Abstract
Background:
Heart failure with preserved ejection fraction (HFpEF), formerly referred to as diastolic heart failure (DHF), accounts for >50% of all HF patients. So far, there has been no specific treatment for impaired left ventricular (LV) relaxation. Data from in vitro and animal studies indicate that ranolazine improves diastolic function by inhibiting the late sodium current.
Hypothesis:
RAnoLazIne for the Treatment of Diastolic Heart Failure (RALI‐DHF) is a prospective, single‐center, randomized, double‐blind, placebo‐controlled proof‐of‐concept study to determine if ranolazine compared with placebo will be more effective in improving diastolic function in patients with HFpEF.
Methods:
Twenty patients with HFpEF (EF ≥ 50% and ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity [E/E′] >15 or N‐terminal pro‐type brain natriuretic peptide >220 pg/mL) will be randomized to receive ranolazine or placebo in a 1.5:1 ratio during their catheterization if the LV end‐diastolic pressure is ≥18 mm Hg and the time constant of relaxation (τ) is ≥50 ms. Treatment will consist of intravenous infusion of study drug (or placebo) for 24 hours, followed by oral treatment for a total of 14 days.
Endpoints:
The study will include the following exploratory endpoints: (1) change from baseline to 30 minutes from initiation of intravenous study drug administration during cardiac catheterization hemodynamic parameters at both resting and paced (120 beats per minute) conditions: τ, LV end‐diastolic pressure, and dP/dtmin.; and (2) change from baseline to day 14 in E/E′, maximal oxygen consumption, and N‐terminal pro‐type brain natriuretic peptide.
Conclusions:
The RALI‐DHF study is designed as a translational study to bridge the gap between basic science and therapeutics and to determine if ranolazine, compared with placebo, will be more effective in improving diastolic function in patients with HFpEF. © 2011 Wiley Periodicals, Inc.
Dr. Maier is funded by the German Research Foundation (DFG) through a Heisenberg grant (MA1982/4‐1) and a Klinische Forschergruppe grant (MA1982/2‐2). The RALI‐DHF trial is funded by a grant from Gilead Sciences, Inc. (Palo Alto, CA). Dr. Belardinelli is employed by Gilead. Dr. Maier has a collaborative/research grant with Gilead. Drs. Maier and Jacobshagen received honoraria from Berlin‐Chemie. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
The prevalence of heart failure (HF) has reached epidemic proportions in Western countries. Diastolic heart failure (DHF) currently accounts for >50% of all patients with HF.1, 2, 3 Diastolic heart failure is also referred to as HF with preserved ejection fraction (HFpEF).2 Patients with HFpEF suffer from symptoms of congestive heart failure (dyspnea on exertion, ankle swelling, hepatomegaly, etc.) due to an impaired relaxation and increased stiffness of the left ventricle (LV). Injuries sustained by the myocardium leading to interstitial fibrosis and myocyte hypertrophy, impaired intracellular calcium homeostasis, reduced elastic recoil due to isomeric changes in sarcomeric proteins, and various neurohormonal activation are involved in the pathophysiology of HFpEF.2, 4, 5, 6
The physical limitations (exercise intolerance) and psychological strain in patients suffering from HFpEF are substantial, and the prognosis is as ominous as that of patients suffering from systolic heart failure (SHF), with a 5‐year mortality of about 50%.1, 3, 7, 8 Diastolic LV dysfunction is not unique to patients with HFpEF, but also occurs in patients with SHF; and in this latter group, the degree of diastolic LV dysfunction correlates even better with symptoms than the left ventricular ejection fraction (LVEF).9, 10 Whereas in SHF a variety of evidence‐based therapeutic options for the improvement of symptoms and prognosis are available, the treatment options for patients with DHF are limited.1, 11, 12, 13 Currently, there is no specific treatment for impaired LV relaxation that results in symptomatic improvement and/or prognostic relevance for these patients.2, 12
