Abstract
Background:
This prospective study investigated the association between preprocedural biomarker levels and incident major adverse cardiac events (MACE) in complex patients undergoing percutaneous coronary intervention (PCI) with sirolimus‐eluting stenting.
Hypothesis:
Lipoprotein(a) (Lp[a]), interleukin‐10 (IL‐10), and high‐sensitivity C‐reactive protein (CRP) have long‐term prognostic value in patients undergoing PCI.
Methods:
Between April 2002 and February 2003, 161 patients were included in the study. Blood was drawn before the procedure, and biomarkers were measured. Patients were followed‐up for MACE (death, nonfatal myocardial infarction, and repeat revascularization). Cox proportional hazard models were used to determine risk of MACE for tertiles of biomarkers. Both 1‐year and long‐term follow‐up (median, 6 years; maximum, 8 years) were evaluated.
Results:
Mean age was 59 years, and 68% were men. During long‐term follow‐up, 72 MACE occurred (overall crude cumulative incidence: 45% [95% confidence interval (CI): 37%‐52%]). Lp(a) was associated with a higher 1‐year risk of MACE, with an adjusted hazard ratio (HR) of 3.1 (95% CI: 1.1‐8.6) for the highest vs the lowest tertile. This association weakened and lost significance with long‐term follow‐up. IL‐10 showed a tendency toward an association with MACE. The 1‐year HR was 2.1 (95% CI: 0.92‐5.0). Long‐term follow‐up rendered a similar result. The association of CRP with MACE did not reach statistical significance at 1‐year follow‐up. However, CRP was associated with long‐term risk of MACE, with an HR of 1.9 (95% CI: 1.0‐3.5).
Conclusions:
In this prospective study, preprocedural Lp(a) level was associated with short‐term prognosis after PCI. The preprocedural CRP level was associated with long‐term prognosis after PCI. Clin. Cardiol. 2012 DOI: 10.1002/clc.21988
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality in the Western world. Percutaneous coronary intervention (PCI) has significantly reduced consequences of CAD.1 Nevertheless, post‐PCI patients still constitute a high‐risk group for recurrent events and cardiovascular mortality. To improve long‐term prognosis in post‐PCI patients, first and foremost, further enhancement of risk stratification is needed. Identification of high‐risk patients may then serve as a guide to apply or withhold more aggressive treatment.
Biomarkers have received much attention as predictors of CAD in the past decade. Several biomarkers have been associated with incident coronary events, both in the general population and in patients with known CAD.2, 3 Furthermore, a body of research is growing on emerging biomarkers.4 Data on the association between preprocedural biomarker levels and prognosis after PCI are less elaborate.
In the current study, which was conceived nearly a decade ago, we postulated that early preprocedural markers may predict later cardiac events. At the time of study commencement, several lines of evidence had already confirmed that inflammation plays a major role in the pathogenesis of atherosclerotic lesions of vascular walls. C‐reactive protein (CRP) was strongly implicated5 and was considered a promising candidate for the present study. Furthermore, attention had also been directed toward interleukin (IL)‐10 as 1 of the most important mediators that physiologically limits and downregulates inflammation.6 With regard to lipid biomarkers, although evidence was not always consistent, lipoprotein(a) (Lp [a]) was deemed a promising novel biomarker.5 Therefore, we have investigated the long‐term prognostic value of these 3 biomarkers in complex patients undergoing PCI with sirolimus eluting stenting.
Apart from assessing prognostic value at 1 year of follow‐up, we also examined the value at a maximum follow‐up of 8 years. Such extensive follow‐up data are currently scarce and enabled us to examine the patterns of the associations between biomarkers and cardiac events over time.
Methods
Patient Population and Baseline Data Collection
The study population consisted of a subset (n = 161) of the RESEARCH registry (Rapamycin‐Eluting Stent Evaluated At Rotterdam Cardiology Hospital), that has been described elsewhere.7 Briefly, RESEARCH is a single‐center registry conducted with the main purpose of evaluating the safety and efficacy of sirolimus‐eluting stent (SES) implantation for patients treated in daily practice. To include a patient population representative of the real world, from April 2002 onward a policy was adopted of using SES (Cypher; Johnson & Johnson‐Cordis, Cordis Europa NV, Roden, The Netherlands) as the default strategy for every PCI. The general indications for PCI included stable angina pectoris, unstable angina pectoris, and acute myocardial infarction (MI). For the current study, patients defined as complex undergoing PCI between April 2002 and February 2003 were included. These complex patients typically had SES implanted in bifurcations, left main coronary, chronic total occlusions, very small vessels, and long stented length (>36 mm). Patients were included during office hours, with the exception of random periods of absence of the responsible investigator. The study was approved by the hospital ethics committee and is in accordance with the Declaration of Helsinki. Written informed consent was obtained from every patient.
