Abstract
Background:
Postoperative atrial fibrillation (POAF) is a frequent complication of coronary artery bypass grafting (CABG) surgery. The objective of this study was to determine the impact of POAF on both short‐ and long‐term mortality following isolated CABG.
Hypothesis:
POAF is associated with a poorer short and long‐term mortality following CABG.
Methods:
We retrospectively analyzed the preoperative and operative data of 6728 consecutive patients undergoing a first isolated CABG.
Results:
The incidence of POAF was 27.8%. Operative mortality was higher in patients with POAF compared to those without POAF (2.3% vs 0.9%, P < 0.001). On multivariate analysis, POAF remained an independent predictor of operative mortality (odds ratio [OR]: 1.78, P = 0.01). Patients with POAF also had reduced long‐term survival (6‐year survival: 85.3% vs 89.2%, P < 0.001). After adjusting for other predictors of mortality, POAF was significantly associated with increased long‐term mortality (hazard ratio [HR]: 1.35, P = 0.04). Of note, after adjustment for potential confounders, statin treatment had a highly protective effect in POAF patients for both operative mortality (OR: 0.38, P = 0.003) and long‐term mortality (HR: 0.62, P = 0.03), whereas it had no significant effect in patients without POAF.
Conclusions:
POAF is an independent predictor of both short‐ and long‐term mortality following CABG. Moreover, statin therapy was independently associated with better survival in patients with POAF.
Dr. Pibarot holds the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research. Dr. Mathieu is a research scholar from the Fonds de Recherches en Santé du Québec, Montreal, Canada. Dr. Després has served as a speaker for Abbott Laboratories, AstraZeneca, Solvay Pharma, GlaxoSmithKline, and Pfizer Canada Inc.; has received research funding from Eli Lilly Canada; and has served on the advisory boards of Novartis, Theratechnologies, Torrent Pharmaceuticals Ltd., and Sanofi‐Aventis. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Postoperative atrial fibrillation (POAF), the most frequent complication of cardiac surgery, is associated with higher morbidity, prolonged hospitalization,1 and increased hospital costs.2 However, conflicting results have been reported with regard to the impact of POAF on survival after cardiac surgery.1., 3., 4., 5., 6., 7., 8., 9., 10., 11., 12.
Statins were shown to decrease the incidence of operative mortality after coronary artery bypass grafting (CABG) surgery.13 The beneficial effect of statins is believed to be partly related to their pleiotropic properties that could attenuate postoperative inflammatory response.14 Given that postoperative inflammatory response is a key mechanism of the genesis of POAF,14., 15., 16., 17. the impact of statin therapy on survival might differ in patients with or without POAF.
The specific objectives of our study were to assess the impact of POAF on operative and long‐term mortality in a large contemporary cohort of patients undergoing first isolated CABG, and to assess the impact of statins on survival after CABG in patients with and without POAF.
Methods
Study Population
The data of 6728 patients, with no preoperative history of paroxysmal or chronic atrial fibrillation (AF), operated on for a first isolated CABG at the Quebec Heart Institute between 2000 and 2007, were retrospectively analyzed. The anesthetic and surgical techniques were standardized for all patients and described previously.18
Data Collection
The preoperative and operative data of all patients undergoing cardiac surgery in our institution were prospectively collected and entered in a database.
Detection and Treatment of AF
AF was defined as any sustained episode recorded during the postoperative hospital stay requiring medical and/or electrical cardioversion. No systematic prophylactic measures were used to prevent POAF. Constant ambulatory electrocardiographic (ECG) monitoring was performed in all patients during the first 48 hours after surgery. Thereafter, a standard 12‐lead ECG was recorded daily and during suspected arrhythmic events. Patients were treated with a standardized protocol for the administration of intravenous amiodarone. Patients with sustained AF who were not responsive to pharmacological treatment or patients with unstable hemodynamic had an electrical cardioversion.
Outcome
Operative mortality was defined as death from any cause in the 30 days following the surgery if the patient was discharged from the hospital, and within any interval if the patient was not discharged. Long‐term survival data were obtained from the death certificates of the Registry Office of the Quebec government. All‐cause mortality was analyzed. The censoring date for long‐term survival was December 31, 2005, whereas operative mortality data were available until December 31, 2007.
