Abstract
Background:
In heart failure with preserved ejection fraction (HFPEF), physiological abnormalities are not solely restricted to diastolic function. Because the tissue Doppler imaging (TDI)‐derived myocardial performance index (MPI) offers the advantage of recording systolic and diastolic tissue velocity simultaneously in the same cardiac cycle, this study aimed to determine whether TDI‐MPI is an informative index for assessing HFPEF, compared with conventional echo parameters.
Hypothesis:
In patients with HFPEF, TDI‐MPI would be an independent predictor for adverse cardiac events.
Methods:
Among 408 patients who had diastolic dysfunction without heart failure (HF) or HFPEF, cardiac function was evaluated by mitral flow (MF) or TDI‐MPI. During the median follow‐up of 32 months, clinical outcomes, which were defined as the composite of cardiovascular death and admission for HF, were assessed.
Results:
Mean MF and TDI‐MPI were significantly greater in the HFPEF group. TDI‐MPI rather than MF had a significant correlation with N‐terminal pro‐brain natriuretic peptide level. The area under the receiver operating characteristic curve of TDI‐MPI for the detection of HFPEF was 0.86. With regard to clinical outcomes, 31 events were identified during follow‐up periods. On a multivariate analysis, TDI‐MPI >0.66 was the best prognostic predictor of events and provided incremental predictive value.
Conclusions:
Compared to MF‐MPI, TDI‐MPI may be a more useful parameter for the evaluation of patients with HFPEF. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
The conventional mitral flow (MF)‐derived myocardial performance index (MPI) by pulsed‐wave Doppler has been considered an independent marker of left ventricular (LV) function in patients with amyloidosis, diabetes, myocardial infarction, hypertrophic cardiomyopathy, and heart failure (HF).1, 2, 3, 4, 5 However, pulsed‐wave Doppler echocardiography requires measurement of ejection time (ET) and isovolumic contraction/relaxation time (ICT/IRT) in different cardiac cycles, whereas tissue Doppler imaging (TDI)‐derived MPI by pulsed tissue Doppler annular velocity has the advantage of simultaneous measurement in a single cardiac cycle.
Therefore, we sought to evaluate the usefulness of TDI‐MPI compared to conventional echoparameters, including MF‐MPI, for the assessment of diastolic HF, also known as HF with preserved ejection fraction (HFPEF).
Methods
Study Patients
Between June 2006 and July 2007, we evaluated 248 patients visiting the outpatient clinic at a single center in a tertiary university hospital. At the time of enrollment, all patients who were clinically stable underwent a transthoracic echocardiography. Patients were eligible for inclusion if they presented with the following criteria: age >18 years and evidence of LV diastolic dysfunction. For diastolic dysfunction, patients who had septal e′ velocity <8 cm/s and a left atrial volume index (LAVI) >34 mL/m2 were included.6, 7 Among the patients with LV diastolic dysfunction, the diagnosis of HFPEF was made for patients satisfying 3 obligatory conditions proposed in the guidelines of the European Society of Cardiology: (1) the presence of signs or symptoms of HF based on the Framingham scores8; (2) the presence of normal or mildly abnormal LV systolic function (EF >50%); and (3) evidence of LV diastolic dysfunction.9, 10 Regarding HFPEF, because the values of E/e′ ratio and N‐terminal pro‐brain natriuretic peptide (NT‐ProBNP) can be easily obtained and are the most frequently used in clinical practice, the E/e′ ratio was used for diagnosing HFPEF in this study. A ratio >15 has been considered diagnostic evidence of the presence of diastolic dysfunction. In addition, a NT‐ProBNP values >220 pg/mL was also used for diagnosing HFPEF. Thus, patients who had an E/e′ ratio >15, NT‐ProBNP >220 pg/mL, or both were considered to have HFPEF. Individuals were excluded if they currently had symptoms or signs indicating acute coronary artery disease (CAD) requiring revascularization, myocardial infarction within the previous 4 weeks, a non‐sinus rhythm, bundle branch block, significant valvular disease (moderate or severe grade), chronic obstructive pulmonary disease, moderate or advanced renal disease, or echocardiographic images that were technically inadequate for visualization. After excluding 20 patients, 228 patients (mean age, 68 ± 11 years) were included. In addition, a control group of 180 patients referred for a clinically indicated echocardiography, who showed diastolic dysfunction without overt HF, were also included in this study. The protocol was approved by the institutional review board. All participants were informed about the study, and each gave written consent to participate.
