Abstract
Background:
The benefits of primary prophylactic implantable cardioverter‐defibrillators (ICDs) are actually debated, as some drawbacks become more apparent and as the natural history of cardiac disease seems to improve. Therefore, contemporary follow‐up data of non‐trial populations treated according to current guidelines remain necessary. The aim of this study was to evaluate mortality and the occurrence of ICD interventions in patients with coronary artery disease (CAD) and dilated cardiomyopathy (DCM) who received in the recent era a primary prophylactic ICD without resynchronization therapy.
Hypothesis:
Survival and event‐free rates from appropriate ICD therapy are different between ischemic and nonischemic ICD patients.
Methods:
Prospective cohort study of 427 consecutive primary prevention ICD patients with ischemic or nonischemic heart disease, excluding patients with resynchronization.
Results:
Ischemic heart disease was present in 290 patients (68%), nonischemic heart disease in 137 patients (32%). During a median follow‐up of 31 months (interquartile range [IQR] 15–45 months), 30 patients (7%) died. Mortality was not different in both disease categories. The incidence of appropriate ICD interventions was similar in CAD and DCM (23% vs 21%). Appropriate ICD intervention occurred more frequently in patients with atrial fibrillation (29% vs 19%). Inappropriate ICD intervention occurred in 11% of patients.
Conclusions:
The clinical course of ischemic and nonischemic heart disease patients treated with a primary prophylactic ICD is similar with respect to mortality and to appropriate and inappropriate ICD interventions, in spite of a younger age at baseline of the DCM patients. © 2011 Wiley Periodicals, Inc.
This work was partly funded by “het College Voor Zorgverzekeringen” (OP 0864207). Dr. D.A.M.J. Theuns received research grants from Biotronik, Boston Scientific, and St. Jude Medical, and he is a consultant to Cameron Health (USA). L. Jordaens received research grants and speaker fees from Biotronik, Boston Scientific, Cameron Health, Medtronic, Sorin, and St. Jude Medical. T. Smith and K. Caliskan had no funding, financial relationships, or conflicts of interest to declare.
Introduction
Real‐world follow‐up data of ICD patients who receive an ICD according to the international guidelines are useful in the translation of clinical trial data to clinical practice.1, 2 The landmark trials on primary prevention3, 4, 5 were indeed interpreted in a variable way by different national cardiac societies and reimbursement authorities,6 resulting in different practices often not consistent with the available evidence7 and the international guidelines.8, 9
The indication for primary prophylactic ICDs in patients with dilated cardiomyopathy (DCM) is especially not fully appreciated by all, and the use of resynchronization therapy (CRT) only confounds the interpretation of the outcome of device therapy.10, 11, 12, 13
Therefore, the aim of the present study was to evaluate the mortality and occurrence of ICD interventions in patients with left ventricular dysfunction due to coronary artery disease (CAD) or DCM, who received primary prophylactic ICD‐only therapy (ie, without CRT) according to the international guidelines in the recent era.
Methods
Study Population
The basis for this study was the prospective ICD registry of the Erasmus MC, including all consecutive patients who received an ICD since 1998 in our institution. All consecutive patients with CAD or DCM who received a first ICD‐only implantation for primary prevention between January 2004 and June 2009 were selected. Patients who received a CRT‐defibrillator (CRT‐D) (all according to the guidelines) were excluded. In addition, patients with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, congenital structural heart disease, the Brugada syndrome, or inherited arrhythmia disorders were excluded.
CAD had to be documented clinically by a history of a myocardial infarction according to the definitions by the American College of Cardiology (ACC), the American Heart Association (AHA), and the European Society of Cardiology (ESC), if coronary artery bypass grafting (CABG) or a percutaneous coronary intervention (PCI) had been performed, or if significant coronary artery stenosis was documented with coronary angiography.
The majority of patients (82%) had the device programmed in a 2‐zone configuration, with the rate cutoff for detection of ventricular tachycardia (VT) at 170 to 180 beats per minute (bpm), and for detection of ventricular fibrillation (VF) at 200 to 220 bpm. Antitachycardia pacing (ATP) in combination with cardioversion/defibrillation therapy features was activated in those with 2 zones. ICD programming was tailored to avoid inappropriate therapy as published.14 In brief, the stability criterion was programmed at 30 to 40 ms, the onset criterion at 15% to 20%, and morphology was activated when available. In all dual‐chamber devices, the respective dual‐chamber discrimination algorithm was activated.