Statistical Analysis
Continuous variables were expressed as mean ± SD and were compared using t tests for independent samples. Differences in proportion were compared using a χ 2 test. We used the Kaplan‐Meier method to estimate survivals. Differences between survival curves were analyzed using the log‐rank test. Independent predictors of operative and long‐term mortality were identified with multivariate binary logistic regression models and Cox models, respectively. Age, gender, and all variables associated with a P value <0.20 on univariate analysis were entered in the models. We then performed a backward selection to retain in the models only the variables with a P value <0.10. In an additional analysis, all events that occurred within the 1st year after surgery were excluded to determine whether the effect of POAF on survival persists beyond the early postoperative phase. A probability value <0.05 was considered significant. All statistical analyses were performed with SPSS version 15 (SPSS Inc., Chicago, IL).
Results
Characteristics of the Patients With and Without POAF
In the study population, 1868 patients (27.8%) had POAF (Table 1). The patients with POAF were older and were more likely to have 3‐vessel disease or impaired left ventricular ejection fraction. The prevalence of statin therapy was similar in patients with and without POAF.
Table 1.
Preoperative and Operative Data of Patients With and Without Postoperative Atrial Fibrillation
| Operative Data | All Patients (N = 6728) | POAF Patients (n = 1868) | No POAF (n = 4860) | P Value |
|---|---|---|---|---|
| Age, y | 64.2 ± 10.1 | 68.3 ± 8.9 | 62.6 ± 10.0 | <0.001 |
| Male gender, % | 77.8 | 77.4 | 78.0 | 0.58 |
| BMI, kg/m2 | 27.9 ± 4.7 | 28.2 ± 4.9 | 27.8 ± 4.6 | 0.008 |
| Previous stroke, % | 4.5 | 5.6 | 4.1 | 0.01 |
| Previous myocardial infarction, % | 47.6 | 48.0 | 47.4 | 0.65 |
| Diabetes mellitus, % | 31.2 | 32.7 | 30.6 | 0.10 |
| Hypertension, % | 65.8 | 69.9 | 64.2 | <0.001 |
| COPD, % | 10.5 | 12.5 | 9.7 | 0.001 |
| Renal failure, % | 5.2 | 6.9 | 4.5 | <0.001 |
| 3‐vessel disease, % | 48.3 | 51.7 | 47.0 | 0.001 |
| LV ejection fraction <50%, % | 22.6 | 24.5 | 21.9 | 0.03 |
| Preoperative medication, % | ||||
| ACEI | 48.8 | 50.0 | 48.3 | 0.23 |
| Aspirin | 78.8 | 78.1 | 79.1 | 0.39 |
| β‐Blockers | 79.3 | 78.6 | 79.6 | 0.39 |
| Statins | 80.2 | 80.1 | 80.3 | 0.84 |
| Operative data | ||||
| Emergent surgery, % | 29.1 | 28.6 | 29.2 | 0.59 |
| Off‐pump cardiac surgery, % | 4.7 | 4.5 | 4.8 | 0.66 |
| CPB time, min | 75.3 ± 24.9 | 76.2 ± 22.7 | 74.8 ± 22.7 | 0.02 |
| Aortic cross‐clamp time, min | 51.7 ± 19.9 | 51.9 ± 17.2 | 51.7 ± 20.8 | 0.74 |
| Perioperative complications, % | ||||
| Operative mortality | 1.3 | 2.3 | 0.9 | <0.001 |
| Renal failure | 7 | 12.5 | 4.7 | <0.001 |
| Myocardial infarction | 8.2 | 8.5 | 8.2 | 0.70 |
| Stroke | 1.6 | 2.6 | 1.2 | <0.001 |
Abbreviations: ACEI, angiotensin conversion enzyme inhibitors; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; LV, left ventricular; POAF, postoperative atrial fibrillation. Data are presented as percentages or mean ± standard deviation.