Echocardiographic Examination
Echocardiographic studies were performed using a GE Vivid 7 digital ultrasound system with EchoPAC software version 6.1 (GE Vingmed Ultrasound, Horten, Norway). LV diastolic filling patterns were assessed by the mitral inflow pulsed‐wave Doppler velocity and the following parameters were obtained: peak early (E) and late (A) diastolic transmitral velocity, deceleration time, ICT, IRT, and ET. With these parameters, conventional MF‐MPI was obtained by the equation (ICT+IRT)/ET. Pulsed TDI was performed and mitral annular peak systolic (s′) velocity as well as early (e′) and late (a′) diastolic velocities were also obtained with the sample volume positioned at the septal annulus on the apical 4‐chamber view. Regarding the TDI‐derived index, TDI‐MPI was calculated as (a − b)/b where a is the time interval from the end to the onset of the mitral annular velocity pattern and b is the duration of s′ wave (Figure 1).
Figure 1.

Tissue Doppler imaging‐derived myocardial performance index (TDI‐MPI) at the mitral septal annulus. TDI‐MPI is defined as (a − b)/b, where a is the time interval from the end of late mitral annular diastolic wave (a′) to the onset of early mitral annular diastolic wave (e′), and b is the time interval between the start and the end of mitral annular systolic wave (s′) of TDI. Abbreviations: ET, ejection time; ICT, isovolumic contraction time; IRT, isovolumic relaxation time.
Study Follow‐Up
With regard to the adverse clinical outcomes associated with HFPEF or diastolic dysfunction without overt HF, all patients were followed up every 3 months. The clinical outcomes of our study were cardiovascular death and worsening HF requiring hospitalization.
Statistics
The data analyses were performed with the Statistical Package for Social Science software (SPSS for Windows 12.0; SPSS Inc., Chicago, IL). All values are presented as the mean ± standard deviation for continuous variables and frequencies for discrete variables. Categorical variables were analyzed using a χ 2 test, and continuous variables were analyzed by the Student t test. Receiver‐operating characteristic (ROC) curves and the area under the curves (AUC) were obtained to compare the predictive value of echoparameters. Univariate and multivariate Cox proportional regression analysis was performed and cumulative survival curves were constituted by the Kaplan‐Meier method. The log‐rank test was used to evaluate the event‐free survival according to the cut‐off value of TDI‐MPI. To determine the incremental prognostic value of TDI‐MPI over clinical data, E/e′ ratio, and NT‐ProBNP level, a sequential Cox model analysis was performed and global χ 2 was calculated. P values were 2‐sided, and a P value <0.05 was considered statistically significant.
Results
Clinical Characteristics of Study Subjects
The clinical characteristics of entire study population are shown in Table 1. Patients with HFPEF were significantly older and more often had CAD, a previous hospital admission for HF, and a history of stroke. Additionally they were more likely to have hypertension, whereas the prevalence of diabetes was similar between the groups. Although no difference in hemoglobin was observed between the groups, patients with HFPEF showed higher NT‐ProBNP level.
Table 1.
