Abstract
Background:
The CHADS2 score (C, congestive heart failure [CHF]; H, hypertension [HT]; A, age ≥75 y; D, diabetes mellitus; S2, prior stroke or transient ischemic attack) is used to assess the risk of ischemic stroke in patients with atrial fibrillation (AF). However, its role in patients without documented AF is not well explored.
Hypothesis:
The goal of the current study was to explore if the incidence of hospitalization with first‐ever AF after stroke increased with increasing CHADS2 score.
Methods:
We identified 57636 patients with nonfatal stroke and no documented AF in the Swedish Stroke Register (Riks‐Stroke) during 2001–2004 and followed them for a mean of 2.2 years through record linkage to the Inpatient and Cause of Death registers. Cox regression hazard models were used to estimate the relative risk (RR) of new AF following stroke and its association with different CHADS2 scores.
Results:
Overall, 2769 patients were hospitalized with new AF (4.8%, 21.7 per 1000 person‐years). The incidence increased from 9.6 per 1000 person‐years in CHADS2 score 0 to 42.7 in CHADS2 score 6, conferring a RR of 4.2 (95% confidence interval [CI]: 2.5–6.8). For CHADS2 scores 3–5, the RRs were approximately 3 (vs CHADS2 score 0). Adjusted RRs were 1.9 (95% CI: 1.7–2.1) for CHF, 1.4 (95% CI: 1.3–1.5) for HT, 2.1 (95% CI: 2.0–2.3) for age ≥75 years, 0.9 (95% CI: 0.8–1.0) for diabetes, and 1.0 (95% CI: 0.91–1.07) for previous stroke. The risk of AF was higher in ischemic than in hemorrhagic stroke.
Conclusions:
In this retrospective register study, the incidence of AF following stroke was strongly influenced by higher CHADS2 scores where age ≥75 years, CHF, and HT were the contributing CHADS2 components. © 2011 Wiley Periodicals, Inc.
Riks‐Stroke is funded by the National Board of Health and Welfare and the Swedish Association of Local Authorities and Regions. S. Åsberg has received a research scholarship from the National Association for Stroke Patients in Sweden. All authors have independent affiliations with universities in Sweden. B. Farahmand and K. Henriksson are employees of AstraZeneca R&D, Sweden. A. Terént has received funding from AstraZeneca. N. Edvardsson serves as medical advisor to AstraZeneca R&D, Sweden. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
About 15% of strokes can be estimated to be of embolic origin and related to atrial fibrillation (AF).1 The AF may have been previously documented or may be ongoing at the time of admission. Patients admitted with sinus rhythm (SR) may have had earlier episodes of paroxysmal or persistent AF, but a high proportion of patients have no history of AF. Investigation may reveal the cause of stroke, but in some patients no explanation can be found, and such patients are said to have had a cryptogenic stroke.
Scores for estimating the risk of an embolic stroke have been developed, eg, the CHADS2 score (C, congestive heart failure [CHF]; H, hypertension [HT]; A, age ≥75 y; D, diabetes mellitus [DM]; S2, prior stroke or transient ischemic attack [TIA]).2 Using these stated clinical variables, giving 1 point to each (except 2 points for a history of stroke), the maximum possible CHADS2 score is 6 points. The higher the score, the higher the risk of having a stroke. Although this score was intended for use in patients with known AF, some of its components are also relevant to the risk of developing AF.
Atrial fibrillation may or may not be symptomatic, and some patients may never feel or know that they are in AF, with a risk of stroke that may be low or high depending on concomitant risk factors. We therefore aimed to examine the relationship between the CHADS2 score on admission and the incidence of hospitalization with an AF diagnosis after stroke.
Subjects and Methods
Subjects
In this retrospective register study, we identified 57636 patients with nonfatal stroke (International Classification of Diseases [ICD]‐10 codes I61, I63, and I64) and with no history of AF (self‐reported questionnaire or hospitalized with AF diagnosis [ICD‐9 code 427.3, ICD‐10 code I489] prior to index stroke) in Riks‐Stroke from 2001 to 2004.