The piperazine derivative ranolazine, marketed as Ranexa for the treatment of chronic angina,14, 15, 16 is an anti‐ischemic agent that has been shown in preclinical and clinical studies to selectively inhibit the late sodium current (INa,late) in cardiac myocytes.17, 18, 19, 20 This might be of particular importance in patients with HF and diastolic dysfunction; it is known that in the failing heart, the late sodium current (INa,late) is increased,21 leading to an Na+ accumulation in cardiac myocytes.5 The increased Na+ concentration reverses the mode direction of the Na+/Ca2+‐exchanger, contributing to a Ca2+ overload in the cell. Increased diastolic Ca2+ impairs relaxation leading to diastolic dysfunction. By inhibiting the INa,late, ranolazine is expected to prevent (or reduce) sodium accumulation in the myocyte. This should improve calcium extrusion through the Na+/Ca2+‐ exchanger and thereby improve relaxation of the myocardium.19 Data from in vitro and animal studies indicate that ranolazine improves diastolic function of the myocardium. We published results of a study that confirms the improvement of diastolic function by ranolazine in ex vivo (isolated) human myocardium from patients suffering from HF and diastolic dysfunction,17 and more recently in another study using right atrial trabeculae from patients with chronic atrial fibrillation.18 Ranolazine significantly reduced the increased diastolic tension in isolated myocardial muscle strips by ∼30%.17 This effect was more pronounced at higher stimulation rates of the muscle strips, and especially in the myocardium with severe diastolic dysfunction.17 This is important because patients with HFpEF typically decompensate at high heart rates (eg, during new onset of atrial fibrillation). Mechanistically, we could show that ranolazine inhibits the INa,late and thereby reduces intracellular diastolic sodium and calcium concentrations. Neither the sarcoplasmic reticulum calcium content nor the systolic function of the myocardium was significantly affected.17 In a canine model of chronic HF, ranolazine significantly decreased the LV end‐diastolic pressure (LVEDP) as a parameter for diastolic dysfunction.22 Furthermore, ranolazine has been shown to improve diastolic function in patients with ischemic heart disease.23, 24
The primary objective of the RALI‐DHF trial is to determine if ranolazine, compared with placebo, will be more effective in improving diastolic function in patients with HFpEF.
Methods
Study Design and Patient Selection
The RALI‐DHF study is a prospective, single‐center, randomized, double‐blind, placebo‐controlled proof‐of‐concept study to investigate the effect of ranolazine on diastolic function in patients with HFpEF. The trial is funded by a grant from Gilead Sciences Palo Alto, Inc. (Foster City, CA). The ClinicalTrials.gov registration number is NCT01163734.
Patients with clinical symptoms of HF undergoing cardiac catheterization as part of the routine diagnostic procedure for standard care will be screened for inclusion into the study. Twenty patients who fulfill the inclusion and exclusion criteria will be randomized to receive ranolazine or placebo in a 1.5:1 ratio (12 patients will receive ranolazine and 8 patients will receive placebo). Inclusion and exclusion criteria are listed in Tables 1 and 2.
Table 1.
Ranolazine for the Treatment of Diastolic Heart Failure (RALI‐DHF) Inclusion Criteria
| 1. Males or females age ≥40 years |
|---|
| 2. Clinical symptoms of heart failure (NYHA class II–III) at time of screening (eg, dyspnea, paroxysmal nocturnal dyspnea, orthopnea, bilateral lower extremity edema) |
| 3. LVEF ≥ 50% at screening |
4. With:
|
| 5. For female patients only: must be postmenopausal (no menses for last 24 mo) or sterilized; or if of child‐bearing potential, is not breastfeeding, has a negative pregnancy test at time of study, has no intention of becoming pregnant during the course of the study, and is using a safe contraceptive regimen. |
| 6. Signed informed consent. Continued eligibility criteria: Patients must continue to meet eligibility criteria and have an average (of 3 measurements) resting LVEDP ≥18 mm Hg and resting τ≥50 ms at time of cardiac catheterization to be eligible to receive study drug. |
Abbreviations: E/E′, ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity; NT‐proBNP, N‐terminal pro‐type brain natriuretic peptide; LVEDP, left ventricular end diastolic pressure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Table 2.
