Abstract
Background:
The effect of smoking on prognosis among patients undergoing percutaneous coronary intervention (PCI) is controversial, and data on the importance of smoking cessation or reductions were lacking.
Hypothesis:
Smoking cessation or reductions could reduce the risk of adverse outcomes in patient after PCI.
Methods:
There were 19 506 consecutive patients who had undergone successful PCI between April 2004 and January 2010 followed. Extensive data, including self‐reported smoking habits, were obtained at baseline and during follow‐up.
Results:
Compared with post‐PCI quitters and persistent smokers, the nonsmokers and pre‐PCI quitters were older and had a higher prevalence of comorbid factors such as hypertension and impaired left ventricle function. The adjusted hazard ratios for mortality were 2.52 (95% confidence interval [CI]: 1.92–3.30) for nonsmokers, 0.52 (95% CI: 0.32–0.84) for pre‐PCI quitters, and 0.11 (95% CI: 0.06–0.22) for post‐PCI quitters, compared to persistent smokers. With respect to additional revascularizations, a higher risk was observed among the quitters (1.70 [95% CI: 1.40–2.08] for pre‐PCI quitters and 1.59 [95% CI: 1.36–1.85] for post‐PCI quitters) as well as the nonsmokers (1.40 [95% CI: 1.20–1.64]). Among persistent smokers, each reduction of 5 cigarettes/day was associated with a 72% decline in mortality risk (P < 0.001) but did not reach statistical significant for repeated revascularizations (0.80 [95% CI: 0.46–1.37], P = 0.4132).
Conclusions:
Despite a higher risk of revascularization, the cessation of smoking either before or after PCI is beneficial in all‐cause mortality. The apparent smoker's paradox may be explained by the differences in baseline risk or the reduced sensitivity to adverse outcomes as well as the reluctance to seek medical help among smokers.
This study received an unrestricted grant from Pfizer Investment Co., China. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Although considerable evidence has demonstrated that percutaneous coronary intervention (PCI) can dramatically improve the patient's clinical condition in the short term,1 the patient's personal characteristics might also influence prognosis after PCI.2, 3, 4, 5, 6 Some factors such as age and sex were not modifiable, whereas others were. One of the modifiable factors linked with poorer outcomes after PCI was cigarette smoking.7, 8, 9, 10 However, conflicting data regarding the role of smoking on the outcomes of PCI have been reported. Cohen et al11 found that cigarette smoking was associated with a lower rate of subsequent target lesion revascularization in patients undergoing PCI. On the contrary, Sherif et al12 reported that smokers treated with drug‐eluting stents had higher rates of mortality and myocardial infarction compared to nonsmokers. Therefore, controversies still exist on this topic. Moreover, few data are available on smoking reduction and outcomes after PCI.
With the data from a prospective and real‐world registry study, our goals were: (1) to examine the effect of different smoking status on the postoperative adverse consequences (eg, all‐cause mortality, repeated revascularization), and especially to determine whether the cessation of smoking before or after the index intervention could attenuate them; and (2) to examine whether cigarette reduction was associated with a lower event rate.
Methods
Study Population
From April 2004 to January 2010, a total of 22 743 consecutive patients were treated with stent implantation at the Fuwai Hospital in Beijing. The current study was based on 19 506 patients (85.76%) who underwent successful PCI with at least 1 stent. This study was approved by the ethics committee of Fuwai Hospital and was conducted in accordance with the principles of the Declaration of Helsinki.
Data Collection and Clinical Follow‐up
Baseline demographic, clinical, and angiographic characteristics as well as procedural details were recorded for all enrolled patients. A comprehensive clinical follow‐up was performed through January 20, 2010 by clinic visit or phone. All patients needed to be followed up for at least 1 year. The study end points were all‐cause mortality and repeated revascularization. The choice for additional coronary revascularization of the target lesion or other segments was up to the operators' decision according to their experiences.