The Swedish Stroke Register, Riks‐Stroke
Riks‐Stroke (http://www.Riks‐Stroke.org) is the national quality register for stroke care in Sweden. It includes all hospitals that admit patients with acute stroke and covers >80% of all stroke events in Sweden. A case‐by‐case validation of Riks‐Stroke indicates that patients who die early, who are not treated at a stroke unit, or who are cared for in a nursing home are less likely to be included in the register.3 The Riks‐Stroke protocol has a confirmed very high validity for certain variables, eg, the stroke diagnoses.4 It is less accurate for others, eg, smoking.
Registration consists of the completion of a case record form at admission of an acute stroke, with 37 variables covering basal information such as sex, age, cardiovascular risk factors, previous stroke, and subtype of stroke, and a follow–up questionnaire 3 months later consisting of 26 variables mainly focusing on the quality of care and daily function abilities, living conditions, and general health.5, 6
The Swedish Hospital Discharge and Cause of Death Registers
All inhabitants have a unique 10‐digit identification number that provides a reliable tool for linking to the many extensive national registers in Sweden. The medical discharge and death registers are kept by the National Board of Health and Welfare and all entries are mandatory. This in combination with a high standard of medical care provided to all citizens makes the databases quite sturdy to biases on population level. The register includes data on main and secondary diagnoses and is updated annually. We collected record‐linked information on comorbidity and mortality data for all study subjects from 1987 until the end of 2005. The linkage between databases is done by the authorities after assessment and approval by the ethical committee. All data are returned without identification numbers to the researcher. This study was approved by the ethics committee of the Umeå University (reg. no. 69106, 2006).
Definition of Stroke and Atrial Fibrillation
A diagnosis was present if identified either by ICD code in the discharge register or reported in the Riks‐Stroke protocol. Stroke and AF were defined according to the World Health Organization definitions, ie, intracerebral hemorrhage, cerebral infarction, and unspecified stroke (ICD‐9 codes 431, 434, and 436; ICD‐10 codes I61 and I63‐I64), and AF (ICD‐9 code 427.3 or ICD‐10 code I48.9). No distinction was made between different types of AF, eg, paroxysmal, persistent or permanent AF, or atrial flutter; or whether the tachyarrhythmia was symptomatic or asymptomatic. Atrial fibrillation could be diagnosed by means of electrocardiography (ECG), in‐hospital ECG monitoring, or long‐term ambulatory ECG monitoring.
CHADS2 Score Calculation
As previously stated, the CHADS2 score ranges from 0 to 6 and is calculated by adding 1 point for each of the first 4 risk factors and 2 points for previous history of stroke or TIA. A CHADS2 score can thus range from 0 (none of the comorbidities and age <75 years) to 6 (all comorbidities and age ≥75 years). In the present study, the ICD‐9 and ICD‐10 codes used were as follows: CHF, ICD‐9 428–429, ICD‐10 I50; HT, self‐reported or hospitalized with diagnosis of ICD‐9 401–405, ICD‐10 I10‐I15; age ≥75 years; DM, self‐reported or hospitalized with diagnosis of ICD‐9 250, ICD‐10 E10‐E14; and previous stroke or TIA, self‐reported or hospitalized with diagnosis of ICD‐9 431, 434–436, ICD‐10 I61, I63, I64, or G45. The CHADS2 scores were calculated based on information collected prior to the registration of the index stroke.
Statistical Analysis
We calculated the incidence of AF per 1000 person‐years by CHADS2 components and score. The occurrence of AF within 2 years following stroke, associated with an increasing CHADS2 score was examined by means of the Kaplan‐Meier method. Cox proportional hazard regression models were used to estimate the adjusted hazard ratios and their 95% confidence intervals (CIs) of AF associated with CHADS2 score, and separately for each component of CHADS2. To investigate the impact of age, we applied the same 4 age categories (<64, 65–74, 75–84, and ≥85) as were originally used in analyses from the Riks‐Stroke database. As age is included in the CHADS2 score (≥75 years yes/no), we adjusted for age as a continuous variable whenever possible. The LIFETEST and PHREG procedures in SAS version 9.1 (SAS Institute, Inc., Cary, NC) were used for the computation. Person‐years were calculated individually for each patient from the date of first registration in the Riks‐Stroke register to the date of first hospitalization for primary or secondary diagnosis of AF, death, or end of follow‐up at December 31, 2005, whichever came first.