Ranolazine for the Treatment of Diastolic Heart Failure (RALI‐DHF) Exclusion Criteria
| 1. Acute cardiac decompensation requiring mechanical ventilation |
|---|
| 2. Hypotension with systolic/diastolic BP <90/50 mm Hg, respectively |
| 3. Primary hypertrophic or restrictive cardiomyopathy or systemic illness associated with infiltrative heart disease (eg, cardiac amyloidosis) |
| 4. Pericardial constriction |
| 5. Hemodynamically significant uncorrected obstructive or regurgitant valvular disease |
| 6. Cor pulmonale or other causes of right heart failure not associated with LV dysfunction |
| 7. MI, UA, or CABG surgery within 90 days prior to screening, or PCI within 30 days prior to screening |
| 8. Stroke within 90 days prior to screening |
| 9. Clinically significant pulmonary disease in the opinion of the investigator, or requiring home oxygen or oral steroid therapy |
| 10. History of serious cardiac dysrhythmias, including AF with resting heart rate >100 bpm |
| 11. Need for treatment with class I or III antiarrhythmic medications |
| 12. Implantable pacemaker, cardioverter‐defibrillator, or LVAD |
| 13. Clinically significant chronic hepatic impairment (Child‐Pugh class B [moderate] or class C [severe]) |
| 14. Severe renal insufficiency defined as creatinine clearance ≤30 mL/min as calculated by Cockcroft‐Gault formula or MDRD equation |
| 15. History of congenital or a family history of long QT syndrome, or known acquired QT‐interval prolongation |
| 16. Inability to exercise due to other comorbidities that may affect performance of CPET (eg, osteoarthritis, PVD) |
| 17. Current treatment with potent and moderate CYP3A inhibitors |
| 18. Current treatment with potent CYP3A inducers (eg, rifampin/rifampicin, St. John's Wort, carbamazepin/carbamazepine) |
| 19. Prior treatment with ranolazine |
| 20. Participation in another trial of an investigational drug or device within 30 days prior to screening |
| 21. Other conditions that in the opinion of the investigator may increase the risk to the patient (eg, weight ≤ 60 kg), prevent compliance with study protocol, or compromise the quality of the clinical trial |
Abbreviations: AF, atrial fibrillation; BP, blood pressure; bpm, beats per minute; CABG, coronary artery bypass graft; CPET, cardiopulmonary exercise test; CYP3A, cytochrome P450, family 3, subfamily A; LV, left ventricular; LVAD, left ventricular assist device; MDRD, Modified Diet in Renal Disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; UA, unstable angina.
Study Conduct and Procedures
Patients with clinical symptoms of HF will be consented and screened for eligibility. Complete medical history and physical examination will be recorded at screening. Echocardiography will be performed to determine cardiac dimensions, regional and global contractility, and cardiac valve functions. The measured and calculated parameters that are obtained by standard 2‐D images, pulsed‐wave and continuous‐wave Doppler tracings, and tissue Doppler are listed in Table 3.
Table 3.
Hemodynamic Data and Echocardiographic Parameters
| Hemodynamic Data and Pressure Measurements |
|---|
| Time constant of relaxation (τ) |
| Relaxation time |
| Left ventricular end‐diastolic pressure (LVEDP) |
| Left ventricular end‐systolic pressure (LVESP) |
| dP/dt minimum |
| Pulmonary capillary wedge pressure (PCWP) |
| Pulmonary artery pressure (systolic/diastolic/mean) |
| Systemic vascular resistance (SVR) |
| Pulmonary vascular resistance (PVR) |
| Cardiac output (CO) and cardiac index (CI) |
| Right atrial pressure (RAP) |
| Echocardiographic Parameters |
| Left ventricular end‐diastolic volume/index (LVEDV) |
| Left ventricular end‐systolic volume/index (LVESV) |
| Left ventricular stroke volume |
| Left ventricular ejection fraction (LVEF; Simpson method) |
| Mitral E wave velocity |
| Mitral A wave velocity |
| Mitral E/A ratio |
| Mitral annular velocity (E′, tissue Doppler) |
| E/E′ ratio |
Blood samples will be collected to determine the level of N‐terminal pro‐type brain natriuretic peptide (NT‐pro‐BNP) at screening. Other laboratory measurements include complete blood count, red blood cells, hemoglobin, hematocrit, platelet count, white blood count, coagulation test/international normalized ratio, aspartate transaminase, alanine transaminase, alkaline phosphatase, bilirubin, blood urea nitrogen, creatinine, gamma‐glutamyl transferase, potassium, and sodium.