Association Between POAF and Operative Mortality
Overall, operative mortality was 1.3% (89 patients). Patients with POAF had increased operative mortality (2.3% vs 0.9%, P < 0.001), postoperative renal failure (12.5% vs 4.7%, P < 0.001), and stroke (2.6% vs 1.2%, P < 0.001) (Table 2). Patients with POAF were at significantly greater risk for operative death (odds ratio [OR]: 2.47, P < 0.001). In the multivariate logistic model, POAF remained associated with higher operative mortality (OR: 1.78, P = 0.01).
Table 2.
Risk Factors for Operative Mortality in the Whole Cohort and in Patients With or Without Postoperative Atrial Fibrillation (N = 6728)
| Univariate Analysis | Multivariate Analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | All Patients | Patients With POAF | Patients Without POAF | |||||||||
| Univariate OR | CI | P Value | OR in the Whole Cohort | CI | P Value | OR in Patients With POAF | CI | P Value | OR in Patients Without POAF | CI | P Value | |
| Age, y | 1.08 | 1.06–1.11 | <0.001 | 1.07 | 1.04–1.10 | <0.001 | 1.06 | 1.01–1.10 | 0.01 | 1.07 | 1.04–1.11 | <0.001 |
| Female gender | 1.99 | 1.29–3.08 | 0.002 | — | — | — | — | — | — | — | — | — |
| History of stroke | 2.41 | 1.20–4.85 | 0.01 | — | — | — | 2.67 | 1.09–6.51 | 0.03 | — | — | — |
| Previous myocardial infarction | 1.49 | 0.97–2.27 | 0.07 | — | — | — | — | — | — | — | — | — |
| Diabetes mellitus | 1.58 | 1.03–2.42 | 0.03 | — | — | — | — | — | — | — | — | — |
| Hypertension | 2.59 | 1.49–4.52 | 0.001 | 2.10 | 1.19–3.84 | 0.01 | 2.10 | 0.90–4.90 | 0.09 | 2.42 | 1.07–5.52 | 0.04 |
| BMI, kg/m2 | ||||||||||||
| <20 | 2.61 | 1.06–6.46 | 0.04 | — | — | — | — | — | — | — | — | — |
| 20 to 25 | Reference | N/A | N/A | — | — | — | — | — | — | — | — | — |
| 25 to 30 | 0.77 | 0.46–1.29 | 0.32 | — | — | — | — | — | — | — | — | — |
| >30 | 0.86 | 0.49–1.50 | 0.59 | — | — | — | — | — | — | — | — | — |
| COPD | 2.51 | 1.52–4.16 | <0.001 | 1.82 | 1.07–3.11 | 0.03 | — | — | — | 2.14 | 1.03–4.44 | 0.04 |
| Renal failure | 3.52 | 1.97–6.30 | <0.001 | 2.17 | 1.16–4.05 | 0.02 | 2.46 | 1.07–5.65 | 0.04 | — | — | — |
| LV ejection fraction <50% | 2.48 | 1.60–3.85 | <0.001 | 1.95 | 1.28–3.17 | 0.004 | 1.98 | 1.03–3.80 | 0.04 | 2.25 | 1.20–4.24 | 0.01 |
| Emergent surgery | 2.15 | 1.41–3.29 | <0.001 | 1.92 | 1.23–3.00 | 0.004 | — | — | — | 2.16 | 1.16–4.03 | 0.02 |
| CPB time >120 minutes | 4.21 | 2.21–8.04 | <0.001 | 4.13 | 2.04–8.34 | <0.001 | 5.73 | 2.28–14.40 | <0.001 | — | — | — |
| Preoperative medication | ||||||||||||
| Statins | 0.59 | 0.37–0.94 | 0.02 | — | — | — | 0.38 | 0.20–0.72 | 0.003 | — | — | — |
| ACEI | 1.29 | 0.85–1.97 | 0.23 | — | — | — | — | — | — | — | — | — |
| Aspirin | 0.87 | 0.53–1.42 | 0.58 | — | — | — | — | — | — | — | — | — |
| β‐Blockers | 0.96 | 0.58–1.60 | 0.87 | — | — | — | — | — | — | — | — | — |
| Perioperative complications | ||||||||||||
| POAF | 2.47 | 1.62–3.75 | <0.001 | 1.78 | 1.14–2.81 | 0.01 | N/A | N/A | N/A | N/A | N/A | N/A |
| Myocardial infarction | 1.58 | 0.84–2.99 | 0.16 | — | — | — | 3.44 | 1.56–7.61 | 0.002 | — | — | — |
Abbreviations: ACEI, angiotensin conversion enzyme inhibitors; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; LV, left ventricular; N/A, not applicable; OR, odds ratio; POAF, postoperative atrial fibrillation. Only the variables with a P value <0.2 are shown.