Results
A total of 2769 patients (4.8%) had their first documented AF after hospitalization of the index stroke, an incidence of 21.7 per 1000 person‐years. The corresponding incidence during the same period for a general population aged >65 years in Sweden was 5.3 per 1000 person‐years. The patients who developed AF were on an average 4 years older than those who did not, and a higher proportion was women. There were twice as many patients with CHADS2 score 0 among patients without AF than with AF (16% vs 8%). Congestive heart failure, HT, and age ≥75 years were more frequent in patients with AF than without (Table 1). The 2 groups did not differ in terms of the prevalence of DM or previous stroke on admission.
Table 1.
Characteristics of Study Subjects
| AF | ||||
|---|---|---|---|---|
| No | Yes | |||
| n = 54 867 | % | n = 2769 | % | |
| Age, y | ||||
| <64 | 12142 | 22.1 | 249 | 9.0 |
| 65–74 | 13 487 | 24.6 | 661 | 23.9 |
| 75–84 | 20153 | 36.7 | 1302 | 47.0 |
| ≥ 85 | 9085 | 16.6 | 557 | 20.1 |
| Mean age, y | 73.3 | 12.0 | 77.2 | 8.9 |
| Mean years of follow‐up | 2.3 | 1.4 | 1.4 | 1.1 |
| Sex | ||||
| M | 28 549 | 52.0 | 1326 | 47.9 |
| F | 26318 | 48.0 | 1443 | 52.1 |
| Type of stroke | ||||
| Hemorrhagic | 7731 | 14.1 | 156 | 5.6 |
| Ischemic | 44459 | 81.0 | 2464 | 89.0 |
| Unspecified | 2677 | 4.9 | 149 | 5.4 |
| Comorbidities | ||||
| C (CHF) | 4327 | 7.9 | 337 | 12.2 |
| H (HT) | 29313 | 53.4 | 1715 | 61.9 |
| A75 (age ≥75 y) | 29238 | 53.3 | 1859 | 67.1 |
| D (DM) | 11596 | 21.1 | 539 | 19.5 |
| S (stroke/TIA) | 13651 | 24.9 | 713 | 25.7 |
| CHADS2 score | ||||
| 0 | 8822 | 16.1 | 226 | 8.2 |
| 1 | 16 484 | 30.0 | 774 | 28.0 |
| 2 | 13525 | 24.7 | 810 | 29.3 |
| 3 | 8354 | 15.2 | 499 | 18.0 |
| 4 | 5515 | 10.1 | 332 | 12.0 |
| 5 | 1882 | 3.4 | 111 | 4.0 |
| 6 | 285 | 0.5 | 17 | 0.6 |
Abbreviations: AF, atrial fibrillation; CHF, congestive heart failure; DM, diabetes mellitus; F, female; HT, hypertension; M, male; TIA, transient ischemic attack.
The Kaplan‐Meier curves for AF after stroke diverged by CHADS2 score. At the end of the 2.2‐year follow‐up, AF had occurred in 2% of patients with CHADS2 score 0, in 4% with score 1, in 6% with scores 2–5, and in 9% with score 6 (Figure 1). We observed a trend toward an increase in the incidence of AF at higher CHADS2 scores. The hazard ratio of AF among those with the highest vs the lowest CHADS2 score was 4.2 (95% CI: 2.54–6.80; Table 2).
Figure 1.
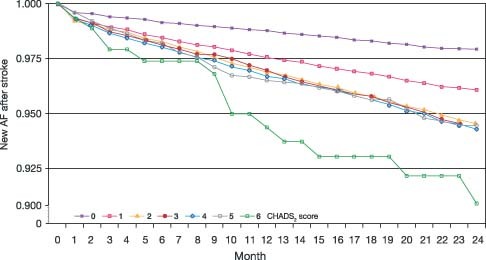
Kaplan‐Meier curves for the detection of the first‐ever atrial fibrillation after stroke by CHADS2 score. Abbreviation: AF, atrial fibrillation.
Table 2.