A standard 12‐lead electrocardiogram (ECG) will be performed and reviewed for any clinically significant abnormalities to ensure patient safety. A symptom‐limited cardiopulmonary exercise test (CPET) will be performed using a bicycle ergometer. The following parameters will be measured: peak oxygen uptake (Pk VO2), maximal oxygen uptake (VO2 max), anaerobic threshold, respiratory exchange ratio (RER), oxygen saturation (SpO2), exercise duration, and ventilation/carbon dioxide production ratio (VE/VCO2).
Patients who fulfill the inclusion and exclusion criteria will be randomized prior to catheterization. The cardiac catheterization itself is part of the diagnostic procedure of the standard of care for these patients. A catheter will be introduced into the right ventricle and into the pulmonary artery for measurement and analysis of right heart pressures. A conductance catheter will be advanced into the LV for measurement of LV pressures and pressure/volume relations. A transient pacemaker probe will be introduced into the right atrium for pacemaker stimulation. If clinically indicated, coronary angiography will be performed before any pressures and hemodynamic data are measured. Once all catheters are in stable position, 3 sets of pressures and hemodynamic assessments will be recorded at resting conditions. In addition, atrial pacing will be performed at 120 bpm and 3 sets of pressure and hemodynamic assessments will again be obtained. The parameters that will be obtained are listed in Table 3.
Administration of the study drug (placebo or ranolazine) will be initiated if the continued eligibility criteria (average resting LVEDP ≥ 18 mm Hg and τ ≥ 50 ms) are met. The study treatment will begin with an initial intravenous (IV) bolus injection at T = 0 minutes of 92 mg ranolazine or placebo (Figure 1). The initial 92 mg of study drug will be administered as 10‐mL loading bolus over a period of 2 minutes. A second bolus injection of 92 mg ranolazine or placebo at the same dilution will be administered 15 minutes (T = 15 min) after initial bolus injection. Continuous infusion of ranolazine or placebo at a dose of 92 mg/hour (23 mL/h) will start at T = 20 minutes (5 min after the start of second bolus injection) and continue through to 24 hours (T = 24 h). To assess pharmacokinetic (PK) parameters, PK blood samples will be drawn at T = 10 minutes, T = 20 minutes (prior to continuous infusion), and T = 30 minutes.
Figure 1.
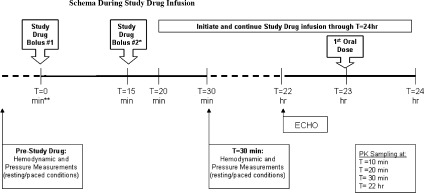
Schema during study‐drug infusion. Abbreviations: ECHO, echocardiography; PK, pharmacokinetic.
The catheter probes stay in place during the initiation of the IV study drug administration. All invasive measurements will be repeated at 30 minutes after initiation of the first loading bolus (T = 30 min) at resting (3 sets) and paced (3 sets) conditions. Three measurements will be taken, and the average of the 3 will represent the value for that variable measured. When all assessments are complete, the catheters are removed and the patient is transferred to the cardiac ward for monitoring (continuous 3‐lead ECG monitoring via telemetry, vital signs every 4 h). The IV study drug infusion will be continued through to 24 hours (T = 24 h). One hour prior to the end of the 24‐hour infusion, patients will be started on oral study drug 1000 mg twice daily and will be continued until the end of the study on day 14. All echocardiography measurements will be repeated at T = 22 hours (within 60 min prior to administration of oral study drug). The 12‐lead ECG will be repeated at this time point. Patients may be discharged at any time after T = 24 hours at the investigator's judgment per standard of care. Oral treatment will last for 13 days. Patients will be instructed to take 2 intact tablets of the study medication with water every day at 12‐hour intervals (in the morning and in the evening).
Patients will be required to return to clinic for the end of study visit on day 14 (±2 d). The end‐of‐study visit procedures (specifically, echocardiography and CPET) will be performed within 2 to 6 hours after the morning dose. The following assessments will be performed: complete PE including vital signs and weight measurements, 12‐lead ECG, echocardiography (prior to CPET), NT‐pro‐BNP determination and safety labs, CPET, serum pregnancy test (for females aged ≤65 years), adverse events, and concomitant medications.
Approximately 14 days after the end‐of‐study visit, the patient will be contacted either by phone or in person to assess and record adverse‐event and concomitant medication status since the last visit (day 28 safety follow‐up contact). The schedule of study events is depicted in Figure 2.