Baseline characteristics were assessed by screening medical records at the time of the procedure and included demographics, medical history, medication use, and cardiovascular risk factors. Information on cardiovascular risk factors included smoking, diabetes mellitus, hypertension, and hypercholesterolemia as diagnosed and registered in the medical records by treating physicians.
Biomarker Measurements
Blood was drawn immediately before PCI. High‐sensitivity CRP (hsCRP) was determined directly at the Clinical Chemistry Department of Erasmus Medical Center by using Rate Near Infrared Particle Immunoassay (Immage Immunochemistry System; Beckman Coulter, Inc., Brea, CA). This system measures concentrations from 0.2 to 1440 mg/L, with a within‐run precision <5% and a total precision <7.5%.
Subsequently, blood samples were stored at −80°C at Erasmus Medical Center, after which they were transported on dry ice to Dunedin, New Zealand, where IL‐10 and lipoprotein (a) (Lp [a]) were measured. IL‐10 was measured using the Quantikine HS immunoassay (HS100B) (R&DSystems, Minneapolis, MN). The minimal detectable dose of this assay is 0.5 pg/mL, the intra‐assay precision is <8.5%, and the interassay precisionis <15.6%. Lp(a) was measured using a double sandwich enzyme‐linked immunosorbent assay as previously described.8 This assay detects all apolipoprotein (a) isoforms on an equivalent molar basis and is considered the most accurate method for Lp(a) measurement.9 This assay measures concentrations from 2 to 600 nmol/L, with coefficients of variation <10%.
End Point Definitions
The primary outcome was the occurrence of major adverse cardiac events (MACE), defined as: 1) death, 2) nonfatal MI, or 3) repeat revascularization. MI was defined as a diagnosis made by a cardiologist based on the combination of typical ischemic chest complaints and objective evidence of myocardial necrosis as demonstrated by the electrocardiogram or elevated cardiac markers. Revascularization was defined as a repeat intervention (surgical or percutaneous) to treat a luminal stenosis in any epicardial vessel.
Follow‐up
Information about in‐hospital outcomes was obtained from an electronic clinical database for patients maintained at Erasmus Medical Center, Rotterdam and by review of hospital records for those discharged to referring hospitals. Postdischarge survival status was obtained from municipal civil registries. Postdischarge repeat interventions and rehospitalizations were prospectively assessed during follow‐up. Yearly questionnaires with information about anginal status and medication use were sent to all living patients, and treating physicians and institutions were contacted whenever necessary for additional information.
Statistical Analysis
We calculated means and proportions of the baseline characteristics and crude cumulative incidence. Subsequently, we divided biomarker levels into categories (tertiles and detectable vs nondetectable when appropriate) and calculated means and proportions of baseline characteristic according to these categories. To test for trends, we used linear regression for continuous variables, logistic regression for dichotomous variables, and multinomial regression for variables with more than 2 categories. We used ln‐transformed continuous levels of biomarkers as the independent variable.
To address the predictive value of baseline biomarker levels, we calculated relative risks of MACE associated with increasing tertiles of CRP (cutpoints 1.9 and 4.5 mg/L), Lp(a) (cutpoints 9.8 and 65.2 nmol/L), and IL‐10 (cutpoints 0.4 and 3.2 ng/mL) by Cox proportional hazards analysis. The proportional hazards assumption was tested by drawing log minus log plots of the survival function, which confirmed that the assumption was met. We adjusted for age and sex (model 1), and subsequently for age, sex, smoking, diabetes mellitus, hypertension, and hypercholesterolemia (model 2). First, we truncated follow‐up time at 1 year. Subsequently, we examined very long‐term outcome by taking complete follow‐up into account. We repeated all analyses using ln‐transformed continuous values of the biomarker levels instead of tertiles to demonstrate trends.