Association Between POAF and Long‐Term Mortality
Long‐term follow‐up was available for all patients included before December 2005 (n = 5411, 80.4%). The mean follow‐up was 2.8 years. Overall, 321 patients died. Six‐year survival in patients with and without POAF were 85.3% vs 89.2%, respectively (P < 0.001) (Figure 1A). On multivariate analysis, POAF remained a significant predictor of long‐term mortality (hazard ratio [HR]: 1.35, P = 0.04) even after adjusting for perioperative complication (myocardial infraction, renal failure, and stroke) (Table 3).
Figure 1.
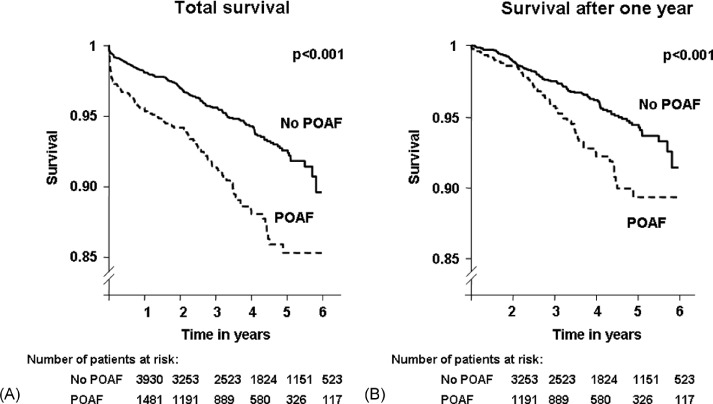
Long‐term survival in patients with and without postoperative atrial fibrillation (POAF): overall mortality (A) and excluding events that occurred within the 1st year (B).
Table 3.
Risk Factors for Long‐Term Mortality in the Whole Cohort and in Patients With or Without Postoperative Atrial Fibrillation (n = 5411)
| Univariate Analysis | Multivariate Analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | All Patients | Patients With POAF | Patients Without POAF | |||||||||
| HR | CI | P Value | HR | CI | P Value | HR | CI | P Value | HR | CI | P Value | |
| Age, y | 1.07 | 1.05–1.08 | <0.001 | 1.05 | 1.03–1.06 | <0.001 | 1.05 | 1.02–1.07 | <0.001 | 1.05 | 1.03–1.07 | <0.001 |
| Female gender | 1.48 | 1.17–1.89 | 0.001 | — | — | — | — | — | — | — | — | — |
| History of stroke | 2.77 | 1.94–3.92 | <0.001 | 1.84 | 1.16–2.90 | 0.009 | 2.46 | 1.31–4.63 | 0.005 | — | — | — |
| Previous myocardial infraction | 1.56 | 1.25–1.95 | <0.001 | — | — | — | — | — | — | — | — | — |
| Diabetes mellitus | 1.74 | 1.39–2.17 | <0.001 | 1.48 | 1.11–1.96 | 0.007 | — | — | — | 1.60 | 1.10–2.30 | 0.01 |
| Hypertension | 1.59 | 1.23–2.03 | <0.001 | — | — | — | 1.61 | 0.99–2.61 | 0.06 | — | — | — |
| BMI, kg/m2 | ||||||||||||
| <20 | 1.60 | 0.90–2.89 | 0.11 | 1.28 | 0.65–2.65 | 0.49 | 2.32 | 0.88–6.09 | 0.09 | — | — | — |
| 20 to 25 | Reference | — | — | Reference | — | — | Reference | — | — | — | — | — |
| 25 to 30 | 0.76 | 0.58–0.99 | 0.04 | 0.71 | 0.50–0.97 | 0.04 | 0.55 | 0.33–0.92 | 0.02 | — | — | — |
| >30 | 0.83 | 0.62–1.12 | 0.22 | 0.77 | 0.52–1.07 | 0.15 | 0.70 | 0.41–1.19 | 0.19 | — | — | — |
| COPD | 2.71 | 2.10–3.52 | <0.001 | 1.77 | 1.27–2.44 | 0.001 | — | — | — | 2.49 | 1.62–3.76 | <0.001 |
| Renal failure | 2.98 | 2.19–4.05 | <0.001 | 1.45 | 0.96–2.20 | 0.08 | — | — | — | 1.75 | 1.00–3.01 | 0.048 |
| 3‐vessel disease | 1.40 | 1.12–1.75 | 0.03 | — | — | — | — | — | — | — | — | — |
| LV ejection fraction <50% | 2.26 | 1.79–2.85 | <0.001 | 2.01 | 1.52–2.65 | <0.001 | 1.72 | 1.13–2.63 | 0.01 | 2.25 | 1.55–3.26 | <0.001 |