Incidence per 1000 Person‐Years and Hazard Ratios of AF After Stroke
| No. of Patients | No. with AF | Days at Risk | Incidence/1000 y | RR | 95% CI | RR1 a | 95% CI | RR2 b | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| C (CHF) | ||||||||||
| No | 52972 | 2432 | 43891162 | 20.22 | 1.00 | 1.00 | 1.00 | |||
| Yes | 4664 | 337 | 2651748 | 46.39 | 2.22 | 1.98–2.48 | 1.89 | 1.68–2.12 | 1.83 | 1.63–2.06 |
| H (HT) | ||||||||||
| No | 26608 | 1054 | 21753369 | 17.71 | 1.00 | 1.00 | 1.00 | |||
| Yes | 31028 | 1715 | 24789541 | 25.29 | 1.42 | 1.32–1.53 | 1.38 | 1.28–1.49 | 1.36 | 1.26–1.47 |
| A75 (age ≥75 y) | ||||||||||
| No | 26539 | 910 | 24675067 | 13.48 | 1.00 | 1.00 | 1.00 | |||
| Yes | 31097 | 1859 | 21867843 | 31.07 | 2.26 | 2.08–2.44 | 2.13 | 1.97–2.31 | 2.39 | 2.06–2.77 |
| 3.52 | 3.07–4.04 | |||||||||
| 4.34 | 3.72–5.06 | |||||||||
| D (DM) | ||||||||||
| No | 45501 | 2230 | 37116918 | 21.96 | 1.00 | 1.00 | 1.00 | |||
| Yes | 12135 | 539 | 9425992 | 20.90 | 0.95 | 0.86–1.04 | 0.88 | 0.80–0.97 | 0.88 | 0.80–0.97 |
| S (stroke) | ||||||||||
| No | 43272 | 2056 | 35540442 | 21.14 | 1.00 | 1.00 | 1.00 | |||
| Yes | 14346 | 713 | 11002468 | 23.69 | 1.11 | 1.02–1.21 | 0.99 | 0.91–1.08 | 0.96 | 0.88–1.05 |
| Sex | ||||||||||
| F | 27761 | 1443 | 21877385 | 24.11 | 1.00 | 1.00 | 1.00 | |||
| M | 29875 | 1326 | 24665525 | 19.65 | 0.82 | 0.76–0.88 | 0.94 | 0.87–1.02 | 0.97 | 0.90–1.05 |
| CHADS2 score | ||||||||||
| 0 | 9048 | 226 | 8571052 | 9.64 | 1.00 | |||||
| 1 | 17258 | 774 | 14504221 | 19.50 | 2.00 | 1.73–2.33 | ||||
| 2 | 14335 | 810 | 11244789 | 26.33 | 2.69 | 2.32–3.11 | ||||
| 3 | 8853 | 499 | 6657935 | 27.39 | 2.79 | 2.38–3.26 | ||||
| 4 | 5847 | 332 | 4197717 | 28.91 | 2.93 | 2.47–3.47 | ||||
| 5 | 1993 | 111 | 1221588 | 33.21 | 3,32 | 2.64–4.16 | ||||
| 6 | 302 | 17 | 145608 | 42.67 | 4.16 | 2.54–6.80 |
Abbreviations: AF, atrial fibrillation; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; F, female; HT, hypertension; M, male; RR, relative risk.
RR1: C, H, A75, D, S, and sex included in the model.
RR2: C, H, age 4 categories, D, S, and sex included in the model.
In an analysis of CHADS2 components, the incidence of AF per 1000 person‐years was higher among patients with vs without CHF (46.4 vs 20.2), with vs without HT (25.3 vs 17.7), and above vs below age 75 years (31.1 vs 13.5), comprising the adjusted HR of 2.4 (95% CI: 2.06–2.77) for age, 1.8 (95% CI: 1.63–2.06) for CHF, and 1.4 (95% CI: 1.26–1.47) for HT. Diabetes and previous stroke were not associated with increased or decreased risk of developing AF after stroke (Table 2).
Ischemic vs Hemorrhagic Stroke
In ischemic stroke, the crude incidence of AF per 1000 person‐years was much higher than in patients with hemorrhagic stroke (23.1 vs 10.1, respectively). This difference was even more emphasized by higher CHADS2 score, so that for a CHADS2 score of 0 the difference was 10.4 vs 4.7 and for a CHADS2 score of 6 it increased to 45.1 vs 15.1 (Tables 3 and 4).
Table 3.