Values for covariates were missing in <1% of the patients, except for previous MI (missing in <2%). Given these low percentages, we chose to perform a complete‐case analysis. All analyses were conducted with SPSS 17.0 for Windows (IBM, Armonk, NY). All tests were 2‐sided.
Results
The mean age of the patients was 59 years, and 68% were male (Table 1). The median follow‐up was 6 years, with a maximum of 8 years. The total number of person‐years of follow‐up amounted to 709 years. A total of 39 and 72 MACE occurred after 1 year and 8 years of follow‐up, respectively. The overall crude cumulative incidence was 45% (95% confidence interval [CI]: 37%‐52%).Occurrence of MACE was highest at the beginning of the follow‐up period and subsequently declined. Crude cumulative incidence over the first year of follow‐up was 24% (95% CI: 18%‐31%).
Table 1.
Baseline Characteristics of the Study Population (n = 161)
| Variable | |
|---|---|
| Age, y | 59.4 ± 11.3 |
| Men | 110 (68%) |
| Hypertension | 63 (39%) |
| Hypercholesterolemia | 125 (78%) |
| Diabetes mellitus | 31 (19%) |
| Current smoking | 51 (32%) |
| History of myocardial infarction | 53 (34%) |
| History of CABG | 18 (11%) |
| History of PCI | 43 (27%) |
| Family history of coronary disease | 60 (38%) |
| Clinical presentation | |
| Stable angina | 85 (54%) |
| Unstable angina | 45 (28%) |
| Acute myocardial infarction | 29 (18%) |
| No. of diseased vessels | |
| 1 vessel | 70 (44%) |
| 2 vessels | 51 (32%) |
| 3 vessels | 38 (24%) |
| C‐reactive protein, mg/L | 3.03 (1.33–5.72) |
| Lipoprotein (a), nmol/L | 20.8 (6.08–84.9) |
| Interleukin‐10, ng/mL | 1.81 (0.00–4.48) |
Abbreviations: CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Categorical variables are expressed as number (percentage). Valid percentages are reported.Values of continuous variables are expressed as mean ± standard deviation or as median (interquartile range) in case of skewed distribution.
The median (interquartile range) levels of biomarkers were 3.03 (1.33–5.72) mg/L for CRP, 20.8 (6.08–84.9) nmol/L for Lp(a), and 1.81 (0–4.48) ng/mL for IL‐10, respectively. All biomarker distributions were right‐skewed. CRP was available in all patients and below the detection limit in 4 patients (2.5%). For trend analysis, the data points in these patients were imputed by dividing the lowest detectable limit of the assay (0.2 mg/L) by 2. Lp(a) measurement was missing in 3 patients and below the detection limit in 16 patients (10%). The lower limit of detection (2 nmol/L), divided by 2, was imputed in these patients for trend analysis. IL‐10 measurement was missing in 3 patients and below the detection limit in 49 patients (31%). Because of this high percentage of undetectable levels, values were not imputed, and the P for trend was not computed. IL‐10 was thus only examined in tertiles and as a dichotomous variable (detectable vs not detectable).
Table 2 displays baseline characteristics according to categories of Lp(a), CRP, and IL‐10 level. CRP showed a positive association with diabetes mellitus (P = 0.004) and with number of diseased vessels (P = 0.04).
Table 2.