| Emergent surgery | 1.31 | 1.02–1.66 | 0.03 | — | — | — | — | — | — | — | — | — |
| Preoperative medication | ||||||||||||
| Statins | 0.61 | 0.48–0.77 | <0.001 | 0.73 | 0.54–0.97 | 0.03 | 0.62 | 0.41–0.94 | 0.03 | — | — | — |
| ACEI | 1.36 | 1.10–1.72 | 0.007 | — | — | — | — | — | — | — | — | — |
| Aspirin | 0.78 | 0.60–1.00 | 0.05 | — | — | — | — | — | — | — | — | — |
| β‐Blockers | 0.68 | 0.53–0.87 | 0.02 | — | — | — | — | — | — | — | — | — |
| Perioperative complications | ||||||||||||
| POAF | 1.85 | 1.48–2.32 | <0.001 | 1.35 | 1.02–1.81 | 0.040 | N/A | N/A | N/A | N/A | N/A | N/A |
| Myocardial infarction | 1.41 | 1.02–1.97 | 0.04 | 1.43 | 0.97–2.13 | 0.08 | 2.04 | 1.19–3.52 | 0.01 | — | — | — |
| Renal failure | 4.16 | 3.07–5.67 | <0.001 | 2.13 | 1.50–3.06 | <0.001 | 2.48 | 1.56–3.94 | <0.001 | 2.03 | 1.17–3.50 | 0.01 |
| Stroke | 7.61 | 5.24–11.11 | <0.001 | 5.19 | 3.34–8.13 | <0.001 | 5.44 | 3.06–9.65 | <0.001 | 4.05 | 1.95–8.32 | <0.001 |
Abbreviations: ACEI, angiotensin conversion enzyme inhibitors; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LV, left ventricular; N/A, not applicable; POAF, postoperative atrial fibrillation. Only the variables with a P value <0.2 are shown.
Survival in patients with POAF was significantly lower when excluding deaths that occurred within the 1st year after surgery (P < 0.001) (Figure 1B). After adjusting for other risk factors, POAF remained independently associated with long‐term mortality beyond 1 year (HR: 1.6, P = 0.02), whereas other perioperative complications no longer had a significant impact.
Differential Impact of Statins on Mortality in Patients With and Without POAF
On univariate analysis, preoperative statin therapy had no impact on operative mortality rates in patients without POAF (1.0% vs 0.7%, P = 0.45), whereas it was associated with a marked reduction in mortality in the patients with POAF (1.6% vs 5.1%, P < 0.001). On multivariate analysis, statin medication remained significantly associated with better operative outcome in the subset of patients with POAF (OR: 0.38, P = 0.003) (Table 2).
Preoperative use of statins was also associated with reduced long‐term mortality in patients with POAF (HR: 0.53, P = 0.001) as well as those without POAF (HR: 0.69, P = 0.02). However, when adjusting for age and gender, statins were significantly associated with better survival only in patients with POAF (POAF, HR: 0.61, P = 0.008 vs No POAF, HR: 0.78, P = 0.12). After further adjustment for other risk factors, statin medication remained an independent predictor of improved long‐term survival in patients with POAF (HR: 0.62, P = 0.03) (Table 3).