Data Collection
Follow‐up started at the time of ICD implantation. All patients were regularly followed at 3‐monthly intervals for the first 12 months, and thereafter every 6 months. The patients were advised to contact the outpatient clinic after a symptomatic event as soon as possible. All spontaneous episodes with stored electrograms that resulted in ventricular therapies were reviewed and classified by 2 of the authors (D.T., L.J.). In case of disagreement between the 2 reviewers about the stored electrograms, a third electrophysiologist was consulted and made the decision. For each episode, the date, type, morphology, and mean cycle length of the tachyarrhythmia, and the type and outcome of delivered ICD intervention were recorded. The arrhythmias were classified as (1) ventricular tachyarrhythmia or (2) atrial tachyarrhythmia without a coexistent ventricular arrhythmia. When an atrial electrogram was available, the presence of atrioventricular dissociation was used to classify a ventricular tachyarrhythmia. Otherwise, a ventricular tachyarrhythmia was defined as an event with a sudden increase in rate combined with a change in electrogram morphology from the baseline rhythm. Intervention triggered by a ventricular tachyarrhythmia was considered appropriate, while intervention delivered for atrial tachyarrhythmia (including atrial fibrillation, atrial flutter, atrial tachycardia, and sinus tachycardia) and interference by other cardiac or extracardiac signals was defined as inappropriate. Atrial fibrillation was diagnosed with all available means. For all patients, the renal function was assessed by estimating the baseline glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation: eGFR (mL/min/1.73 m2 of body surface area) =186× (serum creatinine in mg/dL)−1.154 × (age)−0.203 ×0.742 in female subjects. Impaired renal function was defined as an eGFR <60 mL/min/1.73 m2.
Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation (SD) if normally distributed, or otherwise as the median and interquartile range (IQR). Continuous variables were compared with the Student t test or 1‐way analysis of variance (ANOVA). Categorical data were summarized as frequency. The chi‐square test was used to compare categorical variables. Survival and event‐free rates from ICD intervention were calculated according to the Kaplan‐Meier method. Survival time was defined as the date from ICD implantation to the date of death as verified in the civil registry. Patients who underwent heart transplantation were censored from the moment of transplantation. Differences between pairs of survival curves were tested by the log‐rank test. A Cox proportional hazards model was used to identify predictors of mortality. The proportional hazards assumption was checked graphically by assessing log‐log survival curves. In addition, the proportional hazards assumption was tested for all covariates using Schoenfeld residuals. Hazard ratios (HRs) were reported with corresponding 95% confidence intervals (CIs). A 2‐tailed P value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS for Windows (release 16.0; SPSS, Inc., Chicago, IL) and with STATA for Windows (release 11; StataCorp, TX).
Results
Study Population
The study population consisted of 427 patients who received an ICD for primary prevention of sudden cardiac death. CAD was present in 290 patients (68%) and DCM in 137 patients (32%). The mean age of the study population was 58±14 years, the mean left‐ventricular ejection fraction (LVEF) was 27% ± 9%. Baseline characteristics and differences in characteristics between CAD and DCM patients are presented in Table 1. DCM patients were younger than CAD patients and were more often of the female gender. CAD patients were more often in New York Heart Association (NYHA) class I‐II compared to DCM patients (69% vs 52%, P<0.001). The use of digoxin was not significantly different between CAD patients and DCM patients (18% vs 25%), whereas use of beta‐blockers (82% vs 69%, P = 0.004) and statins (82% vs 19%, P<0.001) was more frequent in CAD patients compared to DCM patients. The implanted devices were 317 single‐chamber ICDs (74%), and 110 dual‐chamber ICDs (26%). Patients with DCM more often received a dual‐chamber device compared to CAD patients (32% vs 23%, P = 0.044)
Table 1.