Baseline Characteristics According to Categories of Lipoprotein (a), Interleukin‐10, and C‐Reactive Protein Level
| Baseline Characteristic | Lp (a), Tertiles | Il‐10, Detectable vs Nondetectable | CRP, Tertiles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 52) | 2 (n = 54) | 3 (n = 52) | P for Trend | Nondetectable (n = 49) | Detectable (n = 109) | P Value | 1 (n = 53) | 2 (n = 54) | 3 (n = 53) | P for Trend | |
| Age, y | 58.0 ± 11.9 | 60.0 ± 11.8 | 60.6 ± 9.9 | 0.20 | 60.1 ± 10.7 | 59.2 ± 11.5 | 0.61 | 57.4 ±12.8 | 61.6 ± 10.4 | 59.4 ± 10.1 | 0.15 |
| Men | 73 | 70 | 62 | 0.43 | 61 | 72 | 0.63 | 68 | 69 | 68 | 0.71 |
| Hypertension | 27 | 40 | 52 | 0.05 | 47 | 34 | 0.13 | 34 | 38 | 47 | 0.08 |
| Hypercholesterolemia | 71 | 77 | 85 | 0.28 | 80 | 77 | 0.70 | 81 | 77 | 76 | 0.31 |
| Diabetes mellitus | 19 | 17 | 23 | 0.87 | 27 | 17 | 0.15 | 9 | 19 | 30 | 0.004 |
| Current smoking | 44 | 25 | 25 | 0.14 | 37 | 29 | 0.32 | 30 | 25 | 40 | 0.78 |
| History of MI | 28 | 42 | 33 | 0.99 | 29 | 36 | 0.40 | 23 | 38 | 41 | 0.11 |
| History of CABG | 8 | 11 | 15 | 0.28 | 8 | 13 | 0.39 | 8 | 11 | 15 | 0.22 |
| History of PCI | 19 | 26 | 37 | 0.15 | 31 | 25 | 0.46 | 28 | 26 | 26 | 0.71 |
| Family history of coronary disease | 29 | 45 | 35 | 0.36 | 43 | 35 | 0.36 | 38 | 26 | 47 | 0.24 |
| Number of diseased vessels | 0.55a | 0.75a | 0.04a | ||||||||
| 1 | 52 | 42 | 37 | Reference | 47 | 44 | Reference | 53 | 42 | 36 | Reference |
| 2 | 29 | 30 | 39 | 0.31b | 33 | 31 | 0.98b | 30 | 30 | 36 | 0.27b |
| 3 | 19 | 28 | 23 | 0.44b | 20 | 25 | 0.48b | 17 | 26 | 28 | 0.02b |
Abbreviations: CABG, coronary artery bypass graft; CRP, C‐reactive protein; Il‐10, interleukin‐10; Lp (a), lipoprotein (a); MI, myocardial infraction; PCI, percutaneous coronary intervention.
Categorical variables are expressed as percentage. Continuous variables are expressed as mean ± standard deviation. P for trend was obtained by linear, logistic, or multinomial regression, whichever was appropriate.
Overall P for trend.
P for trend for specific number of vessels.
Table 3 shows hazard ratios (HRs) for MACE at 1 year of follow‐up, where as Table 4 shows those at maximum follow‐up. Lp(a) was associated with 1‐year risk of MACE, with an HR of 3.1 (95% CI: 1.1‐8.6) for the highest vs the lowest tertile. This association weakened and lost significance with long‐term follow‐up, the HR becoming 1.6 (95% CI: 0.86‐3.1). IL‐10 showed a tendency toward an association with MACE. The 1‐year HR was 2.1 (95% CI: 0.92‐5.0) for the highest vs the lowest tertile. Long‐term follow‐up rendered a similar result, the HR becoming 1.7 (95% CI: 0.94‐3.2). The association of CRP with MACE did not reach statistical significance at 1‐year follow‐up and was 1.7 (95% CI: 0.77‐3.8). However, CRP was associated with long‐term risk of MACE, with a HR of 1.9 (95% CI: 1.0‐3.5) for the highest vs the lowest tertile. The Figure 1 further illustrates these associations.
Table 3.
Hazard Ratios for Major Adverse Cardiac Events at 1 Year of Follow‐Up
| Biomarker | Cumulative Incidence (95% CI) | Hazard Ratio (95% CI) | |
|---|---|---|---|
| MACE, Adjusted for Age and Sex | MACE, Multivariate Adjusted | ||
| Lipoprotein (a) | |||
| Tertile 1 | 10% (2‐18) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 | 31% (19‐44) | 3.7 (1.4‐10.1) | 3.3 (1.2‐9.2) |
| Tertile 3 | 31% (18‐43) | 3.5 (1.3‐9.6) | 3.1 (1.1‐8.6) |
| P for trend | 0.04 | 0.06 | |
| Interleukin‐10 | |||
| Tertile 1 | 17% (7‐28) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 | 24% (13‐35) | 1.5 (0.63‐3.5) | 1.3 (0.52‐3.3) |
| Tertile 3 | 29% (17‐41) | 2.1 (0.92‐5.0) | 2.1 (0.92‐5.0) |
| Detectable vs nondetectable | 1.6 (0.76‐3.4) | 1.5 (0.69‐3.3) | |
| C‐reactive protein | |||
| Tertile 1 | 21% (10‐32) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 | 20% (10‐31) | 0.93 (0.40‐2.2) | 0.87 (0.37‐2.1) |
| Tertile 3 | 32% (20‐45) | 1.6 (0.72‐3.3) | 1.7 (0.77‐3.8) |
| P for trend | 0.20 | 0.17 | |
Abbreviations: CI, confidence interval; MACE, major adverse cardiac events (death, nonfatal myocardial infarction, and repeat revascularization).