Patients under statin medication had lower low‐density lipoprotein (LDL) cholesterol blood levels (0.85 g/L ± 0.33 vs 1.00 g/L ± 0.34, P < 0.001) at baseline. In the patients who were not on statins prior to surgery, the percentage of patients who had LDL cholesterol >1 g/L was 48.5% in patients without POAF and 46.6% in patients with POAF. After further adjustment for preoperative LDL levels, statins remained independently associated with a better long‐term survival (HR: 0.56, P = 0.01).
Discussion
The major findings of this study are that POAF is an independent predictor of both short‐ and long‐term mortality following CABG. Moreover, statin therapy was independently associated with a marked reduction in operative and long‐term mortality in patients with POAF.
Impact of POAF on Operative Mortality
There are conflicting results regarding the association between POAF and increased operative mortality.1., 3., 4., 6., 8., 11. Kalavrouziotis et al found that unadjusted mortality rates were similar in patients with and without POAF (3% vs 2.5% respectively, P = 0.99) in a series of 7347 patients undergoing valvular and/or coronary surgery.6 In contrast, Villareal et al studied patients with isolated CABG and found a strong and independent association between POAF and operative mortality.1 Therefore, it is possible that the determinants of POAF and its impact on operative outcome might be different in patients with CABG vs those with valvular surgery.
Potential causative mechanisms that could be responsible for the POAF‐related increase in operative mortality include adverse hemodynamic effects, thromboembolism, and proarrhythmic effects of the drugs used to prevent or treat POAF. First, it is well established that POAF increases the risk of perioperative stroke by 2 to 4 fold.1., 3., 6. Second, the loss of atrial contraction may substantially reduce cardiac output, which could in turn lead to the development of cardiac and/or renal failure. These perioperative complications may contribute to the increased operative mortality associated with POAF.
It is also well known that the postoperative inflammatory response due to extracorporeal circulation is a key mechanism of the genesis of POAF.15., 16., 17. Exacerbated inflammatory response after surgery has been linked to worse postoperative outcome.19., 20. Therefore, one could speculate that patients who developed POAF following CABG had a low‐grade chronic inflammatory state prior to surgery, thereby predisposing them to exaggerated inflammatory response following cardiopulmonary bypass, which would in turn translate into a higher incidence of perioperative morbidity and mortality.
Impact of POAF on Long‐Term Mortality
Some previous studies have reported that POAF is associated with increased mortality in the long term.1., 3., 5., 8., 11. In the present study, to determine whether or not the impact of POAF on long‐term survival was uniquely due to POAF‐related perioperative complications, we performed further adjustment for perioperative stroke and renal failure, and censored all events occurring within 1 year of the surgery. Even when using this comprehensive adjustment, POAF remained a significant and independent predictor of long‐term mortality. The results of our study, supported by the results of other well‐conducted studies in the field of coronary surgery,1., 5., 8., 11. provide strong evidence for a significant impact of POAF on long‐term survival.
POAF might be a marker for increased susceptibility to chronic AF, which has been linked to worse long‐term prognosis following CABG.21 To this effect, AF could cause lethal stroke. AF can also have deleterious hemodynamic effect, which could contribute to an excess mortality rate. Another possibility is that AF is a surrogate marker for a more advanced stage of the disease and/or for a more severe risk profile, which could contribute to explaine the worse long‐term outcome associated with this perioperative complication. In particular, POAF may be a marker for an increased chronic inflammatory state, which is in turn associated with increased risk of cardiovascular events in the short and long term.22
Differential Impact of Statins in Patients With vs Without POAF
Despite some conflicting results regarding the beneficial impact of statins on perioperative outcome after CABG,13., 23., 24., 25., 26. overall, strong evidence supports the use of statins in patients with CABG.13., 19., 23., 24., 27. As in some other large observational studies,25., 26. we did not find a significant association between statin therapy and operative mortality. However, in this study, statin therapy was associated with a protective effect against both operative and long‐term mortality in the subset of patients who developed POAF. Even after adjustment for baseline LDL levels, statins remained independently associated with better survival in patients with POAF, thus supporting the role of the pleiotropic effects of this class of drug. In particular, the anti‐inflammatory and antioxidant properties of statins could prevent the occurrence of AF and associated complications. Statins have consistently been shown to substantially decrease the risk of stroke, and this effect is in a large part independent of lipid‐lowering effects.28 Furthermore, observational studies have reported that statin therapy is associated with a substantial reduction of the incidence of new‐onset AF in patients with coronary artery disease.29., 30. Therefore, statin therapy may have contributed to preventing the recurrence of AF and to reducing the occurrence of POAF‐related fatal complications. Given that POAF is likely a marker for a more severe risk profile, the differential impact of statins may also be explained by the fact that the protective effects of statins are much more pronounced in high‐risk than in low‐risk patients.