Baseline Demographic and Clinical Characteristics of the Entire Study Group
| Parameters | Silent DD (N = 180) | HFPEF (N = 228) | P Value |
|---|---|---|---|
| Age, y | 62.3 ± 12.2 | 68.3 ± 11.4 | <0.001 |
| Men, n (%) | 75 (41.7) | 89 (39.0) | 0.612 |
| Height, cm | 158.4 ± 14.3 | 157.8 ± 9.7 | 0.595 |
| Weight, kg | 61.1 ± 10.3 | 60.2 ± 11.2 | 0.414 |
| BSA, m2 | 1.53 ± 0.42 | 1.52 ± 0.40 | 0.801 |
| NYHA class | <0.001 | ||
| I/II, n | 85/95 | 21/86 | |
| III/IV, n | 0/0 | 118/3 | |
| Hypertension, n (%) | 73 (40.6) | 114 (50.0) | 0.058 |
| Diabetes, n (%) | 28 (15.6) | 47 (20.6) | 0.201 |
| CAD, n (%) | 17 (9.4) | 49 (21.5) | 0.001 |
| Admission of CHF, n (%) | 0 | 9 (3.9) | 0.006 |
| Stroke, n (%) | 0 | 8 (3.5) | 0.010 |
| Cardiac events | <0.001 | ||
| Cardiac death, n | 0 | 2 | |
| Admission of CHF, n | 3 | 26 | |
| Medications | |||
| ACEi/ARBs, n (%) | 41 (22.8) | 94 (41.2) | <0.001 |
| Calcium blockers, n (%) | 34 (18.9) | 62 (27.2) | 0.060 |
| Beta blockers, n (%) | 43 (23.9) | 85 (37.3) | 0.004 |
| Diuretics, n (%) | 29 (16.1) | 85 (37.3) | <0.001 |
| Hemodynamics | |||
| Systolic BP, mm Hg | 122.7 ± 17.6 | 123.2 ± 19.0 | 0.795 |
| Diastolic BP, mm Hg | 73.5 ± 10.5 | 72.0 ± 12.0 | 0.173 |
| Heart rate, beats/min | 70.0 ± 12.8 | 69.0 ± 14.6 | 0.458 |
| Laboratory results | |||
| Hemoglobin, g/dL | 13.2 ± 1.6 | 12.9 ± 6.8 | 0.604 |
| BUN, mg/dL | 16.4 ± 6.78 | 20.3 ± 12.1 | <0.001 |
| Creatinine, mg/dL | 1.09 ± 1.09 | 1.17 ± 0.72 | 0.402 |
| LogNT‐ProBNP | 4.01 ± 1.08 | 6.22 ± 2.05 | <0.001 |
Abbreviations: ACEi/ARBs, angiotensin converting enzyme inhibitors/ angiotensin receptor blockers; BP, blood pressure; BSA, body surface area; BUN, blood urea nitrogen; CAD, coronary artery disease; CHF, congestive heart failure; DD, diastolic dysfunction; HFPEF, heart failure with preserved ejection fraction; NT‐ProBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Data are expressed as mean ± SD or as a number.
Echocardiographic Characteristics of Study Subjects
In Table 2, the HFPEF group had a significantly lower LVEF, and also LV dimensions, LAVI, and LV mass index (LVMI) were significantly higher. TDI‐derived s′, e′ and a′ were lower in the HFPEF group. Consequently, the E/e′ ratio was significantly higher in the HFPEF group.
Table 2.