Multivariate adjusted: adjusted for age, sex, smoking, diabetes mellitus, hypertension, and hypercholesterolemia.
Table 4.
Hazard Ratios for Major Adverse Cardiac Events at 8 Years Follow‐Up
| Biomarker | Cumulative Incidence (95% CI) | Hazard Ratio (95% CI) | |
|---|---|---|---|
| MACE, Adjusted for Age and Sex | MACE, Multivariate Adjusted | ||
| Lipoprotein (a) | |||
| Tertile 1 | 33% (20‐45) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 | 44% (31‐58) | 1.6 (0.86‐3.0) | 1.5 (0.79‐2.9) |
| Tertile 3 | 56% (42‐69) | 1.9 (1.0‐3.5) | 1.6 (0.86‐3.1) |
| P for trend | 0.11 | 0.20 | |
| Interleukin‐10 | |||
| Tertile 1 | 40% (27‐54) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 | 44% (31‐58) | 1.2 (0.69‐3.3) | 1.2 (0.62‐2.3) |
| Tertile 3 | 48% (34‐62) | 1.7 (0.95‐3.2) | 1.7 (0.94‐3.2) |
| Detectable vs nondetectable | 1.4 (0.83‐2.4) | 1.4 (0.78‐2.4) | |
| C‐reactive protein | |||
| Tertile 1 | 36% (23‐49) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 | 39% (26‐52) | 0.98 (0.52‐1.8) | 0.93 (0.49‐1.8) |
| Tertile 3 | 60% (47‐74) | 1.7 (0.98‐3.1) | 1.9 (1.0‐3.5) |
| P for trend | 0.016 | 0.009 | |
Abbreviations: CI, confidence interval; MACE, major adverse cardiac events (death, nonfatal myocardial infarction, and repeat revascularization).
Multivariate adjusted: adjusted for age, sex, smoking, diabetes mellitus, hypertension, and hypercholesterolemia.
Figure 1.
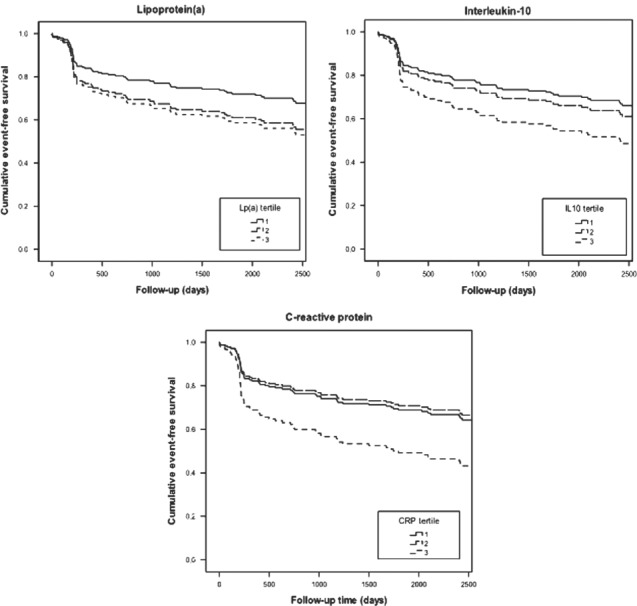
Multivariate‐adjusted event‐free survival until major adverse cardiac events. All curves are adjusted for age, sex, smoking, diabetes mellitus, hypertension, and hypercholesterolemia. Abbreviations: CRP, C‐reactive protein; IL 10, interleukin‐10; Lp (a), lipoprotein (a).
Discussion
In this prospective study, we examined the association between preprocedural biomarker levels and incident MACE in patients undergoing PCI with sirolimus‐eluting stenting. Lp(a) level was associated with MACE at 1‐year follow‐up. CRP level was associated with MACE at 8 years of follow‐up. IL‐10 showed a tendency toward an association with MACE.
