Abstract
Marijuana is the most abused recreational drug in the United States. Cannabinoids, the active ingredients of marijuana, affect multiple organ systems in the human body. The pharmacologic effects of marijuana, based on stimulation of cannabinoid receptors CB1 and CB2, which are widely distributed in the cardiovascular system, have been well described. Activation of these receptors modulates the function of various cellular elements of the vessel wall, and may contribute to the pathogenesis of atherosclerosis. Clinically, there are reports linking marijuana smoking to the precipitation of angina and acute coronary syndromes. Recently, large published clinical trials with CB1 antagonist rimonabant did not show any significant benefit of this agent in preventing progression of atherosclerosis. In light of these findings and emerging data on multiple pathways linking cannabinoids to atherosclerosis, we discuss the literature on the role of cannabinoids in the pathophysiology of atherosclerosis. We also propose a marijuana paradox, which implies that inhalation of marijuana may be linked to precipitation of acute coronary syndromes, but modulation of the endocannabinoid system by a noninhalation route may have a salutary effect on the development of atherosclerosis.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Although cannabinoids are among the oldest known medicinal compounds, their chemistry, pharmacology, and clinical effects have only been recently studied. There is also an increasing recognition of their therapeutic potential in multiple clinical scenarios.
Cannabinoids, the active ingredients in marijuana, are derived from the hemp plant Cannabis sativa. Different parts of this plant, including leaves, flowers, and resin extract, have been used for euphoric effects for centuries. The use of cannabinoids as herbal medicine as an analgesic, muscle relaxant, appetite stimulant, and bronchodilator can be traced back 5000 years in China.1
In the United States, the Marijuana Tax Act, passed in 1937, declared the use of marijuana illegal for any kind of recreational or medicinal use.2 According to the 2009 National Survey on Drug Use and Health, marijuana is the most commonly abused drug in United States.3 Given that cannabinoids may have therapeutic potential in some disease states, lately there has been much social and political push to legalize medical use of marijuana. Currently, 14 US states permit the medical use of marijuana, although there is much variability in the definition of legal use in each state.4
The effects of marijuana smoking on cardiovascular physiology have been well described.2 Here we review the role of cannabinoids in the pathogenesis of atherosclerosis and the relationship of marijuana smoking with precipitation of acute coronary syndromes. This is particularly relevant currently in the United States given the potential for increased medical marijuana use and the ever‐growing epidemic of atherosclerotic heart disease.
Cannabinoid Receptors
In 1967, Mechoulam and Gaoni first isolated delta 9‐tetrahydrocannabinol (THC), the main psychoactive constituent of marijuana, and studied its pharmacological effects.5 This was followed by discovery of 2 distinct membrane receptor subtypes for THC, CB1 and CB2.1 Devane in 19926 and Mechoulam in 19957 first described the endogenous ligands, anadamide (AEA) and 2‐arachidonoglycerol (2‐AG), respectively, for these receptors. Both CB1 and CB2 are G‐protein coupled receptors that modulate second messengers and signaling components such as adenylate cyclase, mitogen activated protein kinases (MAPK), and members of the nuclear factor κ B (NF‐κB) family, in addition to playing a role in direct modulation of cell membrane ion channels.8 CB1 and CB2 receptors are expressed to varying degree in various tissues. The varying degree of selectivity of endogenous and exogenous cannabinoids for CB1 and CB2 receptors account for their various psychotropic and peripheral effects. CB1 receptors are expressed predominantly in the brain and peripheral tissues, including cardiac muscle, the liver, gastrointestinal tract, vascular endothelium, vascular smooth muscle cells, and kidney.8., 9. CB2 receptors are predominantly expressed in immune cells,10 and have been recently identified on endothelial cells where their expression is upregulated by proinflammatory cytokines.11 Some tissues, such as adipocytes,8 platelets,12 and bronchial epithelium,13 express both CB1 and CB2 receptors (Figure 1).
Figure 1.