Incidence per 1000 Person‐Years and Hazard Ratios of AF After Hemorrhagic Stroke
| No. of Patients | No. with AF | Days at Risk | Incidence/1000 y | RRa | 95% CI | RR1 b | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| C (CHF) | ||||||||
| No | 7557 | 145 | 5437116 | 9.73 | 1.00 | 1.00 | ||
| Yes | 330 | 11 | 155211 | 25.87 | 2.61 | 1.41–4.83 | 2.15 | 1.16–4.01 |
| H (HT) | ||||||||
| No | 3984 | 65 | 2702956 | 8.79 | 1.00 | 1.00 | ||
| Yes | 3903 | 91 | 2889371 | 11.51 | 1.32 | 0.96–1.81 | 1.33 | 0.96–1.84 |
| A75 (age ≥75 y) | ||||||||
| No | 4174 | 62 | 3455690 | 6.56 | 1.00 | 1.00 | ||
| Yes | 3713 | 94 | 2136637 | 16.08 | 2.43 | 1.76–3.35 | 2.53 | 1.82–3.53 |
| D (DM) | ||||||||
| No | 6746 | 134 | 4833573 | 10.13 | 1.00 | 1.00 | ||
| Yes | 1141 | 22 | 758754 | 10.60 | 1.05 | 0.67–1.64 | 0.95 | 0.60–1.50 |
| S (stroke) | ||||||||
| No | 6205 | 119 | 4540882 | 9.58 | 1.00 | 1.00 | ||
| Yes | 1682 | 37 | 1051445 | 12.86 | 1.33 | 0.92–1.92 | 1.12 | 0.77–1.63 |
| Sex | ||||||||
| F | 3734 | 68 | 2523210 | 9.85 | 1.00 | 1.00 | ||
| M | 4153 | 88 | 3069117 | 10.48 | 1.07 | 0.78–1.47 | 1.30 | 0.94–1.80 |
| CHADS2 score | ||||||||
| 0 | 1648 | 17 | 1331204 | 4.67 | 1.00 | |||
| 1 | 2772 | 55 | 2062120 | 9.75 | 2.08 | 1.21–3.58 | ||
| 2 | 1679 | 38 | 1130772 | 12.28 | 2.62 | 1.48–4.64 | ||
| 3 | 1013 | 26 | 642719 | 14.79 | 3.13 | 1.70–5.77 | ||
| 4 | 610 | 17 | 344879 | 18.02 | 3.81 | 1.95–7.47 | ||
| 5 | 148 | 3 | 72272 | 15.17 | 3.16 | 0.93–10.80 | ||
| 6 | 17 | 0 | 8361 | 0.00 |
Abbreviations: AF, atrial fibrillation; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; F, female; HT, hypertension; M, male; RR, relative risk.
RR: unadjusted.
RR1: C, H, A75, D, S, and sex included in the model.
Table 4.
Incidence per 1000 Person‐Years and Hazard Ratios of AF After Ischemic Stroke
| No. of Patients | No. with AF | Days at Risk | Incidence/1000 y | RRa | 95% CI | RR1 b | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| C (CHF) | ||||||||
| No | 42883 | 2155 | 36546725 | 21.52 | 1.00 | 1.00 | ||
| Yes | 4040 | 309 | 2371594 | 47.56 | 2.13 | 1.89–2.40 | 1.84 | 1.63–2.08 |
| H (HT) | ||||||||
| No | 21283 | 921 | 18111814 | 18.59 | 1.00 | 1.00 | ||
| Yes | 25640 | 1543 | 20806505 | 27.11 | 1.45 | 1.33–1.57 | 1.40 | 1.29–1.52 |
| A75 (age ≥75 y) | ||||||||
| No | 21381 | 814 | 20305243 | 14.65 | 1.00 | 1.00 | ||
| Yes | 25542 | 1650 | 18613076 | 32.40 | 2.16 | 1.99–2.35 | 2.03 | 1.87–2.22 |
| D (DM) | ||||||||
| No | 36539 | 1972 | 30670952 | 23.50 | 1.00 | 1.00 | ||
| Yes | 10384 | 492 | 8247367 | 21.80 | 0.92 | 0.84–1.02 | 0.86 | 0.78–0.96 |
| S (stroke) | ||||||||
| No | 35020 | 1828 | 29487199 | 22.66 | 1.00 | 1.00 | ||
| Yes | 11903 | 636 | 9431120 | 24.65 | 1.08 | 0.99–1.8 | 0.97 | 0.89–1.07 |
| Sex | ||||||||
| F | 22601 | 1286 | 18362377 | 25.60 | 1.00 | 1.00 | ||
| M | 24322 | 1178 | 20555942 | 20.95 | 0.82 | 0.76–0.89 | 0.94 | 0.87–1.02 |
| CHADS2 score | ||||||||
| 0 | 7108 | 199 | 6966135 | 10.44 | 1.00 | |||
| 1 | 13644 | 675 | 11796446 | 20.91 | 1.98 | 1.69–2.32 | ||
| 2 | 11905 | 728 | 9590001 | 27.75 | 2.61 | 2.23–3.05 | ||
| 3 | 7360 | 447 | 5689858 | 28.71 | 2.69 | 2.77–3.18 | ||
| 4 | 4917 | 297 | 3654171 | 29.71 | 2.77 | 2.32–3.32 | ||
| 5 | 1724 | 102 | 1092172 | 34.13 | 3.13 | 2.46–3.97 | ||