Strengths of the current study include the collection of preprocedural biomarker levels, the availability of extensive long‐term follow‐up, and the measurement of multiple biomarkers. Furthermore, the study population consisted of complex patients, which generally suffer a high event rate as evidenced by the high incidence rate in this study. This enriched population may especially benefit from additional measures for risk stratification, enabling application or withholding of more aggressive treatment. However, at the same time, our focus on this selected patient group limits the generalizability of our findings to a broader PCI population.
Several other aspects of this study warrant consideration. Sample size was limited, which was primarily due to the relative novelty and complexity of the laboratory measurements at the time of study commencement in 2002. Nevertheless, our results on CRP, the marker that has been examined most elaborately in the past, confirm previous findings. As such, we expect that associations between other biomarkers and adverse events, if present, would be demonstrated in this cohort. We had a limited number of MACE available at 1‐year follow‐up (n = 39, 24%). This number of events suffices for the analyses adjusted for age and sex. However, we realize that use of a multivariate‐adjusted model for 1‐year follow‐upis not fully justified. In this regard, the choice to divide the sample into tertiles may also be debated. As such, we also performed the analyses using continuous biomarker levels to demonstrate trends. Nevertheless, using tertiles enables identification of a dose‐response relationship. Lp(a) was below the detection limit in 10% of the patients and IL‐10 in 30%. This is a commonly encountered challenge when measuring these types of biomarkers. An analysis based on tertiles circumvents this issue, and therefore we believe our findings are nevertheless informative.
Although we found an association of CRP, a sensitive marker of inflammation, with MACE at long‐term follow‐up of 6 years, the association was not significant at 1 year of follow‐up. Because the point‐estimate at 1‐year follow‐up was comparable to that at long‐term follow‐up, this is probably due to lack of statistical power. Levels of CRP have been elaborately investigated, and have been found to be associated with poorer cardiovascular outcomes in both healthy populations and patients with coronary artery disease.3 Furthermore, several large studies have shown that higher preprocedural CRP levels are related to a greater long‐term risk of adverse events after PCI.10, 11, 12 These studies examined outcomes for up to 2 years of follow‐up. Fewer data are available on long‐term follow‐up beyond 2 years. Gach et al examined the effect of hsCRP in stable patients undergoing PCI on adverse events during a mean follow‐up of nearly 80 months, and found that increase in CRP after PCI was more predictive of MACE than CRP level before PCI.13 However, this study was confined to 89 patients. Our results confirm association of preprocedural CRP levels with MACE during long‐term follow‐up, and expand these findings to a follow‐up of 8 years.
Lp(a) showed an association with occurrence of MACE at 1‐year follow‐up, but the association lost statistical significance at 8‐year follow‐up. Lp(a) is a lipoprotein that may induce either a prothrombotic/antifibrinolytic effect, as apolipoprotein(a) resembles plasminogen but has no fibrinolytic activity, or may accelerate atherosclerosis, because like low‐density lipoprotein, the Lp(a) particle is cholesterolrich, or both.14 Consequently, numerous studies have been performed on the role of Lp(a) in restenosis, rendering inconsistent results.15, 16, 17, 18, 19, 20, 21 Zairis et al investigated both in‐stent restenosis and incidence of MACE in 483 consecutive patients with either stable or unstable coronary syndromes undergoing PCI with stenting.22 Although they found no associations of Lp(a) with restenosis, they did find that high plasma levels of both CRP and Lp(a) were independently associated with MACE at long‐term follow‐up. They concluded that progression of atherosclerosis to a significant lesion in vessels not previously intervened on may play a significant role in the underlying pathophysiology as opposed to in‐stent restenosis. The results of 1‐year follow‐up in the current study are in accordance with these findings. Several other studies have demonstrated positive associations of Lp(a) with adverse events during long‐term follow‐up.23, 24 Our study confirms these results and provides additional information on very long‐term follow‐up, demonstrating loss of significance of the association in our (specific) patient population.
