Abstract
Heart failure is a frequent complication of myocardial infarction. Several factors, such as recurrent myocardial ischemia, infarct size, ventricular remodeling, stunned myocardium, mechanical complications, and hibernating myocardium influence the appearance of left ventricular systolic dysfunction after myocardial infarction. Importantly, its presence increases the risk of death by at least 3‐ to 4‐fold. The knowledge of the mechanisms and clinical features are essential for the diagnosis and treatment of left ventricular dysfunction and heart failure after myocardial infarction. Therefore, this review will focus on the clinical implications and treatment of heart failure after myocardial infarction. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Heart failure (HF) is a frequent complication of myocardial infarction (MI). Several factors, such as recurrent myocardial ischemia, infarct size, ventricular remodeling, stunned myocardium, mechanical complications, and hibernating myocardium influence the appearance of left ventricular systolic dysfunction with or without clinical HF after MI.1, 2, 3
Of note, the relevance of each factor responsible for HF after MI depends on the time to the establishment of cardiac dysfunction following coronary occlusion (Figure 1). Patients with signs of HF on admission to the hospital are usually elderly, with recurrent ischemia and diabetes. In this setting, ventricular function is related to preexistent comorbidities that reduce tolerance to ischemic injury.1 On the other hand, development of HF during one's hospital stay is usually related to infarct size, mechanical complications, or myocardial stunning. Finally, some patients will develop HF only after being discharged from the hospital. In this setting, myocyte loss, hibernating myocardium, and ventricular remodeling are the principal causes of heart failure. Among these factors, ventricular remodeling is the most important.1
Figure 1.
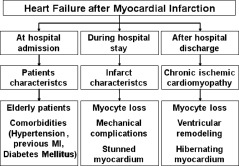
Mechanisms of ventricular dysfunction after myocardial infarction. MI, myocardial infarction.
The knowledge of these mechanisms and clinical features are essential for the diagnosis and treatment of left ventricular dysfunction and HF after MI. As a result, this review will focus on the clinical implications and treatment of heart failure after myocardial infarction.
Clinical Implications of Heart Failure After Myocardial Infarction
Epidemiological studies have reported that the rate of signs and symptoms of heart failure after MI is approximately 25%. Importantly, this finding appears to be in agreement with the registries of several clinical trials. In addition, approximately 40% of myocardial infarctions are accompanied by left ventricular systolic dysfunction. Therefore, the available data suggest that HF after MI is a very frequent event.4
Considering the kind of cardiac dysfunction following MI, most patients present systolic dysfunction. Recent meta‐analysis showed that restrictive mitral filling pattern, the most severe form of diastolic dysfunction, was presented in approximately 10% of the patients with preserved ejection fraction. In addition, restrictive pattern was associated with poor outcome.5 However, the true prevalence and relevance of diastolic dysfunction after MI remains to be elucidated. Another important issue is that the consequences of cardiac dysfunction after MI are well established, and its presence increases the risk of death by at least 3‐ to 4‐fold.6 Compared with patients without heart failure and left ventricular systolic dysfunction after myocardial infarction, patients who have heart failure and left ventricular systolic dysfunction are at higher risk for adverse outcomes, including cardiac rupture, cardiac arrest, stroke, longer hospitalizations, ventricular arrhythmias, recurrent myocardial infarction, and death, including sudden death.4
Assessment of Heart Failure After Myocardial Infarction
The priority is the identification of the mechanisms involved in HF after MI, because this step can determine the treatment. Regardless the mechanism, an adequate history and clinical examination remain the most important tools in the evaluation of ventricular dysfunction after MI.