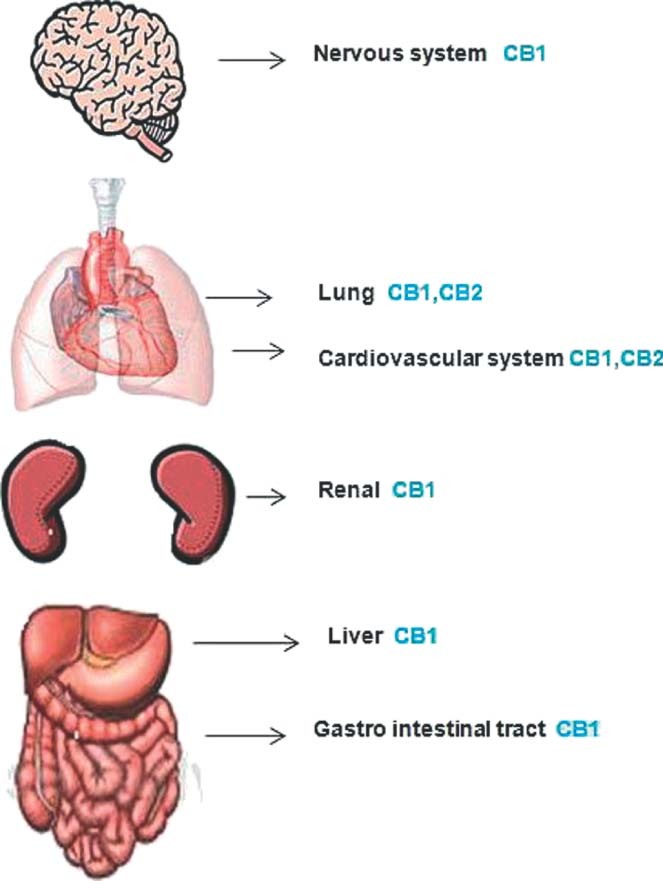
Distribution of cannabinoid receptors in major organ systems. CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2.
There is evidence to suggest that some cannabinoid effects are not mediated by either CB1 or CB2 receptors, suggesting the presence of other receptors, such as transient receptor potential channels of type V1, also known as vanilloid receptors,14 and the nuclear receptor peroxisome proliferator‐activated receptor‐γ (PPAR‐γ).15 The role of these receptors in atherogenesis has not yet been identified.
Cannabinoids and Atherosclerosis
Effect on Immune Cells
Cannabinoids, acting via both CB2 and CB1 receptor modulation, have an important role in immune system regulation.16 Because inflammation plays a key role in atherogenesis,17 cannabinoids can potentially affect atherogenesis via modulation of the immune system (Figure 2).
Figure 2.
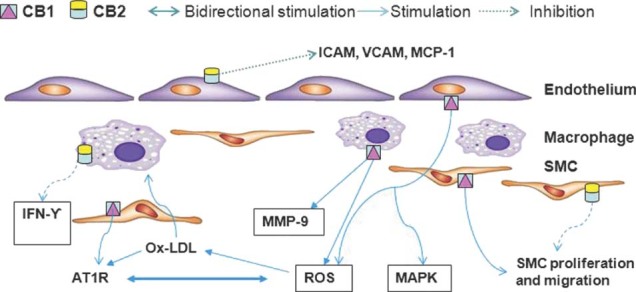
Salient features of cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2) receptor activation in relation to atherosclerosis. Abbreviations: AT1R, angiotensin II receptor type 1; ICAM, intercellular adhesion molecule; IFN‐γ, interferon‐γ; MAPK, mitogen activated protein kinase; MMP‐9, matrix metalloproteinase‐9; MCP 1, monocyte chemoattractant protein‐1; Ox‐LDL, oxidized low‐density lipoprotein; ROS, reactive oxygen species; SMC, smooth muscle cell; VCAM, vascular cell adhesion molecule.