Smoking Status
The population was divided into 4 groups on the basis of information provided by the patients at baseline and during follow‐up. Nonsmokers, defined as patients who had never smoked before or after the index procedure; pre‐PCI quitters, those who had quit smoking at least 1 month before the index procedure; post‐PCI quitters, those who permanently quit smoking 1 month before PCI to the final visit; and persistent smokers, patients who smoked during the year preceding their procedure and at any time during the follow‐up period. Intermittent smoking between interviews was regarded as persistent smokers.13
Given the fact that smoking status may be misclassified by self‐reported information from participants as well as the investigators, we randomly selected 975 (5%) patients and re‐collected the same items without telling the research nurses. Among those participants, 791 provided permission to answer again, and the kappa coefficient reached 0.95 for the nonsmokers, 0.91 for the former smokers, 0.93 for quitters, and 0.97 for persistent smokers.
Statistical Analysis
Continuous variables were described as mean (standard deviation), and differences across smoking categories were tested for significance with 1‐way analysis of variance. Categorical variables were displayed as percentages and compared by χ 2 test. Student t test was executed when comparing the characteristics between the patients excluded and those who remained. Kappa coefficient was used to verify agreement between both research nurses. Cumulative event rates were calculated by the Kaplan‐Meier method, and comparisons were made using the log‐rank test. Cox proportional hazards analysis was used to estimate the hazard ratios (HRs). A further analysis was conducted within persistent smokers to assess the impact of smoking reduction on each event. All P values referred to 2‐tailed significance tests.
Results
Population
Of the 19 506 participants, 1882 (9.6%) patients were excluded due to missing or insufficient information on smoking status during follow‐up. Compared with the remaining patients, those excluded were of similar age (57.50 vs 57.72 years, P = 0.3859), but were more likely to be male (80.61% vs 78.62%, P = 0.0451), nonsmokers (62.49% vs 59.93%, P = 0.0174), and not have hyperlipidemia (29.49% vs 31.84%, P = 0.0374).
Baseline Characteristic
The subsequent analysis was based on the remaining 17 624 patients. Patients were followed for a median of 3.01 (interquartile range, 1.80–4.46) years. The baseline characteristics across the 4 groups of patients are summarized in Table 1. Overall, nonsmokers had a less favorable risk profile with regard to several clinical and angiographic variables, such as hypertension and diabetes mellitus, as well as impaired left ventricle function. In particular, patients who never smoked were approximately 7 years older than the persistent smokers.
Table 1.
Baseline Characteristic According to Smoking Status
| Variables | Nonsmokers | Pre‐PCI Quitters | Post‐PCI Quitters | Persistent Smokers | P |
|---|---|---|---|---|---|
| No. | 7611 | 1524 | 4440 | 4049 | |
| Age (years) | 60.89 ± 10.24 | 60.68 ± 10.09 | 55.47 ± 9.94 | 53.14 ± 9.60 | <0.001 |
| Male (%) | 4233 (55.62) | 1438 (94.36) | 4297 (96.78) | 3888 (96.02) | <0.001 |
| Unstable angina (%)a | 4939 (64.89) | 1011 (66.34) | 3000 (67.57) | 2648 (65.40) | 0.02 |