Comparison of Echocardiographic Measurements of the Entire Study Group
| Parameters | Silent DD (N = 180) | HFPEF (N = 228) | P Value |
|---|---|---|---|
| LVEDD, mm | 48.3 ± 4.8 | 49.2 ± 5.0 | 0.034 |
| LVESD, mm | 30.1 ± 4.1 | 32.1 ± 5.2 | <0.001 |
| LVEF, % | 66.1 ± 5.1 | 62.3 ± 5.3 | <0.001 |
| LV mass index, gm/m2 | 91.2 ± 11.9 | 106.1 ± 10.4 | <0.001 |
| LA volume index, mL/m2 | 38.7 ± 3.57 | 48.5 ± 7.53 | <0.001 |
| IVSTd, mm | 8.9 ± 1.4 | 9.9 ± 2.2 | <0.001 |
| PWTd, mm | 8.7 ± 1.2 | 9.3 ± 1.1 | <0.001 |
| Mitral inflow | |||
| DT, ms | 229.5 ± 40.1 | 241.1 ± 34.3 | 0.023 |
| E‐inflow, m/s | 0.70 ± 0.21 | 0.75 ± 0.16 | 0.035 |
| A‐inflow, m/s | 0.79 ± 0.11 | 0.88 ± 0.15 | <0.001 |
| Tissue Doppler velocity | |||
| s′, cm/s | 7.58 ± 1.74 | 6.30 ± 1.60 | <0.001 |
| e′, cm/s | 5.89 ± 1.34 | 4.37 ± 1.28 | <0.001 |
| a′, cm/s | 8.90 ± 2.07 | 7.90 ± 2.02 | <0.001 |
| E/e′ ratio | 11.08 ± 2.62 | 17.46 ± 5.41 | <0.001 |
| Myocardial performance index | |||
| TDI‐ICT, ms | 56.3 ± 14.6 | 67.2 ± 12.2 | <0.001 |
| TDI‐IRT, ms | 68.0 ± 20.7 | 109.9 ± 25.5 | <0.001 |
| TDI‐ET, ms | 295.8 ± 34.0 | 283.4 ± 33.3 | 0.003 |
| TDI‐MPI | 0.42 ± 0.19 | 0.63 ± 0.12 | <0.001 |
| MF‐ICT, ms | 52.1 ± 12.8 | 51.9 ± 12.4 | 0.343 |
| MF‐IRT, ms | 110.4 ± 21.1 | 129.0 ± 23.2 | <0.001 |
| MF‐ET, ms | 284.9 ± 37.0 | 283.1 ± 34.1 | 0.815 |
| MF‐MPI | 0.57 ± 0.25 | 0.63 ± 0.23 | 0.018 |
Abbreviations: a′, late diastolic mitral annular velocity; DD, diastolic dysfunction; DT, deceleration time; e′, early diastolic mitral annular velocity; E/e′, the ratio of peak early diastolic mitral inflow to mitral annular velocity; ET, ejection time; HFPEF, heart failure with preserved ejection fraction; ICT, isovolumic contraction time; IRT, isovolumic relaxation time; IVSTd, interventricular septal thickness at end‐diastole; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; MF, mitral flow, MPI, myocardial performance index; PWTd, posterior wall thickness at end‐diastole; s′, systolic mitral annular velocity; TDI, tissue Doppler imaging.
Data are expressed as mean ± SD.
In patients with HFPEF, TDI‐ICT and TDI‐IRT were found to be significantly longer, whereas TDI‐ET was also shorter than those with diastolic dysfunction. Accordingly, TDI‐MPI was shown to be higher. As for MF, there were no significant differences in MF‐ICT and ET between the groups. However, MF‐MPI, including MF‐IRT, was higher in the HFPEF group.
Analysis of Echocardiographic Parameters
Significant correlations were found between NT‐ProBNP level and LVMI, LAVI, tissue Doppler velocities, E/e′ ratio, TDI‐IRT/MPI, and MF‐ICT (all parameters, P<0.001). There were significant but weak to modest correlations between echocardiographic indices and NT‐ProBNP level (r = 0.195 to 0.372), whereas a significant correlation was not observed between MF‐MPI and NT‐ProBNP level. Among these parameters, TDI‐MPI showed the strongest correlation with NT‐ProBNP level (r = 0.372, P<0.001). In the ROC analysis for identification of HFPEF, the AUCs were 0.863 (95% confidence interval [CI]: 0.826–0.895) for TDI‐MPI, 0.847 (95% CI: 0.808–0.886) for E/e′ ratio, 0.819 (95% CI: 0.778–0.856) for NT‐ProBNP, and 0.548 (95% CI: 0.485–0.612) for MF‐MPI. The AUC of TDI‐MPI was the greatest among that of other parameters; however, no statistical differences were noted between the AUCs of TDI‐MPI, NT‐ProBNP, and E/e′ ratio.