| 6 | 265 | 16 | 129536 | 45.15 | 4.03 | 2.42–6.71 |
Abbreviations: AF, atrial fibrillation; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; F, female; HT, hypertension; M, male; RR, relative risk.
RR: unadjusted.
RR1: C, H, A75, D, S, and sex included in the model.
The relative risks (RR) of the components of the CHADS2 score were also generally higher in patients with ischemic than in patients with hemorrhagic stroke (Tables 3 and 4).
In hemorrhagic stroke, men were at higher risk than women (RR: 1.30, 95% CI: 0.94–1.80), whereas the opposite was true in ischemic stroke (RR: 0.94, 95% CI: 0.87–1.02).
Discussion
The risk of being hospitalized with a new diagnosis of AF within 2.2 years was 4.8% (2769/54867), consistent with data from 24–72‐hour Holter monitoring, when new AF was identified in 3.8%–6.1% of the patients.7 However, we do not know how many patients may have had episodes of AF but were not hospitalized, or if the AF was actually the first AF episode in these patients. The likelihood of detection is probably high in symptomatic patients, whereas in asymptomatic subjects AF could be present but undetected. If the latter is true, one would expect a risk profile that resembles those with documented AF more than those without. This was actually found in our study; the proportion of patients with CHADS2 score of 0 was lower in patients who got AF, and of the components of the score, high age, HT, and CHF were more common in patients with subsequent AF.
Cryptogenic stroke is a term that implies that the origin of stroke was not identified. This may occur especially if the cause is intermittent in character, such as episodes of asymptomatic AF. Repeated ECG recordings or long‐term ECG recordings increase the chances of capturing these episodes. In particular, implantable loop recorders allow long‐term continuous ECG monitoring for up to 3 years and the automatic capture of predefined arrhythmias as well as manual activation of a recording during symptoms. In a report based on data from 30‐day cardiac event monitoring, the authors found AF in 4 of 20 patients with cryptogenic stroke, concluding that intermittent AF may account for a large proportion of cryptogenic stroke.8
In the present study, 4.8% of the patients were hospitalized with AF during approximately 2 years. This is most likely an underestimation of the true occurrence of AF and a higher proportion of patients may have had incident AF that did not result in hospitalization. The risk of subsequent stroke is represented by the qualitative diagnosis of AF in combination with the comorbidities, and would probably lead to start of anticoagulation unless already ongoing. Cho et al. 9 used magnetic resonance imaging to find a high proportion (39.4%) of unrecognized cerebral infarcts in patients with first‐ever stroke, with an even higher proportion of 58.3% in 36 patients with high cardioembolic risk, 31 of whom had AF. One review suggested that a previous stroke or TIA is an important predictor of further stroke in patients with AF, which underlines the value of a medical history and assessment of the heart rhythm.10 The likelihood of being hospitalized with AF was lower after hemorrhagic stroke, indicating that the comorbidity pattern differs in strokes of different origin.