| Prior MI (%) | 1950 (25.62) | 446 (29.27) | 1681 (37.86) | 1497 (36.97) | <0.001 |
| Prior CABG (%) | 147 (1.93) | 53 (3.48) | 77 (1.73) | 54 (1.33) | <0.001 |
| Diabetes mellitus (%) | 1569 (20.61) | 288 (18.90) | 725 (16.33) | 695 (17.16) | <0.001 |
| Hypertension (%) | 4131 (54.28) | 814 (53.41) | 2111 (47.55) | 1811 (44.73) | <0.001 |
| Dyslipidemia (%) | 2410 (31.66) | 510 (33.46) | 1395 (31.42) | 1296 (32.01) | 0.49 |
| Family history of CHD (%) | 268 (3.52) | 52 (3.41) | 215 (4.84) | 185 (4.57) | 0.001 |
| Ejection fraction <40% | 4225 (55.51) | 844 (55.38) | 2336 (52.61) | 2031 (50.16) | <0.001 |
| RVD (mm) | 3.12 ± 1.84 | 3.17 ± 1.58 | 3.20 ± 1.86 | 3.22 ± 1.62 | 0.002 |
| Lesion length (mm) | 25.04 ± 14.25 | 25.37 ± 14.35 | 26.10 ± 15.30 | 25.58 ± 15.11 | 0.003 |
| DS (%) | 88.18 ± 8.07 | 88.41 ± 7.65 | 89.07 ± 7.88 | 88.64 ± 8.12 | <0.001 |
| Lesion morphology | |||||
| Angulated (%) | 2479 (32.57) | 524 (34.38) | 1542 (34.73) | 1274 (31.46) | 0.01 |
| Calcification (%)b | 313 (4.11) | 63 (4.13) | 140 (3.15) | 118 (2.91) | 0.002 |
| Total occlusion (%) | 1538 (20.21) | 312 (20.47) | 1081 (24.35) | 922 (22.77) | <0.001 |
| Thrombosis (%) | 404 (5.31) | 67 (4.40) | 288 (6.49) | 288 (7.11) | <0.001 |
| TIMI classification (%) | 0.002 | ||||
| 0 | 1518 (19.84) | 307 (20.14) | 1052 (23.69) | 927 (22.89) | |
| 1 | 335 (4.40) | 59 (3.87) | 182 (4.10) | 172 (4.25) | |
| 2 | 982 (12.90) | 183 (12.01) | 554 (12.48) | 494 (12.20) | |
| 3 | 4770 (62.75) | 975 (63.98) | 2652 (59.73) | 2456 (60.66) | |
| Lesion type (%) | |||||
| B2+C | 6342 (83.33) | 1288 (84.51) | 3802 (85.63) | 3370 (83.23) | 0.004 |
| Predilation (%) | 6757 (88.78) | 1362 (89.37) | 4038 (90.95) | 3584 (88.52) | 0.001 |
| Total stent length (mm) | 29.39 ± 14.59 | 29.85 ± 15.04 | 30.53 ± 15.94 | 29.89 ± 15.28 | 0.006 |
| Postdilation (%) | 2683 (35.25) | 585 (37.07) | 1615 (36.37) | 1457 (35.98) | 0.43 |
| Transradial (%) | 5965 (78.37) | 1220 (80.05) | 3612 (81.35) | 3343 (82.56) | <0.001 |
Abbreviations: CABG, coronary artery bypass graft; CHD, coronary heart disease; DS, diameter stenosis; MI, myocardial infarction; PCI, percutaneous coronary intervention; RVD, reference vessel diameter.
Unstable angina was defined according to the Braunwald classification.
Calcification was defined as a radioplaque area before contrast injection and was considered severe if also visible on still frames.
Clinical Outcomes by Smoking Status
The estimated 6‐year rate of death differed substantially among different smoking categories (nonsmokers, 10.69%; pre‐PCI quitters, 1.66%; post‐PCI quitters, 0.81%; persistent smokers, 2.66%; log‐rank, P < 0.001) (Figure 1). When data were adjusted for age and sex, the risk changed slightly with HRs of 2.43 (95% confidence interval [CI]: 1.86–3.17) for nonsmokers, 0.54 (95% CI: 0.34–0.87) for pre‐PCI quitters, and 0.12 (95% CI: 0.06–0.23) for post‐PCI quitters, compared with the unadjusted HRs. Further adjustment for clinical and angiographic characteristic or procedure data did not materially change the results (Table 2). In fully adjusted model, the relative risk of death from cardiac causes for nonsmokers, pre‐PCI quitters, and post‐PCI quitters as compared with persistent smokers was 3.46 (95% CI: 2.52–4.76), 0.58 (95% CI: 0.32–1.03), and 0.15 (95% CI: 0.07–0.31), respectively.
Figure 1.