Baseline Characteristics
| Characteristic (n,%) | All (n = 427) | CAD (n = 290) | DCM (n = 137) | P Value |
|---|---|---|---|---|
| Age, years | 58±14 | 62±10 | 49±15 | <0.001 |
| Male gender | 336 (79%) | 248 (86%) | 88 (64%) | <0.001 |
| Left ventricular ejection fraction, % | 27±9 | 27±7 | 28±12 | NS |
| History of myocardial infarction | 261 (61%) | 261 (61%) | NA | NA |
| NYHA class at time of ICD implantation | ||||
| NYHA I‐II | 347 (82%) | 255 (88%) | 95 (70%) | <0.001 |
| NYHA III‐IV | 77 (18%) | 37 (12%) | 40 (30%) | |
| Previous revascularization | ||||
| CABG | 90 (21%) | 90 (31%) | NA | NA |
| PCI | 141 (33%) | 141 (49%) | NA | NA |
| History of atrial fibrillation | 112 (26%) | 80 (28%) | 32 (24%) | NS |
| QRS duration, ms | 116±26 | 117±26 | 112±27 | NS |
| QRS >0.12 s | 133 (31%) | 100 (35%) | 33 (24%) | 0.034 |
| Serum creatinine (μmol/L) | 97±41 | 99±37 | 94±46 | NS |
| eGFR (mL/min/1.73 m2) | 78±26 | 76±25 | 80±26 | 0.07 |
| Diabetes | 88 (21%) | 70 (24%) | 18 (13%) | 0.01 |
| Renal failure | 106 (25%) | 79 (27%) | 27 (20%) | 0.1 |
| Implanted devices | ||||
| Single‐chamber ICD | 317 (74%) | 224 (77%) | 93 (68%) | 0.044 |
| Dual‐chamber ICD | 110 (26%) | 66 (28%) | 44 (32%) | |
| Cardiovascular medication | ||||
| Amiodarone | 46 (11%) | 29 (10%) | 17 (13%) | NS |
| Beta‐blocker | 332 (78%) | 238 (82%) | 94 (69%) | 0.004 |
| Digoxin | 87 (20%) | 53 (18%) | 34 (25%) | 0.1 |
| ACE inhibitor | 330 (78%) | 228 (79%) | 102 (75%) | NS |
| Diuretic | 252 (59%) | 170 (59%) | 82 (60%) | NS |
| Statin | 265 (62%) | 238 (82%) | 27 (20%) | <0.001 |
Abbreviations: ACE, angiotensin‐converting enzyme; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; NA, not applicable; NS, not significant; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Mortality
During a median follow‐up of 31 months (IQR 15–45 months), 30 (7%) patients died and 14 (3.3%) patients underwent heart transplantation. The total follow‐up consisted of 1070 person‐years; the crude death rate was 2.8 deaths per 100 person‐years. For the total cohort, the cumulative incidence of all‐cause mortality was 2.4%, 4.3%, and 14.7% at 1, 2, and 5 years of follow‐up, respectively. The cumulative incidence of all‐cause mortality was 2.4%, 9.2%, and 15.7% for CAD patients and 2.2%, 7.7%, and 12.5% for DCM patients at 1, 2, and 5 years of follow‐up, respectively. No significant difference in mortality was observed between CAD patients and DCM patients (Figure 1A). Univariate analyses for mortality are shown in Table 2. Patients older than 65 years of age at time of ICD implantation had a higher mortality compared to patients younger than 65 years of age (Figure 1B). Also, mortality was higher in males, and in patients in NYHA class III‐IV.
Figure 1.
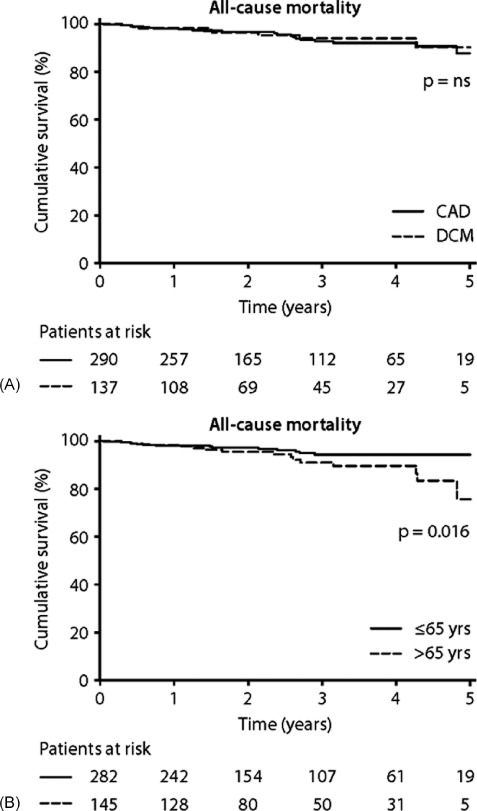
All‐cause mortality. (A) CAD and DCM patients. χ 2 = 0.14, P = 0.71. (B) Patients <65 years of age versus patients ≥ 65 years of age at time of ICD implantation. χ 2 = 5.84, P = 0.0157. Abbreviations: CAD, coronary artery disease; DCM, dilated cardiomyopathy; ICD, implantable cardioverter‐defibrillator; NS, not significant.