The simplest and most widely used method of assessing the severity of heart failure after MI is the Killip classification: class 1, patients have no evidence of heart failure; class 2, patients have rales present in up to one half of the lung fields or a third heart sound and systolic blood pressure >90 mm Hg; class 3, patients have frank pulmonary edema and systolic blood pressure >90 mm Hg; class 4, patients have cardiogenic shock with rales and systolic blood pressure >90 mm Hg. Importantly, recent studies have demonstrated that the Killip classification system is a strong predictor of long‐term mortality after MI.7, 8
Another method to evaluate the severity of HF is the New York Heart Association (NYHA) class. Patients in class I have no limitation of physical activity. Patients in class II present slight limitation of physical activity. Patients in class III refer marked limitation of physical activity. Finally, patients in class IV are unable to carry on any physical activity without discomfort.9
Biochemical markers can be useful to evaluate patients after MI. Indeed, elevated plasma natriuretic peptides (BNP and NT‐proBNP) are usually associated with abnormal ventricular function.9
Considering images methods, chest radiography is also useful for detecting signs of ventricular dysfunction. However, some patients with normal chest x‐rays may have hemodynamic cardiogenic disorders.10 Echocardiography is the most widespread method for determining the degree of ventricular dysfunction following MI and to exclude mechanical complications.11
Other less common modalities to assess cardiac morphology and function after MI include nuclear imaging (SPECT), computed tomography, and magnetic resonance imaging.11
Treatment of Heart Failure After Myocardial Infarction
Acute Treatment
The benefit from myocardial reperfusion with reduced infarct size and associated improvement in later regional and global ventricular function is well established.12 Patients with mild post‐MI heart failure (Killip class 2) could have hypoxemia. In this setting, oxygen supplementation with nasal catheter or facial mask is necessary for the resolution of hypoxemia.13 If wheezing is present, indicating bronchial hyperreactivity, β 2‐agonist inhalation should be used with careful monitoring of the patient's heart rate. Corticosteroid use in the acute phase of MI remains controversial due to concerns about increases in infarct expansion, the development of aneurysms, and left ventricular rupture.14
Diuretics treatments are essential in cases where there is dyspnea and signs of water and sodium retention. Intravenous loop diuretics are widely used given their effects on sodium and water excretion, as well as a possible vasodilator effect. However, if signs and symptoms do not improve with this management, nitrates may be used, mainly nitroglycerin. Intravenous nitrates are useful in reducing preload and relieving symptoms of heart failure after MI.6
In addition, angiotensin‐converting enzyme (ACE) inhibitors should also be used during this phase. Close monitoring of arterial blood pressure, potassium, and creatinine levels are important in the management of ACE inhibitors.1, 6 Patients with severe post‐MI heart failure (Killip class 3) whose hypoxemia does not improve with a nasal catheter or facial mask may require the use of noninvasive ventilation.15
In situations where tissue hypoperfusion occurs without cardiogenic shock, inotropic agents could be an option. However, some inotropes, such as digitalis and dobutamine, have contradictory effects, and others, like milrinone, could worsen prognosis.16 Patients with Killip class 4 have cardiogenic shock. The incidence of cardiogenic shock post‐MI is about 7%, and despite therapeutic advances, it continues to have mortality rates over 50%.17 With regard to medical treatment, the use of inotropic agents in these patients is of special interest. However, it is important to note that despite hemodynamic improvement with dopamine, dobutamine, and levosimendan use, no increase in survival was observed.18
The use of intra‐aortic balloon counterpulsation (IABP) in cardiogenic shock, when not quickly reversed by pharmacologic therapy, is recommended. IABP reduces systolic overload, increases diastolic pressure, and therefore, coronary perfusion, improving left systolic function. The primary limitations of IABP include the lack of active cardiac support, the need for accurate synchronization with the cardiac cycle, and the requirement for a certain level of left ventricular function.19
Usually percutaneous left ventricular assist devices (LVAD) are used as a bridge to recovery and are designed for a maximum use of 14 days.20 In general there are 3 types of devices, which are as follows: percutaneous cardiopulmonary bypass, axial flow pumps, and left atrial‐to‐femoral arterial LVAD.