In both mice and human atherosclerotic lesions, CB2 receptors were first demonstrated to be localized on macrophages and infiltrating T cells.18 In a pioneering study by Steffens et al,18 oral administration of low‐dose THC (1 mg/kg/day) was shown to inhibit progression of atherosclerotic lesions in the aortic root and abdominal aorta in a murine model of atherosclerosis by decreasing monocyte adhesion and infiltrating the subendothelial region via activation of CB2 receptors on these cells. These in vivo observations were further supported by in vitro demonstration of inhibition of macrophage chemotaxis in response to monocyte chemoattractant protein (MCP)‐1 in the presence of THC. Of note, THC was also found to significantly decrease interferon‐γ (T‐helper cell (TH) 1 cytokine) compared to interleukin‐10 (TH2 cytokine),18 suggesting that the antiatherosclerotic properties of cannabinoids might be due to downregulation of TH1 immune response and a shift in cytokine expression.18., 19. This represents an important finding, as TH1 immune cells are the predominant population of T lymphocytes in atherosclerotic lesions.20 Interestingly, the athero‐protective effects of THC have been shown to be dose dependent, with maximum effect at 1 mg/kg/day with concomitant plasma concentration of 0.6 ng/mL, which is much lower than the plasma concentration of THC associated with the psychotropic effects of cannabinoids.18 The athero‐protective effect at such a low concentration may be related to the lipophilic character of THC leading to a high concentration in the atherosclerotic lesions.18., 21. Similar to the findings by Steffens et al, another study in apolipoprotein E (Apo E) knockout mice demonstrated a decrease in the extent of atherosclerotic plaque in the aortic root, without significantly affecting the serum lipid levels when the mice were administered WIN 55212‐2 (a synthetic cannabinoid agonist with CB2 selectivity). This decrease in extent of atherosclerosis was associated with a decrease in proinflammatory cytokine gene expression and attenuated oxidized low‐density lipoprotein (ox‐LDL)‐induced NF‐κB activation.22
Several studies provide evidence for the role of CB1 blockade in modulation of inflammation in atherosclerosis. Sugamura et al23 identified CB1 expression in macrophages of advanced atheromas. In their study, atherosclerotic coronary artery sections from patients with unstable angina had significantly higher expression of CB1 receptors as compared to coronary artery sections from patients with stable angina. In vitro culture studies showed that CB1 receptor expression on macrophages was increased in the presence of macrophage colony stimulating factor and ox‐LDL, and the density of CB1 receptors correlated with increased expression of type A scavenger receptor. Further, in this study CB1 receptor blockade in cultured human macrophages was associated with a decrease in expression of matrix metalloproteinase‐9 (MMP‐9) and inflammatory cytokines.
Han et al24 examined the relative expression of CB1 and CB2 receptors in freshly isolated human monocytes and identified significant upregulation of CB1 receptors as monocytes differentiate into macrophages. This upregulation of CB1 receptor expression correlated with the increase in reactive oxygen species (ROS) production. In another study done with low‐density lipoprotein receptor‐null mice, CB1 receptor antagonist rimonabant (originally developed as an antiobesity agent) was shown to decrease atherosclerotic lesion development independent of its effect on food intake and weight loss.25 In this study, the antiatherosclerotic effects were ascribed to rimonabant‐induced decrease in proinflammatory cytokine gene expression in mouse peritoneal macrophages and in vivo inhibition of macrophage recruitment to plaque site.
Thus, the available evidence suggests a diverse role for CB1 and CB2 receptors in macrophages toward progression of atherosclerosis.
Effect on Endothelial Cells
Endothelial dysfunction plays a critical role in the pathogenesis and complications of atherosclerosis. Cannabinoids have attracted attention in relation to endothelial function as it relates to atherosclerosis.
Liu et al26 identified CB1 receptors on human aortic endothelial cells and demonstrated that CB1 agonism leads to activation of MAPK. In addition, Rajesh et al27 have demonstrated that stimulation of CB1 receptors located on human coronary artery endothelial cells was associated with increased production of ROS, MAPK activation, and endothelial cell injury, and that these effects were inhibited by CB1 receptor blockade.
Earlier, Rajesh et al11 had examined the role of CB2 receptor stimulation on endothelial cell response to proinflammatory molecules such as endotoxin and tumor necrosis factor‐α (TNF‐α). They demonstrated that CB2 agonism leads to attenuation of inflammatory response to TNF‐α, leading to decreased expression of intercellular adhesion molecule‐1, vascular cell adhesion molecule‐1, and MCP on endothelial cells; decreased monocyte adhesion to endothelial cells; and decreased transendothelial migration of monocytes. Similar results were duplicated with CB2 receptor agonism in an in vivo mice model of endotoxin‐induced vascular inflammation.
Zhao et al28 evaluated the effect of WIN 55212‐2 (see above) on expression of adhesion molecules in vascular endothelial cells in the Apo E knockout mice model. Similar to findings of Rajesh et al, it was demonstrated that CB2 agonism was associated with a decrease in expression of inflammation‐associated adhesion molecules on endothelial cells, and these effects were completely blocked in the presence of AM 630 (CB2 antagonist).
The dichotomy of the CB1 and CB2 receptor on endothelial function is similar to effects on macrophages, where CB1 agonism promotes a proatherogenic profile, and CB2 agonism prevents atherogenesis.
Cannabinoids and Lipid Metabolism
The role of lipid peroxidation in the pathogenesis of atherosclerosis has been well described.29 Cannabidiol, a nonpsychoactive component of cannabis, and its dimethyl ether derivative have been shown to have 15‐lipoxygenase (15‐LOX) inhibitor activity.30 As oxidation of LDL by 15‐LOX is a pathophysiologically important process in atherogenesis,31 cannabidiol may be able to decrease the progression of atherosclerosis by preventing formation of ox‐LDL.