Thus, it seems reasonable to screen for AF in the clinical evaluation of a stroke patient. In an earlier study, we reported the mortality of patients admitted with stroke while in AF or in SR, in relation to the CHADS2 score on admission.11 Whereas patients with documented AF had a higher risk of dying, high CHADS2 scores were even more predictive in patients without AF. An increasing risk score in patients without known AF seems to be predictive of the chance of finding the first‐ever episode of AF as well as of the risk of death. Among patients with not yet known AF are the ones with not yet detected AF, those just about to have their first episode and those who are not at risk of AF. It is reasonable to believe that a risk score, eg, the CHADS2, could be of value in identifying the risk of a subsequent documentation of AF. Because we found a correlation between the CHADS2 score and the first‐ever documented AF after stroke, this simple score may help to get attention to important cardiovascular risk factors of stroke and death, which might encourage to a change in current practice.
Delayed detection of AF has been reported in spite of monitoring by repeated ECGs after ischemic stroke.12 Serial ECG recordings significantly improved detection of AF in acute stroke 2.6‐fold,13 and ambulatory 7‐day ECG monitoring detected AF in 5.7% of patients with normal ECG and 24‐hour Holter recordings.14 There is evidence that long‐term ECG recording provides important information in relation to stroke. In A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics (the TRENDS study), the atrial tachyarrhythmia burden was studied in patients with implanted pacemakers or defibrillators who had ≥1 risk factors. Their age had to be ≥65 years, otherwise the risk factors were those included in the CHADS2 score. The results suggested that the thromboembolic risk is a quantitative function of the arrhythmia burden, and a burden of ≥5.5 hours of AF on any given day seemed to double the thromboembolic risk from 1.1% to 2.4% over a mean follow‐up period of 1.4 years.15 In a subgroup analysis of the TRENDS study, new atrial tachyarrhythmia was found in 28% of 163 patients over a mean follow‐up period of 1.1 ± 0.7 years.16 In a similar study, the Italian AT500 Registry investigators found that AF episodes longer than 1 day were independently associated with embolic events.17
Another approach would be to ask the question whether the present risk scores, eg, the CHADS2 score, are also useful in predicting risk in patients without documented AF. It would be reasonable to assume that patients with higher risk scores would suffer a higher risk of having AF and also that AF would develop sooner in patients with a higher, as compared with a lower, risk score. Indeed, our analysis confirmed that with increasing risk scores, a substantial number of patients have their first AF documented in hospital <1 month after being admitted for stroke (Figure 1).
Haft and Teichholz reported that of 932 patients with ischemic stroke, 299 had documented AF, almost half of them paroxysmal. Of the 299 patients, 39.8% did not have AF on the day of admission, and 19.3% had AF only on another day remote from the stroke admission.18 In an attempt to predict which of the stroke patients were likely to have/develop AF and thus might benefit from prolonged monitoring, Haft and Teichholz used the CHADS2 “backward” and concluded that hypertensive patients with many of the characteristic risk factors (CHF, age ≥75 years, DM, coronary artery disease, and ECG findings of enlarged left atria, systolic dysfunction, and left ventricular enlargement) are at high risk of developing AF and an ischemic stroke. Similarly, a new way of estimating the risk score for developing AF in the general population was proposed from the Framingham Heart Study.19 Another score, a Score of the Targeting of Atrial Fibrillation (STAF), was presented by Suissa et al.20 Both these new scores need to be prospectively validated.
Study Limitations
Large register data provide high numbers of patients and events, but the trade‐off is the level of detail and accuracy of diagnoses as compared with specific prospectively designed studies. In an observational register study like ours, the diagnoses can not be ascertained apart from what is registered by the caregivers at the time of the admission or discharge from hospital.
The Riks‐Stroke register is based on a protocol with a fixed setup that is aimed to be feasible to administer in the acute admission phase of stroke patients. For example, HT is, according to the Riks‐Stroke protocol, defined by treatment for the disease and not by digital values of blood pressure. Comorbidities mainly retrieved from the National Hospital Discharge Registry can vary in validation according to how they were confirmed at the time of diagnosis. The Riks‐Stroke data have a very high validity for diagnoses such as myocardial infarction or stroke, but information can be missing for conditions that are often treated in primary‐care settings (eg, DM and HT). However, this potential bias was reduced by the combination of information from the Riks‐Stroke register and the National Hospital Discharge Registry.