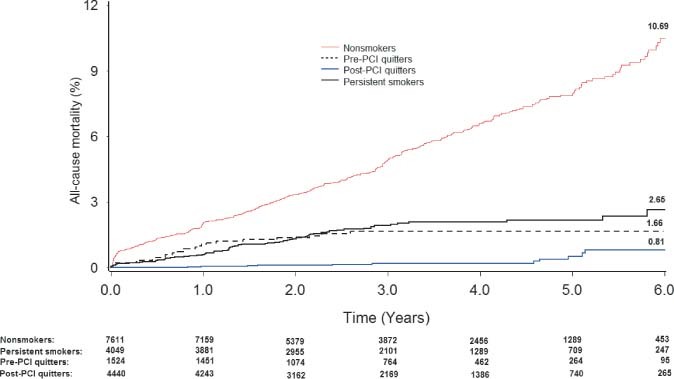
Kaplan‐Meier curves of the cumulative incidence of all‐cause mortality according to different smoking status. Abbreviations: PCI, percutaneous coronary intervention.
Table 2.
Harzad Ratio Estimates for Mortality or Repeated Revascularization Risk After First Percutaneous Coronary Intervention
| Event | Nonsmokers [N = 7611] | Pre‐PCI Quitters [N = 1524] | Post‐PCI Quitters [N = 4440] | Persistent Smokers [N = 4049] |
|---|---|---|---|---|
| Death from all causes | ||||
| No. of cases | 418 | 24 | 11 | 71 |
| Unadjusted | 3.19 (2.48–4.10) | 0.91 (0.58–1.45) | 0.14 (0.08–0.27) | 1 (reference) |
| Age and sex adjusted | 2.43 (1.86–3.17) | 0.54 (0.34–0.87) | 0.12 (0.06–0.23) | 1 (reference) |
| Multivariable adjusteda | 2.56 (1.96–3.34) | 0.56 (0.34–0.89) | 0.12 (0.06–0.23) | 1 (reference) |
| Multivariable adjustedb | 2.52 (1.92–3.30) | 0.52 (0.32–0.84) | 0.11 (0.06–0.22) | 1 (reference) |
| Repeated revascularization | ||||
| No. of cases | 707 | 175 | 478 | 273 |
| Unadjusted | 1.41 (1.23–1.62) | 1.77 (1.47–2.14) | 1.64 (1.41–1.90) | 1 (reference) |
| Age and sex adjusted | 1.42 (1.22–1.66) | 1.73 (1.42–2.10) | 1.62 (1.40–1.88) | 1 (reference) |
| Multivariable adjusteda | 1.43 (1.23–1.67) | 1.73 (1.42–2.10) | 1.62 (1.40–1.88) | 1 (reference) |
| Multivariable adjustedb | 1.40 (1.20–1.64) | 1.70 (1.40–2.08) | 1.59 (1.36–1.85) | 1 (reference) |
Abbreviations: PCI, percutaneous coronary intervention.
Adjusted for age, sex, diabetes mellitus, prior myocardial infarction, hypertension, hyperlipidemia, prior bypass surgery, unstable angina, family history of coronary heart disease, and ejection fraction.
Further adjusted for lesion type, reference vessel diameter, lesion length, restenotic lesion, calcification, angulated, total occlusion, thrombus, predilation, stent length, and postdilation.
For repeated revascularization, we found a lower estimated 6‐year rate among the persistent smokers than the other 3 groups (nonsmokers, 13.22%; pre‐PCI quitters, 15.78%; post‐PCI quitters, 17.29%; persistent smokers, 8.96%; log‐rank, P < 0.05 for the difference between the persistent smokers and the other 3 groups) (Figure 2). Cox regression with full adjustment for baseline confounders yielded respective HRs of 1.40 (95% CI: 1.20–1.64) for nonsmokers, 1.70 (95% CI: 1.40–2.08) for pre‐PCI quitters, and 1.59 (95% CI: 1.36–1.85) for post‐PCI quitters (Table 2). Moreover, among the patients who underwent repeated revascularization at our center, the results persisted for target vessel revascularization (1.55 [95% CI: 1.24–1.92] for nonsmokers, 1.90 [95% CI: 1.44–2.50] for pre‐PCI quitters, and 1.92 [95% CI: 1.56–2.37] for post‐PCI quitters).