Interestingly, a recent study compared Tandem Heart (Cardiac Assist, Inc., Pittsburgh, PA) (left atrial‐to‐femoral arterial LVAD) with IABP in patients with revascularized MI and cardiogenic shock. The LVAD improved hemodynamic status better than IABP.21 Therefore, the use of percutaneous left ventricular assist devices (LVAD) remains an appealing treatment strategy for cardiogenic shock after MI. However, these devices are currently not supported by randomized controlled trials. Another potential strategy is the use of extracorporeal membrane oxygenator (ECMO). In fact, some evidences suggest that ECMO can offer additional benefits in improving outcomes in patients with acute ST‐segment elevation myocardial infarction complicated with cardiogenic shock.22
Chronic Treatment
Chronically, the best treatment approach for HF after MI is neurohormonal blockade. Pharmacologic inhibition of the renin‐angiotensin‐aldosterone and sympathetic nervous systems has been evaluated in a large number of post‐MI clinical trials. Indeed, β‐blockers, ACE inhibitors, and angiotensin‐II receptor blockers (ARB) have been demonstrated to prevent left ventricular remodeling and to reduce post‐MI mortality.2
Ace Inhibitors:
The Survival and Ventricular Enlargement (SAVE) trial was the first to report the benefit of ACE inhibitors. The use of captopril for 42 months reduced the cardiovascular mortality by 21% and the reinfarction rate by 25% in post‐MI patients with left ventricular dysfunction.23 A large number of clinical trials conducted after the SAVE trial have demonstrated a survival benefit when ACE inhibitors were given to all patients with MI and selectively to patients with ventricular dysfunction and heart failure.24., 25, 26
In the Acute Infarction Ramipril Efficacy (AIRE) study, ramipril reduced by 27% all‐cause mortality in post‐MI patients.24 The use of trandolapril for post‐MI patients with left ventricular dysfunction was assessed in the Trandolapril Cardiac Evaluation (TRACE). In this trial, treatment with trandolapril for 50 months reduced cardiovascular mortality, reinfarction, and sudden death.25 In Survival of Myocardial Infarction Long‐term Evaluation (SMILE), zofenopril reduced mortality in post‐MI patients, and in SMILE‐2, zofenopril effects after MI were similar to lisinopril.6, 26 Thus, ACE inhibitors are considered first‐line therapy for all patients after myocardial infarction.
Angiotensin‐II Receptor Blockers:
In the Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan (OPTIMAAL) study, losartan was compared to captopril in patients after MI with heart failure, left ventricular dysfunction, and other high‐risk factors. After 2.7 years, there were no differences in heart failure hospitalization, reinfarction, sudden cardiac death, and all‐cause mortality.27
The Valsartan in Acute Myocardial Infarction (VALIANT) study has compared the effects of captopril and valsartan, alone or in combination, in patients with MI and HF or ventricular dysfunction. After 24.7 months, there were no differences in morbidity and mortality among the groups.28 These results suggest that there is no difference between ACE inhibitors and ARBs in the treatment of HF after MI. In addition, VALIANT results are consistent with the lack of additional outcome benefits by the dual blockade of the renin‐angiotensin system early after MI. Thus, ARBs could be used as an alternative to ACE inhibitors.
β‐Blockers:
The effects of β blockade in patients with left ventricular dysfunction after MI were addressed by the Carvedilol Post‐Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. In this trial, carvedilol treatment was associated with a 23% decrease in all‐case mortality and a 40% reduction in reinfarction after 1.3 years.29 Other large trials in patients with all‐cause systolic heart failure demonstrated that bisoprolol, metoprolol, and nebivolol reduced the rate of hospital admissions and mortality when they were added to standard therapy.30
Aldosterone Antagonists:
The most important trial of aldosterone antagonists in patients with left ventricular dysfunction and MI was the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS).31 In this trial, eplerenone, a selective aldosterone antagonist, was given to post‐MI patients with left systolic dysfunction for 16 months. It was shown to reduce all‐cause mortality by 15%, sudden death by 21%, and hospitalization for heart failure by 15%. Likewise, Hayashi et al have shown that patients with a first anterior myocardial infarction treated with spironolactone for 1 month had a significant improvement in left ventricular remodeling and in ejection fraction.32
Further studies are required to determine whether aldosterone antagonists should be restricted to patients with early evidence of systolic left ventricular dysfunction post‐MI, and whether there is any advantage of eplerenone in comparison with spironaloctone.