Table 2.
Univariate Analysis for All‐Cause Mortality
| Characteristic (n,%) | All (n = 427) | Survived (n = 397) | Deceased (n = 30) | P Value |
|---|---|---|---|---|
| Age <65 years | 282 (66%) | 268 (68%) | 14 (47%) | 0.03 |
| Male gender | 336 (79%) | 307 (77%) | 29 (97%) | 0.01 |
| Left ventricular ejection fraction, % | 27±9 | 27±9 | 25±9 | NS |
| Coronary artery disease | 290 (68%) | 268 (68%) | 22 (73%) | NS |
| History of myocardial infarction | 261 (61%) | 241 (61%) | 20 (67%) | NS |
| NYHA class at time of ICD implantation | ||||
| NYHA I‐II | 347 (82%) | 328 (83%) | 19 (63%) | <0.001 |
| NYHA III‐IV | 77 (18%) | 66 (17%) | 11 (37%) | |
| History of atrial fibrillation | 112 (26%) | 102 (26%) | 10 (33%) | NS |
| QRS >0.12 s | 133 (31%) | 122 (31%) | 11 (37%) | NS |
| Serum creatinin (μmol/L) | 97±41 | 96±89 | 110±98 | NS |
| eGFR (mL/min/1.73 m2) | 78±25 | 78±25 | 74±34 | NS |
| Diabetes | 88 (21%) | 80 (20%) | 8 (27%) | NS |
| Renal failure | 106 (25%) | 95 (24%) | 11 (37%) | NS |
| Cardiovascular medication | ||||
| Amiodarone | 46 (11%) | 43 (11%) | 3 (10%) | NS |
| Beta‐blocker | 332 (78%) | 311 (79%) | 21 (70%) | NS |
| Digoxin | 87 (21%) | 81 (21%) | 6 (20%) | NS |
| ACE inhibitor | 330 (78%) | 303 (77%) | 27 (90%) | 0.1 |
| Diuretic | 252 (59%) | 230 (59%) | 22 (73%) | 0.1 |
| Statin | 265 (62%) | 249 (63%) | 16 (53%) | NS |
Abbreviations: ACE, angiotensin‐converting enzyme; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; NA, not applicable; NS, not significant; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Multivariate Prediction of Mortality
A Cox proportional hazards model was fitted with age, male gender, NYHA class III‐IV, diuretic drug use, angiotensin‐converting enzyme (ACE) inhibitor used, and renal failure as independent variables, and mortality as dependent variable. This model showed that age <65 years (HR 0.43; 95% CI, 0.20–0.90), and NYHA class III‐IV (HR 1.77; 95% CI, 1.20–2.63) were independent predictors of mortality (Table 3).
Table 3.
Cox Proportional Hazards Analysis for All‐Cause Mortality
| Covariate | β | SE(β) | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| Age <65 years | −0.85 | 0.38 | 0.43 | 0.20–0.90 | 0.026 |
| Male gender | 1.96 | 1.02 | 7.13 | 0.96–52.92 | 0.055 |
| NYHA III‐IV | 0.57 | 0.20 | 1.77 | 1.20–2.63 | 0.005 |
| Diuretic use | 0.48 | 0.43 | 1.62 | 0.70–3.79 | NS |
| ACE‐inhibitor use | 0.82 | 0.62 | 2.26 | 0.68–7.52 | NS |
| Renal failure | 0.51 | 0.42 | 1.66 | 0.73–3.77 | NS |
Abbreviations: ACE, angiotensin converting enzyme; β, beta coefficient; CI, confidence interval; HR, hazard ratio; NS, not significant; NYHA, New York Heart Association; SE, standard error.