Once formed, ox‐LDL has been shown to upregulate the endocannabinoid system in macrophages, with an increase in intracellular level of endocannabinoids AEA and 2‐AG, and increased expression of CB1 and CB2 receptors.32 This stimulation of the endocannabinoid system was associated with increased accumulation of lipids in these macrophages. It was demonstrated that exposure of macrophages to exogenous synthetic cannabinoid, WIN 55212‐2 (see above) leads to an increase in lipid accumulation, and that prior treatment with AM251 (CB1 antagonist) decreased this effect. These effects can be explained by the fact that WIN 55212‐2, in addition to CB2 agonism, has mild CB1 agonist activity also. When macrophages were exposed to both WIN 55212‐2 and AM251, there was selective inhibition of CB1 receptor with uninhibited CB2 agonist activity. In this study, it was also shown that exposure of macrophages to WIN55212‐2 was associated with increased levels of PPAR‐γ, which lead to decreased expression of adenosine triphosphate (ATP) binding cassette protein‐A1, a component of lipid export assembly, resulting in increased lipid accumulation in macrophages. This effect was reversed in the presence of CB1 antagonist AM251. Thus, a decrease in lipid accumulation most likely reflects the combined effect of CB2 agonism from WIN 55212‐2 and CB1 antagonism by AM251.
Sugamura et al33 further demonstrated upregulation of scavenger receptor B1 (SR B1) and ATP binding cassette transporter G1 (ABCG1), components of reverse cholesterol transport, in Apo E knockout mice macrophages with CB1 antagonism. In this study, CB1 antagonism was also associated with increased adiponectin secretion. Adiponectin in itself has been shown to accelerate reverse cholesterol transport in macrophages and thus reduce atherosclerosis.34
Acyl coenzyme A transferase 1 (ACAT‐1) esterifies the cholesterol obtained from ox‐LDL in macrophages located in intima of vessel wall. Inhibition of ACAT‐1 by rimonabant was shown in both in vitro and in vivo experiments to lead to decreased lipid accumulation in macrophages and thus decrease foam cell formation. Interestingly, inhibition of ACAT‐1 by rimonabant was independent of its CB1 blocking properties.35 It is not known if this finding represents an effect unique to rimonabant or a class effect of CB1 antagonist.
The concept of beneficial impact of CB1 antagonism on lipid profile was tested in a clinical trial in obese patients with dyslipidemia, and was shown to favorably affect the lipid profile by decreasing serum triglycerides and increasing high‐density lipoprotein fraction.36 These effects were independent to some extent of the weight loss achieved by rimonabant.
Overall, it appears that similar to the effect on macrophages and endothelial cells, CB1 antagonism has a favorable effect on lipid homeostasis, whereas CB2 agonism promotes an antiatherogenic profile.
Cannabinoids and Vascular Smooth Muscle Cells
Platelet‐derived growth factor (PDGF) plays an important role in proliferation and growth of vascular smooth muscle cells (VSMC), and thus contributes to the pathogenesis of atherosclerosis and stent restenosis. The opposite effects of CB1 and CB2 agonism on smooth muscle cell (SMC) growth have been demonstrated. Rajesh et al showed that CB2 agonism leads to decrease in TNF‐α–induced proliferation and migration of human coronary artery SMC.37 They further demonstrated that CB1 antagonism decreases PDGF‐dependent proliferation and migration of human coronary artery SMC by attenuating activation of Ras and extracellular signal‐regulated kinase 1/2.38
Additionally, CB1 receptor modulation has been shown to have a direct effect on angiotensin II type 1receptors (AT1R) expression, where CB1 agonism leads to an increase and CB1 antagonism leads to a decease in receptor expression. An increase in AT1R expression secondary to CB1 agonism in turn leads to increased generation of ROS by VSMC.39 The antioxidative effect of CB1 inhibition on VSMC was also shown to contribute to better endothelial function.
Once again, the difference between CB1 and CB2 activation in VSMC is evident; CB1 agonism leads to a proatherogenic profile, and CB2 agonism inhibits this phenomenon.
Cannabinoids and Ischemia‐Reperfusion Injury
It is well established that sudden reperfusion of the ischemic myocardium leads to additional myocardial damage, induced at least in part by release of ROS and the subsequent onset of inflammation.40 Exposure to cannabinoids has been shown to have a cardioprotective effect by decreasing the area of necrosis during ischemia‐reperfusion (I‐R), without affecting collateral blood flow to the myocardium.41 In 1 study of a mice model, a single intraperitoneal dose of CB2 agonist administered during ischemia reduced the infarct size following reperfusion.42 This protective effect was shown to be mediated via attenuation of ROS generation, activation of protein kinases, and a decrease in neutrophil recruitment in the ischemic‐reperfused areas of the heart. Defer et al43 provided additional evidence for the protective effect of CB2 agonism, by demonstrating extensive injury following I‐R in CB2 knockout mice. In further studies, these investigators demonstrated abrogation of I‐R injury by the CB2 agonist in the wild‐type mice. The protective effects of CB2 agonism were associated with a decrease in the number of apoptotic cells and activation of the prosurvival kinase, Akt.