Figure 2.
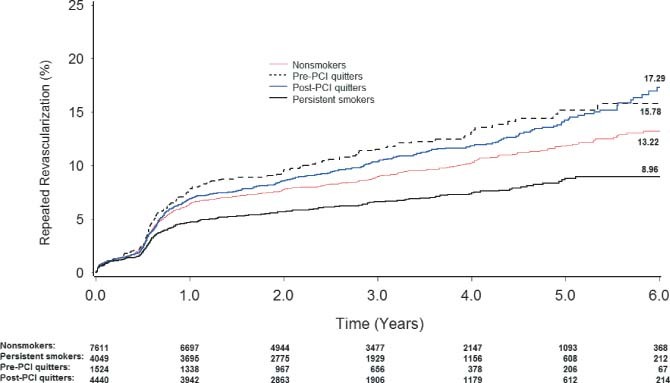
Kaplan‐Meier curves of the cumulative incidence of repeated revascularization according to different smoking status. Abbreviations: PCI, percutaneous coronary intervention.
Smoking Reduction and Clinical Outcomes
Among persistent smokers, each reduction of 5 cigarettes smoked/day was associated with an HR of 0.28 (95% CI: 0.18–0.43, P < 0.0001) in mortality risk after adjustment for baseline characteristics and pre‐PCI intensity. For the repeated revascularization, the risk declined 20%, but did not reach statistical significance (HR = 0.80, 95% CI: 0.46–1.37, P = 0.4132).
Discussion
In this study, nearly 60% of patients were smokers at baseline, of whom only 44% successfully quit smoking after treatment. With the adjustment for baseline differences, patients who gave up smoking before or after PCI significantly reduced their risk of dying, as compared with persistent smokers. Paradoxically, those who never smoked had a higher mortality risk or additional coronary revascularization than those who continued to smoke after PCI.
A large number of previous studies had reported beneficial effects from smoking cessation among the general population as well as patients with coronary heart diseas.7, 14, 15, 16, 17 In a systematic review, Critchley and Capewell16 found a 36% reduction of mortality risk in cardiac patients who quit smoking. Moreover, van Berkel et al17 reviewed the published studies on the impact of tobacco cessation and found that the relative risk of mortality for quitters compared to permanent smokers varied from 0.13 to 0.72. Thus, our figure of a 48% reduction of the mortality risk among pre‐PCI quitters was consistent with other studies, although the magnitude seemed larger for the post‐PCI quitters. In addition, reduction of daily cigarettes among persistent smokes conferred survival advantages. This was in line with previous studies.18, 19
Unlike the large benefit in mortality from smoking cessation, our study found a higher risk in repeated revascularization. Similar results had been reported in previous studies.7, 11 One contributing factor to the seemingly worse prognosis might be that our doctors were less likely to perform additional revascularization for smokers in actual clinical practice, although we did not approve this. Another explanation was that smokers may be more reluctant to seek medical help despite recurrent angina,11 but the quitters paid more attention to their health status before serious problems occurred. This may result in lower hard events (eg, death) but higher unnecessary additional revascularization procedures.