Hydralazine and Isosorbide Dinitrate:
There are no studies that specifically evaluate hydralazine and isosorbide dinitrate in patients with left ventricular dysfunction due to MI. However, in all‐cause systolic heart failure, this association could be used in self‐identified African Americans.30
Lipid Lowering Therapy:
Statins have an antiinflammatory action, and inflammation is thought to play a role in the pathophysiology of heart failure. In fact, 10 mg rosuvastatin daily, in patients with ischemic systolic heart failure, reduced cardiovascular death, myocardial infarction, and stroke mainly in patients with high sensitivity‐C reactive protein ≥2.0 mg/L.33 However, further studies are required to recommend routine use of statins in HF after MI.30
Implantable Cardioverter Defibrillators:
Patients with left ventricular dysfunction after myocardial infarction have an increased risk of sudden death due to lethal arrhythmias. As a result, implantable cardioverter defibrillators are recommended for primary prevention of sudden cardiac death in patients with ischemic heart disease at least 40 days after MI, with an ejection fraction lower than 35%, with an NYHA class of II or III while receiving chronic optimal medical therapy, and with an expectation of survival with good functional status for more than 1 year.30
Stem Cell Therapy:
Another recent approach to heart failure after MI is stem cell therapy. Stem cells are self‐replicating cells that can generate, sustain, and replace differentiated cells. Bone marrow‐derived cells include many progenitor cells including mesenchymal stem cells, endothelial progenitor cells, and hematopoietic progenitor cells. Endothelial progenitor cells can also be collected from peripheral blood and other fetal tissues. Some clinical trials have demonstrated the positive effects of stem cells on ventricular function post‐MI; nevertheless, the exact mechanism of this effect has yet to be established. Neovascularization, paracrine effects on surrounding tissues, and regeneration of viable myocardium are some of the hypotheses concerning its mechanism.34 There are 2 important trials that have used stem cells in HF after MI, the Re‐infusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR‐AMI) trial and the BALANCE (Clinical Benefit and Long‐Term Outcome After Intracoronary Autologous Bone Marrow Cell Transplantation in Patients With Acute Myocardial Infarction) study.35, 36
In the first trial, 204 patients were randomized to receive intracoronary bone marrow‐derived stem cell transplantation 3 to 7 days after MI. After 12 months, transplanted patients had a reduction in revascularization, reinfarction, and all‐cause mortality.35 In the BALANCE study, 62 patients received intracoronary stem cell transplantation; after 5 years they had improved end systolic and diastolic volumes as well as an increased ejection fraction.36
Conclusion
Knowledge of the mechanisms responsible for ventricular dysfunction after MI is of major importance. In addition to understanding these mechanisms, clinicians should seek to determine the presence of signs and symptoms of heart failure, after which ventricular dysfunction should be confirmed. β‐blockers, ACE inhibitors, aldosterone antagonists, and angiotensin‐II receptor blockers improve the prognosis of patients with HF after MI and should be started in the acute phase and maintained indefinitely.
References
- 1. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. [DOI] [PubMed] [Google Scholar]
- 2. Zornoff LAM, Paiva SAR, Duarte DR, et al. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol. 2009;92:157–164. [DOI] [PubMed] [Google Scholar]
- 3. Cleland JGF, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart. 2005;91:ii7–ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albert NM, Lewis C. Recognizing and managing asymptomatic left ventricular dysfunction: after myocardial infarction. Crit Care Nurse. 2008;28:20–37. [PubMed] [Google Scholar]
- 5. Meta‐Analysis Research Group in Echocardiography (MeRGE). AMI Collaborators , Møller JE, Whalley GA, Dini FL, et al. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta‐analysis: Meta‐Analysis Research Group in Echocardiography acute myocardial infarction. Circulation. 2008;117:2591–2598. [DOI] [PubMed] [Google Scholar]
- 6. Gheorghiade M, Fonarow GC. Management of post‐myocardial infarction patients with left ventricular systolic dysfunction. Am J Med. 2007;120:109–120. [DOI] [PubMed] [Google Scholar]
- 7. Parakh K, Thombs BD, Bhat U, et al. Long‐term significance of Killip class and left ventricular systolic dysfunction. Am J Med. 2008;121:1015–1018. [DOI] [PubMed] [Google Scholar]