There are also some data to suggest a role for CB1 receptor agonism in I‐R injury. Lepicier et al44 suggested a small role for CB1 agonism in an ex vivo rat heart model in protection against I‐R injury. Wagner et al showed that activation of CB1 receptors by 2‐AG led to nitric oxide induced delayed cardioprotection. In contrast to the above‐mentioned studies,41., 42., 43. Wagner et al failed to show a significant role of CB2 receptors in cardioprotection against I‐R injury. Whether this study reflects a role for CB1 agonism, specifically in a late phase of cardioprotection mediated through nitric oxide, needs to be explored further.
In addition to CB1 and CB2 receptor mediated mechanisms, a nonpsychoactive cannabinoid agonist, cannabidiol has been shown to limit I‐R injury acting via a CB1, CB2 independent pathway.45 One potential mechanism for this is modulation of adenosine receptor signaling.
Studies so far show a promising effect of modulation of the cannabinoid system on myocardial I‐R injury. In contrast to atherosclerosis, both CB1 and CB2 agonism seem to play a protective role in I‐R. Although decrease in infarct size leads to preservation of ejection fraction, the long‐term effects of cannabinoids on left ventricular remodeling are not known due to the lack of long‐term follow‐up studies.
Cannabinoids and Clinical Syndromes of Atherosclerotic Heart Disease
Cannabinoids and Stable Coronary Artery Disease
In contrast to the athero‐protective effects of THC,21 exposure to marijuana smoke has been linked to the precipitation of angina in the presence of established coronary artery disease (CAD). Marijuana smoking has complex actions on cardiovascular physiology, which include a dose‐dependent increase in heart rate, decrease in stroke volume, increase in cardiac output, and a minimal effect on systemic blood pressure.2 Marijuana smoking leads to premature achievement of maximum heart rate during exercise and decreases the maximum exercise capacity in normal healthy individuals.2 This possibly leads to lowering of the angina threshold, as shown in 1 study comparing marijuana smoking with nicotine smoking. In this study, a single marijuana cigarette decreased the exercise time to angina by an average of 48% as compared to 23% after smoking a tobacco cigarette.46 The impact of marijuana smoking on mortality in patients with known CAD was investigated in a study by Mukamal et al.47 In this retrospective study, there was a trend toward increased cardiovascular mortality in marijuana users (hazard ratio: 1.9; 95% confidence interval: 0.6‐6.3). Interestingly, there was ∼3‐fold increased mortality following myocardial infarction in marijuana users as compared to nonmarijuana users.
Cannabinoids and Acute Coronary Syndrome
Marijuana smoking may also be related to the precipitation of acute coronary syndrome (ACS). In a retrospective study, Mittleman et al48 assessed the risk of ACS after exposure to marijuana smoke and observed that the first hour after smoking marijuana represented a high‐risk period for occurrence of myocardial infarction.48 This risk declined rapidly after the first hour of exposure to marijuana. The mechanism by which marijuana use may precipitate ACS is not clear yet, but potentially, marijuana smoking may lead to the disruption of vulnerable plaque due to hemodynamic stresses and formation of an occlusive thrombus.48 In this regard, how cannabinoids affect thrombogenesis is unclear. THC has been shown to activate platelets via CB1 and CB2 receptors leading to increased GPIIb‐IIIa and P selectin expression,12 and activation of factor VII.49 On the other hand, Keown et al50 suggested the effects on platelets are not mediated via CB1 or CB2 activation. They demonstrated that platelet aggregation mediated by endocannabinoid 2‐AG is due to activation of a cycloxygenase pathway leading to the formation of thromboxane A2. This effect was completely blocked by aspirin but not by the CB1 or CB2 antagonist. Some investigators have even suggested that cannabinoids may inhibit platelet aggregation by some yet unknown mechanism.51
In addition to hemodynamic stress, another proposed mechanism of plaque rupture is the release of MMP‐9 from macrophages of lipid‐rich plaques via CB1 receptor activation.23 MMPs can degrade components of the subendothelial basement membrane, extracellular matrix constituents, and disrupt the protective collagen‐rich fibrotic cap covering the plaque.17 Coronary vasospasm precipitated by marijuana smoking has also been proposed as a mechanism for precipitation of acute myocardial infarction in individuals with either normal or minimally diseased coronary arteries.52 The underlying mechanism for coronary vasospasm following marijuana smoking may be related to an increase in sympathetic discharge.53
Clinical Trials on the Role of Cannabinoids in Coronary Artery Disease
Information on the potential role of endocannabinoids and their synthetic analogs in cardiovascular physiology and pathology has attracted the attention of clinicians and the pharmaceutical industry alike.