Appropriate ICD Interventions
During follow‐up, 92 patients (22%) experienced at least 1 episode of a ventricular tachyarrhythmia triggering ICD intervention. ATP was observed in 68 patients (16%) and ICD shock in 55 patients (13%). The first appropriate device intervention occurred at a median interval of 6.8 months (IQR 1.7–15.1 months) after ICD implantation. The mean cycle length of ventricular arrhythmias triggering appropriate ICD intervention was 290±55 ms. The proportion of patients free from ICD interventions was 85.4%, 78.8%, and 71.7%, at 1, 2, and 5 years of follow‐up, respectively. No significant difference was observed in the occurrence of appropriate ICD interventions between CAD patients and DCM patients (23% vs 21%, P = 0.71) (Figure 2A). Death or heart transplantation without prior appropriate ICD intervention was observed in 28 (64%) of the deceased/transplanted patients, whereas 36% of patients received appropriate device intervention prior to death or heart transplantation. Univariate analysis showed that appropriate ICD interventions occurred significantly more frequent in patients with AF compared to patients without AF (29% vs 19%, P = 0.023) (Figure 2B).
Figure 2.
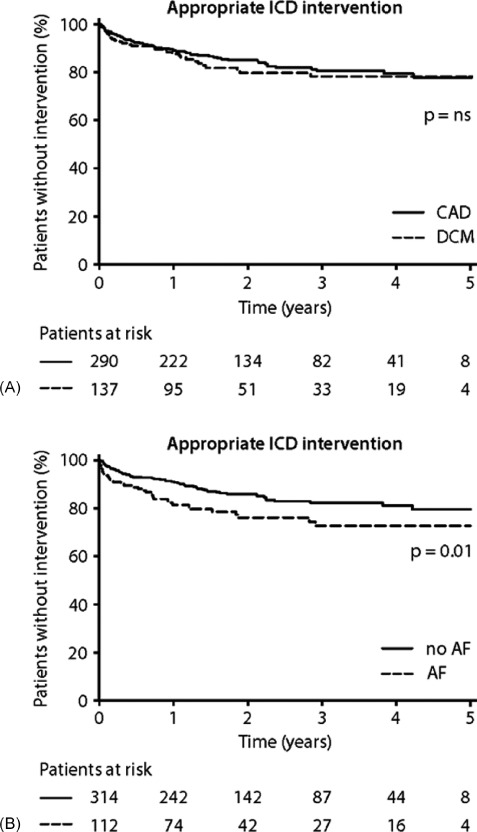
Appropriate ICD interventions. (A) CAD and DCM patients; χ 2 = 0.43, P = 0.5103. (B) Patients with AF versus patients without AF; χ 2 = 6.30, P = 0.0121. Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; DCM, dilated cardiomyopathy; ICD, implantable cardioverter‐defibrillator; NS, not significant.
Inappropriate ICD Interventions
Forty‐six patients (10.8%) experienced at least 1 inappropriately delivered ICD intervention, which occurred at a median interval of 7.3 months (IQR 1.3–14.6 months). The proportion of patients free from inappropriate ICD interventions was 92.0%, 88.9%, and 85.7%, at 1, 2, and 5 years of follow‐up, respectively. A total of 23 patients (5.4%) received an inappropriate ICD shock. The proportion of patients free from inappropriate ICD shocks was 96.2%, 95.2%, and 92.2%, at 1, 2, and 5 years of follow‐up, respectively. There was no significant difference in the occurrence of any inappropriate ICD intervention (ATP or shock) during follow‐up between patients with CAD and DCM. No significant difference in any inappropriate ICD therapy was observed between patients with AF and those without AF. Univariate analysis identified no factors associated with the occurrence of inappropriate ICD interventions.
Discussion
The present study evaluated the mortality and occurrence of ICD interventions in 2 major disease categories for which primary prophylactic ICDs are used: left ventricular dysfunction due to CAD or DCM. All received the device according to the international guidelines in the recent era (2004–2009). The major findings of this study are as follows: (1) after primary prophylactic ICD implantation, mortality is the same in CAD and DCM patients despite the younger age of the latter; (2) the incidence of appropriate ICD intervention was similar in CAD and DCM patients; (3) higher age at time of ICD implantation and appropriate ICD interventions during follow‐up are independent predictors of mortality; (4) AF is a predictor of appropriate ICD interventions; (5) inappropriate ICD interventions occur equally frequently in CAD patients and DCM patients.