Figure 2.
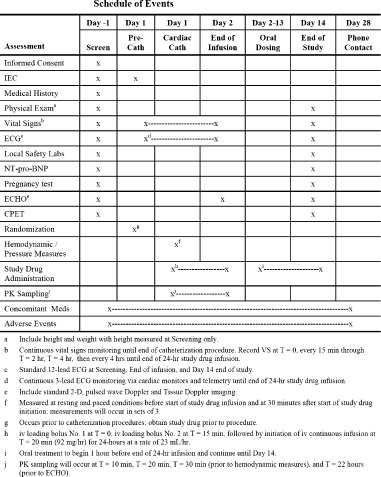
Schedule of events. Abbreviations: cath, catheterization; CPET, cardiopulmonary exercise test; ECG, electrocardiogram; ECHO, echocardiography; iv, intravenous; NT‐proBNP, N‐terminal pro‐type brain natriuretic peptide; PK, pharmacokinetic.
Endpoints
The study will include the following exploratory endpoints:
-
1.Change from baseline to 30 minutes from initiation of study drug bolus No. 1 (T = 30 min) in cardiac catheterization hemodynamic parameters at both resting and paced conditions:
- Time constant of relaxation (τ).
- LVEDP.
- dP/dt min (minimal rate of LV pressure change).
-
2.Change from baseline to day 14 in:
- Mitral E wave velocity/mitral annular velocity (E/E′) ratio assessed by tissue Doppler echocardiography.
- VO2 max assessed by CPET.
- NT‐pro‐BNP.
Baseline is defined as the average of 3 measurements of each hemodynamic parameters taken at rest conditions and during pacing prior to start of study‐drug infusion.
Detailed analyses of hemodynamic data and pressure measurements, echocardiography, and CPET parameters will be performed, pending on blinded review of the distributions of each measurement.
Statistical Considerations
The current study is considered exploratory, and therefore the sample size selection is based on practical considerations.
All statistical tests for treatment difference between ranolazine vs placebo will be performed using 2‐sided hypothesis tests at 5% significance level with no adjustment for multiple comparisons. Descriptive summaries will show counts and percentages for categorical variables and sample size, mean, SD, or SEM, minimum, median, and maximum for continuous variables by treatment. The Wilcoxon rank sum test will be applied to evaluate whether the reductions from baseline in primary and secondary efficacy endpoints are statistically significantly different between placebo vs ranolazine. The Cochran‐Mantel‐Haenszel test may be used to compare categorical variables between randomized treatments: row mean scores test for ordered categorical variables and general association test for nominal categorical variables. Analysis of variance, including effects for treatment, may be used to analyze continuous variables. All patients who receive ≥1 dose of randomized study medication will be included in the analyses for both efficacy and safety endpoints. Patients will be assigned to treatment group according to the treatment actually received.
Discussion
Despite the high prevalence, morbidity, and poor prognosis of HFpEF, its treatment is still poorly defined. Compared with trials in patients with reduced EF, fewer trials have included patients with HFpEF, and often the results have been disappointing.11, 25, 26 Thus far, there has been no specific treatment for impaired LV relaxation.2 In preclinical studies we could demonstrate, that ranolazine improves relaxation in myocardium from patients with HF and diastolic dysfunction due to normalization of altered intracellular sodium and calcium homeostasis.17, 18 The RALI‐DHF study provides a bench‐to‐bedside translational approach to evaluate if ranolazine, compared with placebo, will be more effective in improving diastolic function in patients with HFpEF by inhibiting the INa,late, as described in the background section of this paper. However, diastolic heart failure is not only caused by an abnormal Ca2+ and Na+ homeostasis. Increased interstitial deposition of collagen and modified matricellular proteins also contributes to increased myocardial stiffness and slowed LV relaxation.27, 28 It is not expected that ranolazine will affect this structural pathophysiology. Therefore, the chances for a positive result will depend on the relative contribution of the dysregulated cellular Na+ and Ca2+ homeostasis to the mechanism underlying diastolic dysfunction. Furthermore, it has to be considered that the results in isolated myocardium might not reflect the in vivo situation in patients with HFpEF.