Nissen et al tested the concept of CB1 blockade by rimonabant and its effect on the progression of atherosclerosis in humans in the Strategy to Reduce Atherosclerosis Development Involving Administration of Rimonabant–The Intravascular Ultrasound Study (STRADIVARIUS) trial.54 This was a randomized controlled trial (RCT) that involved intravascular ultrasound‐based assessment of atherosclerotic lesions. Although the primary end point of the percent of atheroma volume was not significantly different in the control and rimonabant group, total atheroma volume, which was a secondary outcome, was reduced significantly with rimonabant therapy, suggesting a role of CB1 receptors in the determination of plaque load. Although the primary end point of the percent of atheroma volume was not significantly decreased, an 18‐month period (duration of the trial) may be too short to assess the effect of a drug on a process that typically runs for decades.
Similar to STRADIVARIUS, the Atherosclerosis Underlying Development Assessed by Intima‐Media Thickness in Patients on Rimonabant (AUDITOR) trial showed no difference in progression of carotid artery intima‐media thickness in patients with abdominal obesity and metabolic syndrome given CB1 antagonist rimonabant or placebo.55 This was a multicenter RCT with median follow‐up of 30 months. In this trial, carotid artery intima‐media thickness was used as a surrogate marker for atherosclerosis.
In another large multicenter trial, Topol et al56 examined the concept of whether CB1 antagonism with rimonabant would lead to decreased vascular events. This trial had to be stopped prematurely because of severe neuropsychiatric events (increased risk of suicide). There was no difference between the rimonabant and placebo group for the occurrence of primary end point (composite of cardiovascular death, myocardial infarction and stroke) over the mean follow‐up period of 13.8 months.
All of these 3 trials essentially demonstrated that CB1 antagonism probably does not alter the natural history of atherosclerosis or its clinical outcomes. Based on this observation and studies discussed above, it appears that CB2 receptor agonism may play a more important role in modulation of atherosclerosis as compared to CB1 antagonism. A recently published study by Netherland et al provides further support for this hypothesis.57 It is conceivable that the future provision of drugs with CB2 agonism in addition to CB1 antagonism may provide a more effective therapy for altering the course and complications of atherosclerosis. Also, future CB1 antagonist drugs will need to address an adverse neuropsychiatric profile as seen with rimonabant, which led to its withdrawal from the market.
Marijuana Paradox
Although there is evidence to suggest that smoking marijuana may decrease the angina threshold and precipitate acute coronary events, there are convincing data from animal models to suggest modulation of atherogenesis by cannabinoids administered systemically in appropriate doses. The mechanistic basis for this potential paradox is unclear. One possible explanation could be that smoking marijuana exposes the individual to other particulate and gaseous material arising from plant combustion.48 Exposure to particulate air pollution has been linked to the increased risk of precipitation of unstable coronary syndromes and progression of atherosclerosis.58., 59. Future studies with selective CB2 agonism alone, or in combination with CB1 antagonism, may provide answers for this interesting paradox.
Conclusion
Based on the studies discussed here, modulation of the cannabinoid system holds promise in preventing the progression of atherosclerosis. Although the spectrum of cannabinoid therapy cuts across a multitude of clinical conditions,4 modulation of the cannabinoid system for treatment of atherosclerosis cannot be recommended at this time because of legal and clinical reasons.
References
- 1. Mendizabal VE, Adler‐Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: A tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannabis, 1977.. Ann Intern Med. 1978;89:539–549. [DOI] [PubMed] [Google Scholar]
- 3.White House. Office of National Drug Control Policy. Marijuana facts and figures. http://www.whitehousedrugpolicy.gov/drugfact/marijuana/marijuana_ff.html. Accessed September 18, 2010.