Mortality
The observed all‐cause mortality in this cohort study is low; the cumulative incidence of all‐cause mortality at 5‐years of follow‐up was 15%, with a median follow‐up of 31 months, whereas Multicenter Automatic Defibrillator Implantation Trial II (MADIT‐II) reported a mortality of 16% at 2 years.4 Mortality was also lower compared to the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT), which also included CAD and DCM patients.5 In SCD‐HeFT, the cumulative 5‐year mortality was 29% with a median follow‐up of 46 months. The crude death rate in SCD‐HeFT was 5.7 deaths per 100 person‐years, compared to 2.8 deaths per 100 person‐years in our study cohort. Long‐term data of MADIT‐II show a 52% cumulative mortality at 8 years of follow‐up in patients with CAD.15 Recent European registries had a comparable low mortality.1, 2 The observed low mortality in our study group can possibly be explained by the age of the patients, which was certainly lower than that of MADIT‐II patients (mean 64 years) and the patients in the 2 cited registries (mean 63 and 66 years, respectively).1, 2, 4 The low observed mortality cannot be explained by differences in pharmacological therapy, as these are small; beta‐blockers were used by 82% of CAD patients (vs 70% in MADIT‐II), ACE inhibitors were used by 79% of CAD patients (vs 68% in MADIT‐II), and statins by 82% (vs 67% in MADIT‐II). This low mortality might indeed be a reason to reanalyze the value of ICDs for this indication, if it is assumed that disadvantages of ICD therapy became more important in recent times.
Appropriate ICD Interventions
In the present study, appropriate ICD interventions were delivered in 22% of patients. This is comparable with the reported proportions of patients with appropriate ICD interventions in the primary prevention trials (range 17.8%–31.4%).3, 4, 5, 16, 17, 18, 19 No differences in the incidence of appropriate interventions between CAD and DCM patients were observed. These data show that in a “real‐world” population of patients with prophylactic ICD implantation, appropriate ICD intervention is delivered in the same proportion of patients as in the landmark randomized clinical trials. The occurrence of appropriate ICD interventions is predicted by high age and by AF, as was previously shown by our group, and several others in ICD patients in general.20, 21, 22 This is not surprising, as AF can be a consequence of advancing heart failure, and as this can initiate ventricular arrhythmias in those who are prone for such events. The excess mortality in those receiving appropriate ICD interventions during follow‐up was also observed in recent studies.23 Whether this is due to progression of the disease remains unclear. Our data were not influenced by the addition of CRT, which has a moderate effect on mortality, but a substantial effect on morbidity, if given to the right patients.10, 24, 25 The observed rate of appropriate ICD interventions in both disease categories can be regarded as an indication that the actual guidelines remain valid.
Inappropriate ICD Interventions
Delivery of inappropriate interventions due to misclassification of atrial tachyarrhythmias as ventricular tachyarrhythmias is the most reported adverse event in ICD recipients.2, 14, 26 Frequencies of inappropriate device interventions up to 21% were reported in the primary prevention trials.18, 27 The proportion of patients with any inappropriate ICD intervention (ATP or shock) is only 11% in our study, with a cumulative incidence of 14% at 5 years of follow‐up. This probably is due to consequent programming.14 Strategic programming can further reduce inappropriate device interventions.28 The frequency of inappropriate delivered shocks can probably be further lowered, both by adaptations from software and hardware.29, 30
Limitations
Because this is an observational study without control group, no statements about the benefit of ICD therapy can be made. We did include a significant number of DCM patients. This can be due to the fact that we serve as a tertiary referral center for heart transplantation, which would imply that several of these patients have a rather unfavorable prognosis. This can be the explanation that they have the same mortality, in spite of their younger age.
Conclusion
In conclusion, the present study shows that the mortality and the occurrence of appropriate and inappropriate ICD interventions in a “real‐world” population are similar after primary prophylactic ICD‐only implantation for both disease categories. Mortality is predicted by high age at time of ICD implantation and by symptoms in NYHA class III‐IV. ICD interventions are predicted by the presence of AF. This suggests that DCM, even at a younger age, is a strong indication for prophylactic ICD therapy, as described in the current guidelines, but which is unfortunately not always appreciated in current practice. It has to be pointed out that these data excluded patients with a CRT‐D indication.