Cardiovascular Outcomes of Entire Study Group
During the median follow‐up period of 32 months, there were 31 clinical cardiac adverse events (2 cardiac deaths and 29 admissions for HF). Kaplan‐Meier survival curves were constructed using the cut‐off value for TDI‐MPI of 0.66, which was determined by the ROC curve built for the optimal value for adverse clinical events. This showed the significant outcome risk increased with increasing TDI‐MPI (Figure 2A). In univariate Cox analysis, clinical risk factors, such as age, diabetes, diuretics, CAD, HF, blood urea nitrogen, and NT‐ProBNP level, and echo parameters such as TDI‐MPI > 0.66, tissue Doppler velocities, E/e′ ratio, LVMI, LAVI, and EF, were significant predictors, whereas New York Heart Association (NYHA) class, hypertension, and MF‐MPI were not (Table 3). Multivariate Cox regression analysis showed that a TDI‐MPI > 0.66 in addition to diabetes and NT‐ProBNP level were associated with clinical events even after adjustment for confounding factors. Patients with a TDI‐MPI > 0.66 had nearly 3‐fold higher risk of clinical outcomes (HR 2.9, 95% CI: 1.0–8.3; P = 0.030). Furthermore, TDI‐MPI provided incremental prognostic value to the baseline clinical data, E/e′ ratio, and NT‐ProBNP level for the prediction of clinical outcomes, although the addition of E/e′ ratio to the clinical data did not provide incremental prognostic information (Figure 2B).
Figure 2.
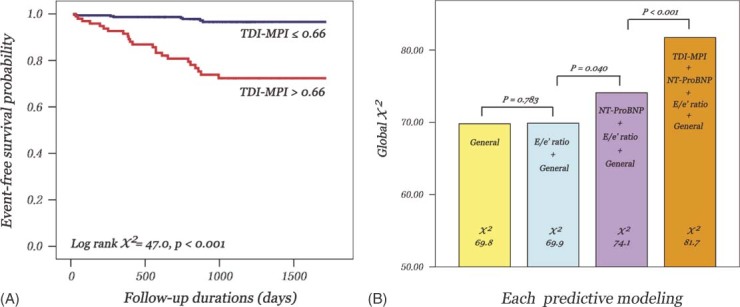
(A) Kaplan‐Meier survival curves of entire study population showing freedom from cardiac outcomes according to tissue Doppler imaging‐derived myocardial performance index (TDI‐MPI). (B) Incremental prognostic value of TDI‐MPI for the prediction of cardiac events. Addition of TDI‐MPI to clinical data, E/e′ ratio and N‐terminal pro‐brain natriuretic peptide (NT‐ProBNP) level provides significant incremental information. Abbreviation: E/e′, the ratio of peak early diastolic mitral inflow to mitral annular velocity.
Table 3.