Though the CHADS2 score estimates the risk of patients with AF getting a stroke, some strokes that occurred may not have been caused by AF, and at higher CHADS2 scores the risk of stroke may have been increased also in the absence of AF. However, the true presence or absence of AF in patients like ours would have to be assessed prospectively with continuous long‐term ECG recording.
Conclusion
In patients admitted for stroke and in SR at the time of admission, hospitalization with a new diagnosis of AF is not uncommon. The number is likely to greatly underestimate the true number of patients with new or undetected AF. In patients with ischemic stroke, AF should be actively looked for and the patient risk assessed by means of, for example, of the CHADS2 score, so that treatment can be directed against underlying risk factors.
Acknowledgements
We are indebted to the steering committee of the Riks‐Stroke register, which is run by the Riks‐Stroke Collaboration and sponsored by the National Board of Health and Welfare and the Federation of Swedish County Councils.
References
- 1. Åsberg S, Henriksson KM, Farahmand B, et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish Stroke Register. Stroke. 2010;41:1338–1342. [DOI] [PubMed] [Google Scholar]
- 2. Rietbrock S, Heeley E, Plumb J, et al. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156:57–64. [DOI] [PubMed] [Google Scholar]
- 3. Swedish National Board of Health and Welfare . StrokesjukvÃ¥rd i Sverige. 2007. 39–42.
- 4. Glader EL. Stroke Care in Sweden: Hospital Care and Patient Follow‐UpBased onRiks‐Stroke, the National Quality Register for Stroke Care [doctoral dissertation]. Umeå, Sweden: Medical Dissertations of Umeå University; 2003. [Google Scholar]
- 5. Stegmayr B, Asplund K, Hulter‐Asberg K, et al. Stroke units in their natural habitat: can results of randomized trials be reproduced in routine clinical practice? Riks‐Stroke Collaboration. Stroke. 1999;30:709–714. [DOI] [PubMed] [Google Scholar]
- 6. Glader EL, Stegmayr B, Norrving B, et al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34:1970–1975. [DOI] [PubMed] [Google Scholar]
- 7. Liao J, Khalid Z, Scallan C, et al. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38:2935–2940. [DOI] [PubMed] [Google Scholar]
- 8. Elijovich L, Josephson SA, Fung GL, et al. Intermittent atrial fibrillation may account for a large proportion of otherwise cryptogenic stroke: a study of 30‐day cardiac event monitors. J Stroke Cerebrovasc Dis. 2009;18:185–189. [DOI] [PubMed] [Google Scholar]
- 9. Cho AH, Kwon SU, Kim TW, et al. High prevalence of unrecognized cerebral infarcts in first‐ever stroke patients with cardioembolic sources. Eur J Neurol. 2009;16:838–842. [DOI] [PubMed] [Google Scholar]
- 10. Stroke Risk in Atrial Fibrillation Working Group . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554.17679673 [Google Scholar]
- 11. Henriksson KM, Farahmand B, Johansson S, et al. Survival after stroke: the impact of CHADS(2) score and atrial fibrillation. Int J Cardiol. 2010;141:18–23. [DOI] [PubMed] [Google Scholar]
- 12. Kamel H, Lees KR, Lyden PD, et al; Virtual International Stroke Trials Archive Investigators . Delayed detection of atrial fibrillation after ischemic stroke. J Stroke Cerebrovasc Dis. 2009;18:453–457. [DOI] [PubMed] [Google Scholar]
- 13. Douen AG, Pageau N, Medic S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6‐fold in patients with acute stroke. Stroke. 2008;39:480–482. [DOI] [PubMed] [Google Scholar]
- 14. Jabaudon D, Sztajzel J, Sievert K, et al. Usefulness of ambulatory 7‐day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35:1647–1651. [DOI] [PubMed] [Google Scholar]
- 15. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 16. Ziegler PD, Glotzer TV, Daoud EG, et al. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke. 2010;41:256–260. [DOI] [PubMed] [Google Scholar]
- 17. Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 18. Haft JI, Teichholz LE. Atrial fibrillation, left ventricular hypertrophy, left atrial enlargement, ejection fraction and hypertension in patients with nonhemorrhagic stroke. Am J Cardiol. 2008;102:1348–1351. [DOI] [PubMed] [Google Scholar]
- 19. Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suissa L, Bertora D, Lachaud S, et al. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40:2866–2868. [DOI] [PubMed] [Google Scholar]