Il‐10 displayed a tendency toward a positive association with long‐term occurrence of MACE in the current study. IL‐10 downregulates inflammatory activation of monocytes and macrophages by transcriptional and post‐transcriptional inhibition of the entire range of proinflammatory cytokines.25 The largest prospective study on the prognostic value of IL‐10 levels in CAD examined 1090 patients with non–ST‐segment elevation acute coronary syndrome during 4 years of follow‐up for all‐cause mortality and nonfatal MI, and found that a high IL‐10 level is associated with better prognosis.26 Other prospective studies in patients with known CAD mostly had follow‐up time limited to several months and showed inconsistent results.27, 28, 29, 30 Data on IL‐10 levels and restenosis after stenting are scarce, and results are also conflicting.31, 32 Further elucidation of the mechanisms involved and of the role of IL‐10 herein is warranted to disclose the reasons behind these seemingly inconsistent findings.
Conclusion
In this prospective study of patients undergoing PCI, preprocedural Lp(a) level was associated with short‐term prognosis, and preprocedural CRP level was associated with long‐term prognosis. IL‐10 showed a tendency toward an association with long‐term prognosis. Large studies with extensive follow‐up and simultaneous measurement of multiple biomarkers are needed to provide further insight into the role of biomarkers in risk stratification of patients undergoing PCI.
References
- 1. Garg S, Serruys PW. Coronary stents: current status. J Am CollCardiol. 2010;56:S1–S42. [DOI] [PubMed] [Google Scholar]
- 2. Koenig W, Khuseyinova N. Lipoprotein‐associated and secretory phospholipase A2 in cardiovascular disease: the epidemiological evidence. Cardiovasc Drugs Ther. 2009;23:85–92. [DOI] [PubMed] [Google Scholar]
- 3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. [DOI] [PubMed] [Google Scholar]
- 4. Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am CollCardiol. 2006;48:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109:III15–III19. [DOI] [PubMed] [Google Scholar]
- 6. Girndt M, Kohler H. Interleukin‐10 (IL‐10): an update on its relevance for cardiovascular risk. Nephrol Dial Transplant. 2003; 18:1976–1979. [DOI] [PubMed] [Google Scholar]
- 7. Lemos PA, Serruys PW, van Domburg RT, et al. Unrestricted utilization of sirolimus‐eluting stents compared with conventional bare stent implantation in the “real world”: the Rapamycin‐Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation. 2004;109:190–195. [DOI] [PubMed] [Google Scholar]
- 8. Jones GT, van Rij AM, Cole J, et al. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin Chem. 2007;53:679–685. [DOI] [PubMed] [Google Scholar]
- 9. Marcovina SM, Albers JJ, Scanu AM, et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 10. Delhaye C, Maluenda G, Wakabayashi K, et al. Long‐term prognostic value of preprocedural C‐reactive protein after drug‐eluting stent implantation. Am J Cardiol. 2010;105:826–832. [DOI] [PubMed] [Google Scholar]
- 11. Iijima R, Byrne RA, Ndrepepa G, et al. Pre‐proceduralC‐reactive protein levels and clinical outcomes after percutaneous coronary interventions with and without abciximab: pooled analysis of four ISAR trials. Heart 2009;95:107–112. [DOI] [PubMed] [Google Scholar]
- 12. Razzouk L, Muntner P, Bansilal S, et al. C‐reactive protein predicts long‐term mortality independently of low‐density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J. 2009;158:277–283. [DOI] [PubMed] [Google Scholar]
- 13. Gach O, Legrand V, Biessaux Y, et al. Long‐term prognostic significance of high‐sensitivity C‐reactive protein before and after coronary angioplasty in patients with stable angina pectoris. Am J Cardiol. 2007;99:31–35. [DOI] [PubMed] [Google Scholar]
- 14. Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alaigh P, Hoffman CJ, Korlipara G, et al. Lipoprotein(a) level does not predict restenosis after percutaneous transluminal coronary angioplasty. ArteriosclerThrombVasc Biol. 1998;18:1281–1286. [DOI] [PubMed] [Google Scholar]