Univariate and Multivariate Cox Regression Analysis for the Prediction of Clinical Outcomes in the Patients of the Entire Study Group
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age | 1.05 (1.01–1.08) | 0.010 | 1.01 (0.94–1.04) | 0.919 |
| CAD | 6.70 (3.29–13.62) | <0.001 | 2.27 (0.97–5.30) | 0.058 |
| Heart failure | 3.01 (1.15–7.92) | 0.025 | 1.20 (0.38–3.74) | 0.757 |
| Diabetes | 3.61 (1.78–7.32) | <0.001 | 2.91 (1.25–6.82) | 0.014 |
| Log NT‐ProBNP | 1.70 (1.10–2.29) | <0.001 | 1.45 (1.05–2.00) | 0.025 |
| BUN | 1.03 (1.00–1.05) | 0.038 | 0.99 (0.98–1.35) | 0.543 |
| Diuretics | 3.11 (1.53–6.30) | 0.002 | 0.81 (0.33–1.99) | 0.640 |
| Ejection fraction | 0.90 (0.86–0.94) | <0.001 | 0.95 (0.88–1.01) | 0.115 |
| LVESD | 2.15 (1.15–4.03) | 0.016 | 0.94 (0.85–1.04) | 0.214 |
| LAVI | 1.02 (1.01–1.03) | <0.001 | 1.04 (0.97–1.12) | 0.229 |
| IVSTd | 1.27 (1.11–1.44) | <0.001 | 1.19 (0.91–1.56) | 0.214 |
| PWTd | 1.24 (1.00–1.54) | 0.048 | 0.74 (0.52–1.04) | 0.085 |
| LVMI | 1.01 (1.01–1.02) | <0.001 | 1.01 (0.97–1.13) | 0.235 |
| s′ | 0.53 (0.40–0.69) | <0.001 | 0.72 (0.47–1.12) | 0.134 |
| e′ | 0.61 (0.46–0.80) | <0.001 | 1.10 (0.72–1.67) | 0.666 |
| a′ | 0.71 (0.59–0.86) | <0.001 | 1.19 (0.91–1.57) | 0.201 |
| E/e′ | 1.15 (1.01–1.23) | 0.032 | 0.99 (0.91–1.07) | 0.763 |
| TDI‐MPI > 0.66 | 10.1 (4.52–22.6) | <0.001 | 2.90 (1.01–8.28) | 0.030 |
Abbreviations: a′, late diastolic mitral annular velocity; BUN, blood urea nitrogen; CAD, coronary artery disease; CI, confidence interval; e′, early diastolic mitral annular velocity; E/e′, the ratio of peak early diastolic mitral inflow to mitral annular velocity; HR, hazard ratio; IVSTd, interventricular septal thickness at end‐diastole; LAVI, left atrial volume index; LVESD, left ventricular end‐systolic dimension; LVMI, left ventricular mass index; NT‐ProBNP, N‐terminal pro‐brain natriuretic peptide; PWTd, posterior wall thickness at end‐diastole; s′, systolic mitral annular velocity; TDI‐MPI, tissue Doppler imaging‐myocardial performance index.
Discussion
The characteristic findings of HFPEF have been reported with respect to structural changes such as LV stiffness and hypertrophy.9, 11, 12, 13 Comparing the ROC curves, we could not find any discriminative power of mitral inflow velocities between both groups (data not shown), despite the fact that mitral inflow Doppler studies have been the most widely used methods to reflect diastolic function. The failure of mitral inflow Doppler study to discriminate HFPEF may indicate that mitral inflow per se would not exactly reflect or not be sufficient to explain the features of HFPEF. This is in concordance with previous observations that mitral inflow provided little incremental information for the diagnosis of HFPEF.14 Contrary to the mitral inflow data, TDI velocity may be a more sensitive method to evaluate LV function. The ability of TDI to quantify myocardial velocity has been attractive in the context of HFPEF to detect subtle systolic dysfunction associated with LV hypertrophy or stiffness.15, 16 The reduced tissue velocities noted in this study were also consistent with the results of previous studies showing that persistently decreased systolic function was observed in spite of normal LVEF.15, 17 Moreover, the TDI‐derived index proved to be useful for HFPEF, in which high LV‐filling pressure was revealed by E/e′ ratio.18 Together with these findings, our results also identified TDI‐MPI to have the strongest correlation with NT‐ProBNP level, which has been regarded as a useful parameter in the evaluation of systolic or diastolic HF.10, 19., 20
Cardiac dysfunction may be associated with the inappropriate coupling of the time intervals in both systolic and diastolic phases. In healthy subjects, the time interval between the 2 methods is equal or similar.21 These time differences are usually affected by myocardial ischemia or asynchrony of contraction. Isovolumic times are reported to be the most responsible factors for the differences between 2 methods.21 Therefore, we speculated that the assessment of the differences of cardiac interval time between 2 methods may shed light on the pathophysiology of HFPEF. In our data, the discrimination ability of TDI‐MPI for HFPEF would be attributed to its sensitivity to alterations in LV stiffness or LV end‐diastolic pressure (LVEDP). This may be explained by the fact that IRT or ICT measured with TDI coincides with myocardial movement and may account for the altered LV function due to LVH or HF.21 Accordingly, LV stiffness or impairment of relaxation contribute to the increased sum of IRT and ICT as well as the shortened ET (or the prolongation of IRT) of LV possibly leading to a relative increase in TDI‐MPI.