By contrast, it was surprising to find a higher mortality or repeated revascularization procedures in nonsmokers over patients who had permanently smoked. This was contrary to several recent published papers.18, 20 We believed that the conflicting results could be partly explained by the relatively shorter duration of follow‐up and the different study population, in which the smoking paradox was commonly reported.7, 11, 21, 22, 23
Why do smokers have a lower incidence of adverse events following PCI? Some investigators attribute this phenomenon to the younger age and more favorable clinical and angiographic profile among smokers or insufficient adjustment of confounding factors.7, 18, 24 Moreover, others believe that the smoker may have less damage to microvascular function after primary coronary intervention.25 Additionally, it has been proposed that coronary obstruction in patients who smoke may be more thrombogenic and less atherosclerotic than that in nonsmokers.26 In our study, we further propose that because a large proportion of smokers die prior to hospital admission,27 the survivors who already have a higher baseline level of sympathetic activity may have reduced sensitivity to respond to postoperative stress in the same fashion as nonsmokers.11, 28 Moreover, nonsmokers may be confident about their prognosis and unwilling to practice strict risk‐factor modification.12
There were several limitations to this study. First, the potential bias from misclassification of smoking status may exist because of the inaccurateness of a patient's reporting of smoking status. However, to assess the bias during the research nurses' collection, we randomly selected 5% of the participants and found a high kappa coefficient between interobservers. Second, all participants were from a single high‐volume coronary treatment center, which would restrict the generalization of our findings. Finally, because some preoperative factors (ie, socioeconomic status, triglyceride level) and postoperative information (ie, lifestyle modification, antiplatelet therapy) was not adequately collected in our database, it limited us to control these potential confounders. However, within these limitations, we believe our work will stimulate more research to verify our results and explore the pathophysiology of adverse outcomes in different smoking status of patients undergoing PCI.
Conclusion
The present study suggested that patients who quit smoking either before or after PCI were at a significantly lower risk for death, and a reduction of mortality was also observed among the persistent smokers who intended to reduce the number of cigarettes. According to the evidences from previous studies,15, 16, 17, 18 our cardiologists should aggressively advise their patients to stop smoking.
References
- 1. Gao RL. Current status and perspective of percutaneous coronary intervention in China. Chin Med J (Engl). 2006;119:531–532. [PubMed] [Google Scholar]
- 2. Woo JS, Kim W, Ha SJ, et al. Impact of gender differences on long‐term outcomes after successful percutaneous coronary intervention in patients with acute myocardial infarction. Int J Cardiol. 2010;145:516–518. [DOI] [PubMed] [Google Scholar]
- 3. Gaglia MA Jr, Steinberg DH, Pinto Slottow TL, et al. Racial disparities in outcomes following percutaneous coronary intervention with drug‐eluting stents. Am J Cardiol. 2009;103:653–658. [DOI] [PubMed] [Google Scholar]
- 4. Rathore S, Rhys J, Buchalter MB, et al. Impact of age on the outcomes of women following percutaneous coronary intervention in the bare‐metal stent era. J Interv Cardiol. 2006;19:245–249. [DOI] [PubMed] [Google Scholar]
- 5. Blake GJ, Ridker PM. C‐reactive protein and prognosis after percutaneous coronary intervention. Eur Heart J. 2002;23:923–925. [DOI] [PubMed] [Google Scholar]
- 6. Jensen LO, Maeng M, Thayssen P, et al. Long‐term outcomes after percutaneous coronary intervention in patients with and without diabetes mellitus in Western Denmark. Am J Cardiol. 2010;105:1513–1519. [DOI] [PubMed] [Google Scholar]
- 7. Hasdai D, Garratt KN, Grill DE, et al. Effect of smoking status on the long‐term outcome after successful percutaneous coronary revascularization. N Engl J Med. 1997;336:755–761. [DOI] [PubMed] [Google Scholar]