- 16. Cooke T, Sheahan R, Foley D, et al. Lipoprotein(a) in restenosis after percutaneous transluminal coronary angioplasty and coronary artery disease. Circulation. 1994;89:1593–1598. [DOI] [PubMed] [Google Scholar]
- 17. Desmarais RL, Sarembock IJ, Ayers CR, et al. Elevated serum lipoprotein(a) is a risk factor for clinical recurrence after coronary balloon angioplasty. Circulation. 1995;91:1403–1409. [DOI] [PubMed] [Google Scholar]
- 18. Gazzaruso C, Garzaniti A, Falcone C, et al. Restenosis after intracoronary stent placement: can apolipoprotein(a) polymorphism play a role? Int J Cardiol. 2003;87:91–98. [DOI] [PubMed] [Google Scholar]
- 19. Miyata M, Biro S, Arima S, et al. High serum concentration of lipoprotein(a) is a risk factor for restenosis after percutaneous transluminal coronary angioplasty in Japanese patients with single‐vessel disease. Am Heart J. 1996;132:269–273. [DOI] [PubMed] [Google Scholar]
- 20. Ribichini F, Steffenino G, Dellavalle A, et al. Plasma lipoprotein(a) is not a predictor for restenosis after elective high‐pressure coronary stenting. Circulation. 1998;98:1172–1177. [DOI] [PubMed] [Google Scholar]
- 21. Wehinger A, Kastrati A, Elezi S, et al. Lipoprotein(a) and coronary thrombosis and restenosis after stent placement. J Am CollCardiol. 1999;33:1005–1012. [DOI] [PubMed] [Google Scholar]
- 22. Zairis MN, Ambrose JA, Manousakis SJ, et al. The impact of plasma levels of C‐reactive protein, lipoprotein (a) and homocysteine on the long‐term prognosis after successful coronary stenting: The Global Evaluation of New Events and Restenosis After Stent Implantation Study. J Am CollCardiol.2002;40: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 23. Igarashi Y, Aizawa Y, Satoh T, et al. Predictors of adverse long‐term outcome in acute myocardial infarction patients undergoing primary percutaneous transluminal coronary angioplasty: with special reference to the admission concentration of lipoprotein (a). Circ J. 2003;67:605–611. [DOI] [PubMed] [Google Scholar]
- 24. Rahel BM, Visseren FL, Suttorp MJ, et al. Preprocedural serum levels of acute‐phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res. 2003;60: 136–140. [DOI] [PubMed] [Google Scholar]
- 25. Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin‐10 in atherosclerosis. Circ Res. 1999;85:e17–e24. [DOI] [PubMed] [Google Scholar]
- 26. Oemrawsingh RM, Lenderink T, Akkerhuis KM, et al. Multimarker risk model containing troponin‐T, interleukin 10, myeloperoxidase and placental growth factor predicts long‐term cardiovascular risk after non‐ST‐segment elevation acute coronary syndrome. Heart. 2011;97:1061–1066. [DOI] [PubMed] [Google Scholar]
- 27. Anguera I, Miranda‐Guardiola F, Bosch X, et al. Elevation of serum levels of the anti‐inflammatory cytokine interleukin‐10 and decreased risk of coronary events in patients with unstable angina. Am Heart J. 2002;144:811–817. [DOI] [PubMed] [Google Scholar]
- 28. Chang LT, Yuen CM, Sun CK, et al. Role of stromal cell‐derived factor‐1alpha, level and value of circulating interleukin‐10 and endothelial progenitor cells in patients with acute myocardial infarction undergoing primary coronary angioplasty. Circ J 2009;73:1097–1104. [DOI] [PubMed] [Google Scholar]
- 29. Heeschen C, Dimmeler S, Hamm CW, et al. Serum level of the antiinflammatory cytokine interleukin‐10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003;107:2109–2114. [DOI] [PubMed] [Google Scholar]
- 30. Yip HK, Youssef AA, Chang LT, et al. Association of interleukin‐10 level with increased 30‐day mortality in patients with ST‐segment elevation acute myocardial infarction undergoing primary coronary intervention. Circ J. 2007;71:1086–1091. [DOI] [PubMed] [Google Scholar]
- 31. Ezhov MV, Sumarokov AB, RaimbekovaIR, Masenko VP, Naumov VG. Interleukin 6 but not interleukin 10 is associated with restenosis after coronary stenting. Atherosclerosis. 2003;169:193–194. [DOI] [PubMed] [Google Scholar]
- 32. Zurakowski A, Wojakowski W, Dzielski T, et al. Plasma levels of C‐reactive protein and interleukin‐10 predict late coronary in‐stent restenosis 6 months after elective stenting. Kardiol Pol. 2009;67:623–630. [PubMed] [Google Scholar]