In CAD, IRT interval, which is the main component of MPI, is usually shorter with TDI,21 whereas IRT as well as ICT tend to increase in diastolic HF and even more in the systolic HF.22 Generally, impaired relaxation without elevated LVEDP showed prolonged MF‐IRT, whereas increased LV filling pressure with overt HF causes shortening MF‐IRT. However, TDI‐IRT in impaired relaxation without elevated LVEDP is not known clearly. Similar observations can be found in other study of diastolic HF, where TDI‐IRT was shorter than MF‐IRT in the patients with LV diastolic dysfunction with preserved EF.23 Particularly, in that study, TDI‐IRT to MF‐IRT ratio was believed to be a positive correlation with LVEDP. Considering these results, as well as our own, we speculated that longer TDI‐IRT than MF might be an early marker of impaired relaxation without elevated LVEDP.
Another principal finding is that TDI‐MPI could predict cardiovascular adverse outcomes with reliability similar to E/e′ ratio and NT‐ProBNP level. TDI‐MPI has been reported to have an independent prognostic value in various cardiomyopathies or to have significant validity with conventional MF‐MPI.1, 2, 3, 24 Additionally, it provided incremental value to clinical data and traditional echoparameters for the prediction of cardiac events, thus allowing for the risk stratification of high‐risk patients.
Limitation
There are some limitations worth noting in our findings when considering the results. The main limitation is that the TDI measurements were performed only at the septal part of the mitral annulus, and thus only 1 septal TDI would not be sufficient to reflect the myocardial function in patients with septal infarction. Another potential limitation is the low cardiac death or events rate in our patients. This may be explained by several variables. Two thirds of patients showed diastolic dysfunction without overt HF symptoms or HFPEF with NYHA I‐II class, and only 50% of patients had hypertension, and 20% had CAD in the present study. These figures are lower than values reported in other previous studies.25, 26 Furthermore, specific criteria of a preserved EF were defined as EF with 50% or higher, whereas an LVEF of 40% or more has been used in most articles.25, 26, 27 In particular, patients with atrial fibrillation or significant renal and valvular dysfunction were excluded from the present study. Therefore, differences in baseline study populations and conductance from a single, tertiary, university hospital center are a possible explanation for the low incidence of clinical events rate.
Conclusion
TDI‐MPI would be helpful in assessing patients with HFPEF, and becomes a significant predictor of adverse clinical outcomes, providing incremental value over E/e′ ratio, NT‐ProBNP level, and other clinical variables.