Clinical Implications
Although POAF appears to be a benign complication in the majority of patients, the present study and other recent studies1., 5., 8., 12. have pinpointed that POAF is a strong and independent predictor of both operative and late mortality. Patients who develop POAF should be considered at higher risk for adverse outcomes in the short and long term. Closer follow‐up and aggressive secondary prevention programs (lifestyle modification, pharmacotherapy) might thus be applied in these high‐risk patients. Whether POAF is a risk factor or solely a risk marker remains unclear, and further randomized studies are needed to determine if preventing POAF translates into a significant reduction in short‐ and long‐term mortality following CABG.
As recommended, unless contraindicated for adverse side effects, statins should be prescribed in every patients undergoing CABG.19 To this effect, it has been reported that patients undergoing CABG are undertreated with respect to statin therapy.31., 32. Furthermore, as pleiotropic effects have been shown to be dose dependent,33 it is possible that high doses of statins may confer a greater protection in patients with POAF, regardless of the patients' LDL blood levels.
Limitations
Given that this was an observational retrospective study, unmeasured or unknown confounders might have influenced the results, and causality regarding the impact of statin therapy on survival cannot be ascertained. The medication at follow‐up was not available in this study. Importantly, some patients who were free of statins before CABG may have been prescribed statins after surgery. If so, some patients considered statin‐free would have benefited from the protective effect of statins, which would have resulted in an underestimation of the association. Another limitation of this study is that episodes of recurrent AF after discharge were not collected, and thus it was not possible to assess whether recurrent AF in the late follow‐up could have explained the higher mortality rate in patients with POAF.
Conclusion
POAF is independently associated with increased risk of both operative and long‐term mortality following CABG. Moreover, statin therapy is associated with a powerful protective effect against fatal events in the patients who develop POAF. These results provide further support for the implementation of preventive strategies to reduce the incidence of POAF in the population undergoing CABG.
References
- 1. Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. [DOI] [PubMed] [Google Scholar]
- 2. Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. [DOI] [PubMed] [Google Scholar]
- 3. Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–511; discussion 511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56: 539–549. [DOI] [PubMed] [Google Scholar]
- 5. Filardo G, Hamilton C, Hebeler RF Jr, et al. New‐onset postoperative atrial fibrillation after isolated coronary artery bypass graft surgery and long‐term survival. Circ Cardiovasc Qual Outcomes. 2009;2:164–169. [DOI] [PubMed] [Google Scholar]
- 6. Kalavrouziotis D, Buth KJ, Ali IS. The impact of new‐onset atrial fibrillation on in‐hospital mortality following cardiac surgery. Chest. 2007;131:833–839. [DOI] [PubMed] [Google Scholar]
- 7. Mariscalco G, Engstrom KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009;88:1871–1876. [DOI] [PubMed] [Google Scholar]
- 8. Mariscalco G, Klersy C, Zanobini M, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612–1618. [DOI] [PubMed] [Google Scholar]
- 9. Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. [DOI] [PubMed] [Google Scholar]
- 10. Stamou SC, Dangas G, Hill PC, et al. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000;86:64–67. [DOI] [PubMed] [Google Scholar]
- 11. Bramer S, van Straten AH, Soliman Hamad MA, et al. The impact of new‐onset postoperative atrial fibrillation on mortality after coronary artery bypass grafting. Ann Thorac Surg. 2010;90:443–449. [DOI] [PubMed] [Google Scholar]