References
- 1. Santini M, Russo M, Botto G, et al. Clinical and arrhythmic outcomes after implantation of a defibrillator for primary prevention of sudden death in patients with post‐myocardial infarction cardiomyopathy: The Survey to Evaluate Arrhythmia Rate in High‐risk MI patients (SEARCH‐MI). Europace. 2009;11:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gradaus R, Block M, Brachmann J, et al. Mortality, morbidity, and complications in 3344 patients with implantable cardioverter defibrillators: results from the German ICD Registry EURID. Pacing Clin Electrophysiol. 2003;26:1511–1518. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 5. Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 6. Foley PWX, Addison CE, Whinney SB, et al. Implantable cardioverter defibrillator therapy for primary prevention of sudden cardiac death after myocardial infarction: implications of international guidelines. Pacing Clin Electrophysiol. 2009;32:S131–S134. [DOI] [PubMed] [Google Scholar]
- 7. Israel CW. Do some implant too many defibrillators or others too few? Europace. 2009;11:982–984. [DOI] [PubMed] [Google Scholar]
- 8. Zipes DP, Camm AJ, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace. 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 9. Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary. Heart Rhythm. 2008;5: 934–955. [DOI] [PubMed] [Google Scholar]
- 10. Rivero‐Ayerza M, Theuns DA, Carcia‐Carcia HM, et al. Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta‐analysis of randomised controlled trials. Eur Heart J. 2006;27:2682–2688. [DOI] [PubMed] [Google Scholar]
- 11. Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . et al: The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 12. Bristow MR, Saxon LA, Boehmer J, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 13. Schaer B, Kuhne M, Koller MT, et al. Therapy with an implantable cardioverter defibrillator (ICD) in patients with coronary artery disease and dilated cardiomyopathy: benefits and disadvantages. Swiss Med Wkly. 2009:139:647–653. [DOI] [PubMed] [Google Scholar]
- 14. Theuns DA, Klootwijk AP, Goedhart DM, et al. Prevention of inappropriate therapy in implantable cardioverter‐defibrillators: results of a prospective, randomized study of tachyarrhythmia detection algorithms. J Am Coll Cardiol. 2004;44: 2362–2367. [DOI] [PubMed] [Google Scholar]
- 15. Cygankiewicz I, Gillespie J, Zareba W, et al. Predictors of long‐term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter‐defibrillators. Heart Rhythm. 2009;6:468–473. [DOI] [PubMed] [Google Scholar]
- 16. Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105: 1453–1458. [DOI] [PubMed] [Google Scholar]
- 17. Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter‐defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia‐AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–1712. [DOI] [PubMed] [Google Scholar]
- 18. Kadish A, Dyer A, Daubert J, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 19. Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter‐defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 20. Theuns DA, Klootwijk AP, Simoons ML, et al. Clinical variables predicting inappropriate use of implantable cardioverter‐defibrillator in patients with coronary heart disease or nonischemic dilated cardiomyopathy. Am J Cardiol. 2005;95:271–274. [DOI] [PubMed] [Google Scholar]
- 21. Gronefeld GC, Mauss O, Li YG, et al. Association between atrial fibrillation and appropriate implantable cardioverter defibrillator therapy: results from a prospective study. J Cardiovasc Electrophysiol. 2000;11:1208–1214. [DOI] [PubMed] [Google Scholar]
- 22. Smit MD, Van Dessel PF, Rienstra M, et al. Atrial fibrillation predicts appropriate shocks in primary prevention implantable cardioverter‐defibrillator patients. Europace. 2006;8:566–572. [DOI] [PubMed] [Google Scholar]
- 23. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 25. Cleland JG, Daubert JC, Erdmann E, et al. Longer‐term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization‐Heart Failure (CARE‐HF) trial extension phase]. Eur Heart J. 2006;27:1928–1932. [DOI] [PubMed] [Google Scholar]
- 26. Sweeney MO, Wathen MS, Volosin K, et al. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111: 2898–2805. [DOI] [PubMed] [Google Scholar]
- 27. Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 28. Wilkoff BL, Williamson BD, Stern RS, et al. Strategic programming of detection and therapy parameters in implantable cardioverter‐defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–550. [DOI] [PubMed] [Google Scholar]
- 29. Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous only implantable cardioverter defibrillator. N Engl J Med. 2010;363: 36–44. [DOI] [PubMed] [Google Scholar]
- 30. Hauser RG, Hayes DL, Epstein AE, et al. Multicenter experience with failed and recalled implantable cardioverter‐defibrillator pulse generators. Heart Rhythm. 2006;3:640–644. [DOI] [PubMed] [Google Scholar]