References
- 1. Tei C, Dujardin KS, Hodge DO, et al. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–664. [DOI] [PubMed] [Google Scholar]
- 2. Ascione L, De Michele M, Accadia M, et al. Myocardial global performance index as a predictor of in‐hospital cardiac events in patients with first myocardial infarction. J Am Soc Echocardiogr. 2003;16:1019–1023. [DOI] [PubMed] [Google Scholar]
- 3. Arnlov J, Ingelsson E, Riserus U, et al. Myocardial performance index, a Doppler‐derived index of global left ventricular function, predicts congestive heart failure in elderly men. Eur Heart J. 2004; 25:2220–2225. [DOI] [PubMed] [Google Scholar]
- 4. Orem C, Kucukosmanoglu M, Hacihasanoglu A, et al. Association of Doppler‐derived myocardial performance index with albuminuria in patients with diabetes. J Am Soc Echocardiogr. 2004;17:1185–1190. [DOI] [PubMed] [Google Scholar]
- 5. Okawa M, Kitaoka H, Matsumura Y, et al. Functional assessment by myocardial performance index (Tei index) correlates with plasma brain natriuretic peptide concentration in patients with hypertrophic cardiomyopathy. Circ J. 2005;69:951–957. [DOI] [PubMed] [Google Scholar]
- 6. Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 7. Rakowski H, Appleton C, Chan KL, et al. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr. 1996;9:736–760. [DOI] [PubMed] [Google Scholar]
- 8. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 9. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 10. Kindermann M. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2686; author reply 2687. [DOI] [PubMed] [Google Scholar]
- 11. Borlaug BA, Lam CS, Roger VL, et al. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe T, Iwai‐Takano M, Oikawa M, et al. Optimal noninvasive assessment of diastolic heart failure in patients with atrial fibrillation: comparison of tissue doppler echocardiography, left atrium size, and brain natriuretic peptide. J Am Soc Echocardiogr. 2008;21:689–696. [DOI] [PubMed] [Google Scholar]
- 14. Emery WT, Jadavji I, Choy JB, et al. Investigating the European Society of Cardiology Diastology Guidelines in a practical scenario. Eur J Echocardiogr. 2008;9:685–691. [DOI] [PubMed] [Google Scholar]
- 15. Surucu H, Tatli E, Degirmenci A, et al. Subtle systolic dysfunction may be associated with the tendency to develop diastolic heart failure in patients with preserved left ventricular ejection fraction. Echocardiography. 2009;26:365–370. [DOI] [PubMed] [Google Scholar]
- 16. Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler‐conductance catheterization study. Circulation. 2007;116:637–647. [DOI] [PubMed] [Google Scholar]
- 17. Nishikage T, Nakai H, Lang RM, et al. Subclinical left ventricular longitudinal systolic dysfunction in hypertension with no evidence of heart failure. Circ J. 2008;72:189–194. [DOI] [PubMed] [Google Scholar]
- 18. King G, Foley JB, Royse CF, et al. Myocardial stiffness and the timing difference between tissue Doppler imaging Ea and peak mitral valve opening can distinguish physiological hypertrophy in athletes from hypertrophic cardiomyopathy. Eur J Echocardiogr. 2006;7:423–429. [DOI] [PubMed] [Google Scholar]
- 19. Executive summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:10–38. [DOI] [PubMed] [Google Scholar]
- 20. Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–e82. [DOI] [PubMed] [Google Scholar]
- 21. Rojo EC, Rodrigo JL, Perez de Isla L, et al. Disagreement between tissue Doppler imaging and conventional pulsed wave Doppler in the measurement of myocardial performance index. Eur J Echocardiogr. 2006;7:356–364. [DOI] [PubMed] [Google Scholar]
- 22. Jarnert C, Mejhert M, Ring M, et al. Doppler tissue imaging in congestive heart failure patients due to diastolic or systolic dysfunction: a comparison with Doppler echocardiography and the atrio‐ventricular plane displacement technique. Eur J Heart Fail. 2000;2:151–160. [DOI] [PubMed] [Google Scholar]
- 23. Rudko R, Przewlocki T, Pasowicz M, et al. IVRT′/IVRT index is a useful tool for detection of elevated left ventricular filling pressure in patients with preserved ejection fraction. Echocardiography. 2008;25:473–481. [DOI] [PubMed] [Google Scholar]
- 24. Dujardin KS, Tei C, Yeo TC, et al. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic‐dilated cardiomyopathy. Am J Cardiol. 1998;82:1071–1076. [DOI] [PubMed] [Google Scholar]
- 25. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 26. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 27. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. [DOI] [PubMed] [Google Scholar]