- 4. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362:1453–1457. [DOI] [PubMed] [Google Scholar]
- 5. Mechoulam R, Gaoni Y. The absolute configuration of delta‐1‐tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967;12:1109–1111. [DOI] [PubMed] [Google Scholar]
- 6. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. [DOI] [PubMed] [Google Scholar]
- 7. Mechoulam R, Ben‐Shabat S, Hanus L, et al. Identification of an endogenous 2‐monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. [DOI] [PubMed] [Google Scholar]
- 8. Steffens S, Mach F. Cannabinoid receptors in atherosclerosis. Curr Opin Lipidol. 2006;17:519–526. [DOI] [PubMed] [Google Scholar]
- 9. Larrinaga G, Varona A, Perez I, et al. Expression of cannabinoid receptors in human kidney. Histol Histopathol. 2010;25:1133–1138. [DOI] [PubMed] [Google Scholar]
- 10. Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. [DOI] [PubMed] [Google Scholar]
- 11. Rajesh M, Mukhopadhyay P, Batkai S, et al. CB2‐receptor stimulation attenuates TNF‐alpha‐induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte‐endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293:H2210–H2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deusch E, Kress HG, Kraft B, et al. The procoagulatory effects of delta‐9‐tetrahydrocannabinol in human platelets. Anesth Analg. 2004;99:1127–1130, table of contents. [DOI] [PubMed] [Google Scholar]
- 13. Gkoumassi E, Dekkers BG, Droge MJ, et al. (Endo)cannabinoids mediate different Ca2+ entry mechanisms in human bronchial epithelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2009;380: 67–77. [DOI] [PubMed] [Google Scholar]
- 14. Begg M, Pacher P, Batkai S, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. [DOI] [PubMed] [Google Scholar]
- 15. Burstein S. PPAR‐gamma: a nuclear receptor with affinity for cannabinoids. Life Sci. 2005;77:1674–1684. [DOI] [PubMed] [Google Scholar]
- 16. Klein TW, Newton C, Larsen K, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. [DOI] [PubMed] [Google Scholar]
- 17. Libby P. Inflammation in atherosclerosis. Nature. 200;420: 868–874. [DOI] [PubMed] [Google Scholar]
- 18. Steffens S, Veillard NR, Arnaud C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. [DOI] [PubMed] [Google Scholar]
- 19. Yuan M, Kiertscher SM, Cheng Q, et al. Delta 9‐tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. 2002;133:124–131. [DOI] [PubMed] [Google Scholar]
- 20. Benagiano M, Azzurri A, Ciervo A, et al. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 2003;100:6658–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steffens S, Mach F. Towards a therapeutic use of selective CB(2) cannabinoid receptor ligands for atherosclerosis. Future Cardiol. 2006;2:49–53. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Liu Y, Zhang W, et al. WIN55212‐2 ameliorates atherosclerosis associated with suppression of pro‐inflammatory responses in ApoE‐knockout mice. Eur J Pharmacol. 2010;649:285–292. [DOI] [PubMed] [Google Scholar]
- 23. Sugamura K, Sugiyama S, Nozaki T, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119:28–36. [DOI] [PubMed] [Google Scholar]
- 24. Han KH, Lim S, Ryu J, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–386. [DOI] [PubMed] [Google Scholar]
- 25. Dol‐Gleizes F, Paumelle R, Visentin V, et al. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor‐deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:12–18. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Gao B, Mirshahi F, et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346(pt 3):835–340. [PMC free article] [PubMed] [Google Scholar]
- 27. Rajesh M, Mukhopadhyay P, Hasko G, et al. Cannabinoid‐1 receptor activation induces reactive oxygen species‐dependent and ‐independent mitogen‐activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol. 2010;160:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Y, Yuan Z, Liu Y, et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol. 2010;55:292–298. [DOI] [PubMed] [Google Scholar]
- 29. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. [DOI] [PubMed] [Google Scholar]
- 30. Takeda S, Usami N, Yamamoto I, et al. Cannabidiol‐2′,6′‐dimethyl ether, a cannabidiol derivative, is a highly potent and selective 15‐lipoxygenase inhibitor. Drug Metab Dispos. 2009;37:1733–1737. [DOI] [PubMed] [Google Scholar]
- 31. Kuhn H, Heydeck D, Hugou I, et al. In vivo action of 15‐lipoxygenase in early stages of human atherogenesis. J Clin Invest. 1997;99:888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang LS, Pu J, Han ZH, et al. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res. 2009;81:805–813. [DOI] [PubMed] [Google Scholar]