- 8. Ivanusa M, Milicic D. 40 years since Killip clinical classification. Int J Cardiol. 2009;134:420–421. [DOI] [PubMed] [Google Scholar]
- 9. Dickstein K, Cohen‐Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 10. Battler A, Karliner JS, Higgins CB, et al. The initial chest x‐ray in acute myocardial infarction. Prediction of early and late mortality and survival. Circulation. 1980;61:1004–1009. [DOI] [PubMed] [Google Scholar]
- 11. Flachskampf FA, Schmid M, Rost C, et al. Cardiac imaging after myocardial infarction. Eur Heart J. 2011;32:272–283. [DOI] [PubMed] [Google Scholar]
- 12. White WD, Cross DB, Elliott JM, et al. Long‐term prognostic importance of patency of the infracted‐related coronary artery after thrombolytic therapy for acute myocardial infarction. Circulation. 1994;89:61–67. [DOI] [PubMed] [Google Scholar]
- 13. Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST‐segment elevation. The Task Force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. [DOI] [PubMed] [Google Scholar]
- 14. Giugliano GR, Giugliano RP, Gibson CM, et al. Meta‐analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol. 2003;91:1055–1059. [DOI] [PubMed] [Google Scholar]
- 15. Lenique F, Habis M, Lofaso F, et al. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Respir Crit Care Med. 1997;155:500–555. [DOI] [PubMed] [Google Scholar]
- 16. Poole‐Wilson PA. Treatment of acute heart failure: out with the old, in with the new. JAMA. 2002;287:1578–1580. [DOI] [PubMed] [Google Scholar]
- 17. Ruiz‐Bailén M, Rucabado‐Aguilar L, Expósito‐Ruiz M, et al. Cardiogenic shock in acute coronary syndrome. Med Sci Monit. 2009;15:RA57–RA66. [PubMed] [Google Scholar]
- 18. Archan S, Toller W. Levosimendan: current status and future prospects. Curr Opin Anaesthesiol. 2008;21:78–84. [DOI] [PubMed] [Google Scholar]
- 19. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:e82–e292. [PubMed] [Google Scholar]
- 20. Thiele H, Smalling RW, Schuler GC. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2007;28:2057–2063. [DOI] [PubMed] [Google Scholar]
- 21. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intraaortic balloon support versus a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005; 26:1276–1283. [DOI] [PubMed] [Google Scholar]
- 22. Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator‐assisted primary percutaneous coronary intervention improved 30‐day clinical outcomes in patients with ST‐segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810–1817. [DOI] [PubMed] [Google Scholar]
- 23. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with Leith ventricular dysfunction alter myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 24. AIRE Study Investigators . Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342:821–828. [PubMed] [Google Scholar]
- 25. Torp‐Pedersen C, Kober L. Effect of ACE inhibitor trandolapril on life expectancy of patients with reduced left‐ventricular function after acute myocardial infarction. TRACE Study Group. Trandolapril Cardiac Evaluation. Lancet. 1999;354: 9–12. [DOI] [PubMed] [Google Scholar]
- 26. Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensinconverting‐enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long‐Term Evaluation (SMILE) Study Investigators. N Engl J Med. 1995;332:80–85. [DOI] [PubMed] [Google Scholar]
- 27. Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–760. [DOI] [PubMed] [Google Scholar]
- 28. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 29. CAPRICORN Investigators . Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 30. McMurray JJV. Systolic heart failure. N Engl J Med. 2010; 362:228–238. [DOI] [PubMed] [Google Scholar]
- 31. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 32. Hayashi M, Tsutamoto T, Wada A, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post‐infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107:2559–2565. [DOI] [PubMed] [Google Scholar]
- 33. McMurray JJ, Kjekshus J, Gullestad L, et al. Effects of statin therapy according to plasma high‐sensitivity C‐reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): a retrospective analysis. Circulation. 2009;120:2188–2196. [DOI] [PubMed] [Google Scholar]
- 34. Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post‐infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. [DOI] [PubMed] [Google Scholar]
- 35. Schaechinger V, Erbs S, Elaesser A, et al. Intracoronary bone marrow‐derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006. 355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 36. Yousef M, Schannwell CM, Kostering M, et al. The BALANCE study . Clinical benefit and long‐term outcome after intracoronary autologous bone‐marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262–2269. [DOI] [PubMed] [Google Scholar]