- 12. El‐Chami MF, Kilgo P, Thourani V, et al. New‐onset atrial fibrillation predicts long‐term mortality after coronary artery bypass graft. J Am Coll Cardiol. 55:1370–1376. [DOI] [PubMed] [Google Scholar]
- 13. Pan W, Pintar T, Anton J, et al. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110:1145–1149. [DOI] [PubMed] [Google Scholar]
- 14. Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA‐3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 15. Echahidi N, Pibarot P, O'Hara G, et al. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:793–801. [DOI] [PubMed] [Google Scholar]
- 16. Abdelhadi RH, Gurm HS, Van Wagoner DR, et al. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am J Cardiol. 2004;93:1176–1178. [DOI] [PubMed] [Google Scholar]
- 17. Bruins P, te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C‐reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. [DOI] [PubMed] [Google Scholar]
- 18. Echahidi N, Pibarot P, Despres JP, et al. Metabolic syndrome increases operative mortality in patients undergoing coronary artery bypass grafting surgery. J Am Coll Cardiol. 2007;50:843–851. [DOI] [PubMed] [Google Scholar]
- 19. Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340–e437. [PubMed] [Google Scholar]
- 20. van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–568. [DOI] [PubMed] [Google Scholar]
- 21. Bramer S, van Straten AH, Soliman Hamad MA, et al. The impact of preoperative atrial fibrillation on early and late mortality after coronary artery bypass grafting. Eur J Cardiothorac Surg. 38:373–379. [DOI] [PubMed] [Google Scholar]
- 22. Kangasniemi OP, Biancari F, Luukkonen J, et al. Preoperative C‐reactive protein is predictive of long‐term outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29:983–985. [DOI] [PubMed] [Google Scholar]
- 23. Clark LL, Ikonomidis JS, Crawford FA Jr, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8‐year retrospective cohort study. J Thorac Cardiovasc Surg. 2006;131:679–685. [DOI] [PubMed] [Google Scholar]
- 24. Powell BD, Bybee KA, Valeti U, et al. Influence of preoperative lipid‐lowering therapy on postoperative outcome in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;99:785–789. [DOI] [PubMed] [Google Scholar]
- 25. Ali IS, Buth KJ: Preoperative statin use and outcomes following cardiac surgery. Int J Cardiol. 2005;103:12–18. [DOI] [PubMed] [Google Scholar]
- 26. Subramaniam K, Koch CG, Bashour A, et al. Preoperative statin intake and morbid events after isolated coronary artery bypass grafting. J Clin Anesth. 2008;20:4–11. [DOI] [PubMed] [Google Scholar]
- 27. Kulik A, Brookhart MA, Levin R, et al. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation. 2008;118:1785–1792. [DOI] [PubMed] [Google Scholar]
- 28. Liao JK. Beyond lipid lowering: the role of statins in vascular protection. Int J Cardiol. 2002;86:5–18. [DOI] [PubMed] [Google Scholar]
- 29. Ramani G, Zahid M, Good CB, et al. Comparison of frequency of new‐onset atrial fibrillation or flutter in patients on statins versus not on statins presenting with suspected acute coronary syndrome. Am J Cardiol. 2007;100:404–405. [DOI] [PubMed] [Google Scholar]
- 30. Young‐Xu Y, Jabbour S, Goldberg R, et al. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003;92:1379–1383. [DOI] [PubMed] [Google Scholar]
- 31. Khanderia U, Townsend KA, Eagle K, et al. Statin initiation following coronary artery bypass grafting: outcome of a hospital discharge protocol. Chest. 2005;127:455–463. [DOI] [PubMed] [Google Scholar]
- 32. Yam FK, Akers WS, Ferraris VA, et al. Interventions to improve guideline compliance following coronary artery bypass grafting. Surgery. 2006;140:541–547; discussion 547–552. [DOI] [PubMed] [Google Scholar]
- 33. Ray KK, Cannon CP. The potential relevance of the multiple lipid‐independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46:1425–1433. [DOI] [PubMed] [Google Scholar]