- 8. Ishikawa T, Yagi H, Ogawa T, et al. Deteriorative effect of smoking on target lesion revascularization after implantation of coronary stents with diameter of 3.0 mm or less. Circ J. 2005; 69:227–231. [DOI] [PubMed] [Google Scholar]
- 9. Taira DA, Seto TB, Ho KK, et al. Impact of smoking on health‐related quality of life after percutaneous coronary revascularization. Circulation. 2000;102:1369–1374. [DOI] [PubMed] [Google Scholar]
- 10. Haddock CK, Poston WS, Taylor JE, et al. Smoking and health outcomes after percutaneous coronary intervention. Am Heart J. 2003;145:652–657. [DOI] [PubMed] [Google Scholar]
- 11. Cohen DJ, Doucet M, Cutlip DE, et al. Impact of smoking on clinical and angiographic restenosis after percutaneous coronary intervention: another smoker's paradox? Circulation. 2001;104:773–778. [DOI] [PubMed] [Google Scholar]
- 12. Sherif MA, Nienaber CA, Toelg R, et al. Impact of smoking on the outcome of patients treated with drug‐eluting stents: 1‐year results from the prospective multicentre German Drug‐Eluting Stent Registry (DES.DE). Clin Res Cardiol. 2011;100:413–423. [DOI] [PubMed] [Google Scholar]
- 13. Rea TD, Heckbert SR, Kaplan RC, et al. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. [DOI] [PubMed] [Google Scholar]
- 14. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5‐year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. [DOI] [PubMed] [Google Scholar]
- 15. van Domburg RT, Meeter K, van Berkel DF, et al. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20‐year follow‐up study. J Am Coll Cardiol. 2000;36:878–883. [DOI] [PubMed] [Google Scholar]
- 16. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 17. van Berkel TF, Boersma H, Roos‐Hesselink JW, et al. Impact of smoking cessation and smoking interventions in patients with coronary heart disease. Eur Heart J. 1999;20:1773–1782. [DOI] [PubMed] [Google Scholar]
- 18. Gerber Y, Rosen LJ, Goldbourt U, et al. Smoking status and long‐term survival after first acute myocardial infarction a population‐based cohort study. J Am Coll Cardiol. 2009;54:2382–2387. [DOI] [PubMed] [Google Scholar]
- 19. Bjartveit K, Tverdal A. Health consequences of smoking 1‐4 cigarettes per day. Tob Control. 2005;14:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frey P, Waters DD, DeMicco DA, et al. Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the Treating to New Targets [TNT] and the Incremental Decrease in End Points Through Aggressive Lipid Lowering [IDEAL] trials). Am J Cardiol. 2011;107:145–150. [DOI] [PubMed] [Google Scholar]
- 21. Andrikopoulos GK, Richter DJ, Dilaveris PE, et al. In‐hospital mortality of habitual cigarette smokers after acute myocardial infarction; the “smoker's paradox” in a countrywide study. Eur Heart J. 2001;22:776–784. [DOI] [PubMed] [Google Scholar]
- 22. Weisz G, Cox DA, Garcia E, et al. Impact of smoking status on outcomes of primary coronary intervention for acute myocardial infarction—the smoker's paradox revisited. Am Heart J. 2005;150:358–364. [DOI] [PubMed] [Google Scholar]
- 23. Aygul N, Ozdemir K, Abaci A, et al. Comparison of traditional risk factors, angiographic findings, and in‐hospital mortality between smoking and nonsmoking Turkish men and women with acute myocardial infarction. Clin Cardiol. 2010;33:E49–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrikopoulos GK, Chimonas ET, Toutouzas PK. Paradoxical clinical value of another smoker's paradox. Circulation. 2002;105:e55. [PubMed] [Google Scholar]
- 25. Katayama T, Iwasaki Y, Sakoda N, et al. The etiology of ‘smoker's paradox’ in acute myocardial infarction with special emphasis on the association with inflammation. Int Heart J. 2008;49:13–24. [DOI] [PubMed] [Google Scholar]
- 26. Barbash GI, White HD, Modan M, et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation. 1993;87:53–58. [DOI] [PubMed] [Google Scholar]
- 27. Herlitz J, Karlson BW, Sjolin M, et al. Five‐year mortality in patients with acute chest pain in relation to smoking habits. Clin Cardiol. 2000;23:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yun AJ, Bazar KA, Lee PY, et al. The smoking gun: many conditions associated with tobacco exposure may be attributable to paradoxical compensatory autonomic responses to nicotine. Med Hypotheses. 2005;64:1073–1079. [DOI] [PubMed] [Google Scholar]