The RALI‐DHF study is considered exploratory, and therefore the sample size (20 patients) is based on practical considerations. If ranolazine significantly improves diastolic function in this proof‐of‐concept study, a larger, multicenter trial will be warranted to confirm whether ranolazine, over a longer period of treatment, provides meaningful clinical benefit for these patients.
Diastolic LV dysfunction is not unique to patients with HFpEF; it also occurs in patients with HF and reduced EF, and in these patients parameters of diastolic dysfunction even correlate better with symptoms than LVEF.9, 10 Dyspnea and edema of the lower extremities are symptoms of CHF that is caused by increased left atrial and LV filling pressures. Therefore, it is also reasonable to investigate the effect of ranolazine on diastolic function in patients with SHF. A separate clinical study, RALI‐SHF, will be also initiated to address this issue.
In conclusion, ranolazine by inhibiting INa,late has been shown to improve diastolic function in isolated human myocardium and animal models as shown previously. In summary, RALI‐DHF is designed to investigate the effect of ranolazine on LV diastolic function in patients with HFpEF.
Acknowledgements
The authors thank Gilead for sponsoring the study and their clinical investigators for their contribution to the design of the study.
References
- 1. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 2. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:1539–1550. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 4. Hasenfuss G, Schillinger W, Lehnart SE, et al. Relationship between Na+‐Ca2+‐exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99: 641–648. [DOI] [PubMed] [Google Scholar]
- 5. Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt U, Hajjar RJ, Helm PA, et al. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 8. Gorelik O, Moznino‐Sarafian D, Shteinshnaider M, et al. Clinical variables affecting survival in patients with decompensated diastolic versus systolic heart failure. Clin Res Cardiol 2009;1;98:224–232. [DOI] [PubMed] [Google Scholar]
- 9. Hadano Y, Murata K, Yamamoto T, et al. Usefulness of mitral annular velocity in predicting exercise tolerance in patients with impaired left ventricular systolic function. Am J Cardiol. 2006;97:1025–1028. [DOI] [PubMed] [Google Scholar]
- 10. Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation. 2004;109:972–977. [DOI] [PubMed] [Google Scholar]
- 11. Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J. 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 12. Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–537. [DOI] [PubMed] [Google Scholar]
- 13. Kamp O, Metra M, De Keulenaer G, et al. Effect of the long‐term administration of nebivolol on clinical symptoms, exercise capacity and left ventricular function in patients with heart failure and preserved left ventricular ejection fraction: background, aims and design of the ELANDD study. Clin Res Cardiol. 2010;99:75–82. [DOI] [PubMed] [Google Scholar]
- 14. Morrow DA, Scirica BM, Karwatowska‐Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non–ST‐elevation acute coronary syndromes: the MERLIN‐TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. [DOI] [PubMed] [Google Scholar]
- 15. Chaitman BR, Skettino SL, Parker JO, et al. Anti‐ischemic effects and long‐term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375–1382. [DOI] [PubMed] [Google Scholar]
- 16. Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. [DOI] [PubMed] [Google Scholar]
- 17. Sossalla S, Wagner S, Rasenack EC, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. [DOI] [PubMed] [Google Scholar]
- 18. Sossalla S, Mazur M, Kallmeyer B, et al. Anti‐arrhythmic properties of ranolazine and improved diastolic function in isolated human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–2342. [DOI] [PubMed] [Google Scholar]
- 19. Hasenfuss G, Maier LS. Mechanism of action of the new anti‐ischemia drug ranolazine. Clin Res Cardiol 2008;97:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burashnikov A, Di Diego JM, Zygmunt AC, et al. Atrium‐selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valdivia CR, Chu WW, Pu J, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. [DOI] [PubMed] [Google Scholar]
- 22. Rastogi S, Sharov VG, Mishra S, et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–H2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayashida W, van Eyll C, Rousseau MF, et al. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther 1994;8: 741–747. [DOI] [PubMed] [Google Scholar]
- 24. Figueredo VM, Pressman GS, Romero‐Corral A, et al. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. J Cardiovasc Pharmacol Ther 2010;doi:10.1177/1074248410382105. [DOI] [PubMed] [Google Scholar]
- 25. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 26. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. [DOI] [PubMed] [Google Scholar]
- 27. Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. [DOI] [PubMed] [Google Scholar]
- 28. Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. [DOI] [PubMed] [Google Scholar]


