythm management devices and electroconvulsive therapy: a critical review apropos of a depressed patient with a pacemaker. J ECT. 2011;27: 214–220. [DOI] [PubMed] [Google Scholar]