- 33. Sugamura K, Sugiyama S, Fujiwara Y, et al. Cannabinoid 1 receptor blockade reduces atherosclerosis with enhances reverse cholesterol transport. J Atheroscler Thromb. 2010;17:141–147. [DOI] [PubMed] [Google Scholar]
- 34. Tsubakio‐Yamamoto K, Matsuura F, Koseki M, et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–394. [DOI] [PubMed] [Google Scholar]
- 35. Netherland C, Thewke DP. Rimonabant is a dual inhibitor of acyl CoA:Cholesterol acyltransferases 1 and 2. Biochem Biophys Res Commun. 2010;398:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Despres JP, Golay A, Sjostrom L, Rimonabant in Obesity‐Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. [DOI] [PubMed] [Google Scholar]
- 37. Rajesh M, Mukhopadhyay P, Hasko G, et al. CB2 cannabinoid receptor agonists attenuate TNF‐alpha‐induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol. 2008;153:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rajesh M, Mukhopadhyay P, Hasko G, et al. Cannabinoid CB1 receptor inhibition decreases vascular smooth muscle migration and proliferation. Biochem Biophys Res Commun. 2008;377: 1248–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tiyerili V, Zimmer S, Jung S, et al. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res Cardiol. 2010;105:465–477. [DOI] [PubMed] [Google Scholar]
- 40. Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia‐reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ugdyzhekova DS, Krylatov AV, Bernatskaya NA, et al. Activation of cannabinoid receptors decreases the area of ischemic myocardial necrosis. Bull Exp Biol Med. 2002;133:125–126. [DOI] [PubMed] [Google Scholar]
- 42. Montecucco F, Lenglet S, Braunersreuther V, et al. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612–620. [DOI] [PubMed] [Google Scholar]
- 43. Defer N, Wan J, Souktani R, et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion‐induced cardiomyopathy. FASEB J. 2009;23:2120–2130. [DOI] [PubMed] [Google Scholar]
- 44. Lepicier P, Bouchard JF, Lagneux C, et al. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003;139:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Durst R, Danenberg H, Gallily R, et al. Cannabidiol, a nonpsychoactive cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;293:H3602–H3607. [DOI] [PubMed] [Google Scholar]
- 46. Aronow WS, Cassidy J. Effect of smoking marihuana and of a high‐nicotine cigarette on angina pectoris. Clin Pharmacol Ther. 1975;17:549–554. [DOI] [PubMed] [Google Scholar]
- 47. Mukamal KJ, Maclure M, Muller JE, et al. An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am Heart J. 2008;155:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. [DOI] [PubMed] [Google Scholar]
- 49. Heiden D, Rodvien R, Jones R, et al. Effect of oral delta‐9‐tetra‐ hydrocannabinol on coagulation. Thromb Res. 1980;17: 885–889. [DOI] [PubMed] [Google Scholar]
- 50. Keown OP, Winterburn TJ, Wainwright CL, et al. 2‐arachidonyl glycerol activates platelets via conversion to arachidonic acid and not by direct activation of cannabinoid receptors. Br J Clin Pharmacol. 2010;70:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Formukong EA, Evans AT, Evans FJ. The inhibitory effects of cannabinoids, the active constituents of cannabis sativa L. on human and rabbit platelet aggregation. J Pharm Pharmacol. 1989;41:705–709. [DOI] [PubMed] [Google Scholar]
- 52. Caldicott DG, Holmes J, Roberts‐Thomson KC, et al. Keep off the grass: marijuana use and acute cardiovascular events. Eur J Emerg Med. 2005;12:236–244. [DOI] [PubMed] [Google Scholar]
- 53. Gash A, Karliner JS, Janowsky D, et al. Effects of smoking marihuana on left ventricular performance and plasma norepinephrine: studies in normal men. Ann Intern Med. 1978;89:448–452. [DOI] [PubMed] [Google Scholar]
- 54. Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. [DOI] [PubMed] [Google Scholar]
- 55. O'Leary DH, Reuwer AQ, Nissen SE, et al. Effect of rimonabant on carotid intima‐media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: the AUDITOR trial. Heart. 2011;97:1143–1150. [DOI] [PubMed] [Google Scholar]
- 56. Topol EJ, Bousser MG, Fox KA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo‐controlled trial. Lancet. 2010;376:517–523. [DOI] [PubMed] [Google Scholar]
- 57. Netherland CD, Pickle TG, Bales A, et al. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic ldlr‐null mice. Atherosclerosis. 2010;213:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chuang KJ, Coull BA, Zanobetti A, et al. Particulate air pollution as a risk factor for ST‐segment depression in patients with coronary artery disease. Circulation. 2008;118:1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bauer M, Moebus S, Mohlenkamp S, et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (heinz nixdorf recall) study. J Am Coll Cardiol. 2010;56:1803–1808. [DOI] [PubMed] [Google Scholar]


