Abstract
Background:
According to published evidence, treatment of infective endocarditis (IE) associated with cardiovascular implantable electronic devices (CIEDs) should include complete removal of the system. Several publications have shown that transvenous removal is an effective and safe nonthoracotomy approach in patients with large vegetations, but experiences with vegetations larger than 20 mm have rarely been reported.
Hypothesis:
Our aim was to describe our experience in percutaneous removal of CIEDs in patients with IE with large vegetations.
Methods:
The data were collected retrospectively and analyzed prospectively. We evaluated in‐hospital morbidity and mortality related to percutaneous removal of vegetations ≥20 mm. This included 8 cases with a follow‐up period of 20 months. We removed 100% of leads in the study population.
Results:
Two patients experienced minor complications. No patient experienced subclavian vein laceration, hemothorax and lead fracture, or severe tricuspid regurgitation. After the removal procedure, 2 patients had symptoms compatible with pulmonary embolism. Both in‐hospital mortality and mortality at follow‐up were zero.
Conclusions:
Transvenous extraction of pacing leads with larger vegetations is a feasible technique. There was a tendency toward symptomatic pulmonary embolism in patients with vegetations larger than 20 mm; however, morbidity and mortality were not influenced. We agree with the consensus that this procedure is highly useful and that the selection of the removal techniques will depend not only on the size of vegetation but also on prior cardiopulmonary conditions, concomitant cardiac surgery, atrial septal defect with risk of paradoxical embolism, center experience, and the possibility of complete removal of the device. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Several publications and scientific lectures on percutaneous extraction of cardiovascular implantable electronic devices (CIEDs) in patients with infective endocarditis (IE) have focused on factors associated with complications related to the procedure. Investigators have discussed fibrosis around the electrode and adherence to the surrounding cardiac tissue (myocardium, tricuspid valve, and vein) with risk of damage during removal.1
Transvenous extraction of large vegetations in patients with IE associated with CIED is controversial. Vegetations >10 mm have historically been removed by surgery because of the risk of embolization and hemodynamic compromise. At present, management of patients with IE associated with CIED and large vegetations is still under discussion.2, 3 The latest update on CIED infection recommends that removal of leads with vegetations larger than 2 cm should be individualized and considered based on the patient's clinical parameters and the extractor's evaluation.2
Herein, we describe our experience in percutaneous removal of CIED in patients with IE with vegetations ≥20 mm.
Methods
Study Population
Between July 2002 and March 2009, patients diagnosed with IE associated with CIED and echocardiographic evidence of intracardiac vegetations ≥20 mm were admitted into the Universidad Abierta Interamericana Hospital, a tertiary referral center for complicated endocarditis.
Endpoints
The primary endpoint was to determine the efficacy and complications related to percutaneous removal of vegetations ≥20 mm, in‐hospital morbidity and mortality, and outcomes after a follow‐up period of 20 months.
Definitions
The diagnosis of endocarditis was confirmed using the modified Duke criteria in patients with a history of implantation of a CIED (pacemaker or implantable cardioverter defibrillator [ICD]).
Lead extraction included surgical backup, personnel, facilities, training, and outcomes pertaining to leads implanted for at least 1 year or requiring the assistance of specialized equipment not included as part of the typical implant tool set.4
Pocket involvement included local signs of inflammation at the site of the generator together with erythema, heat, swelling, wound dehiscence, erosion, tenderness, or purulent drainage.
Immunosuppression was defined as a state in which the ability of the body's immune system to respond decreased, as occurs in cases of human immunodeficiency virus infection, and treatments included corticosteroids and cytotoxic drugs.
Laboratory data included leukocytosis (>10 000/mm3), high erythrocyte sedimentation rate (>0 mm in first hours), and elevated C‐reactive protein (>5 mg/L).
Transesophageal echocardiography (TEE) was performed in all cases when device endocarditis was suspected. Intracardiac vegetation was defined as a discrete, echogenic, oscillating mass found on a valve, lead, or endocardial surface and confirmed by echocardiography in multiple views. Echocardiograms were reviewed by an echocardiographer to determine vegetation size and location and to distinguish true vegetations from false echodensities.
The mobility of the vegetation was classified as follows5: absent, low, moderate, or severe. Diagnosis of pulmonary embolism was based on the classical clinical criteria and confirmed by ventilation‐perfusion scans. Definition of major or minor complications was construed according the Heart Rhythm Society Expert Consensus.4
Current Protocol
Twenty‐one patients had a diagnosis of IE associated with CIED; 13 patients who did not present vegetations ≥20 mm in TEE and/or who underwent open surgery technique were excluded from the study (including 1 case with vegetation <20 mm and a lead abandoned in its right ventricle with impossibility of complete transvenous extraction).
Transthoracic echocardiography (TTE) and TEE studies were performed using a commercially available (USA) HP Sonos echocardiographic system. In all patients, the transesophageal study was performed immediately after the transthoracic study. To rule out new tricuspid regurgitation (TR), A TTE study was performed before discharge.
The same protocol was used for each patient, and the information has been recorded retrospectively and analyzed prospectively. In all patients, medical history, physical examination, blood tests, microbiologic studies, TTE and TEE images, and vegetation morphology were analyzed. A descriptive analysis of the different epidemiologic variables regarding in‐hospital outcomes and outcomes after a 20‐month period was carried out. Intravenous (IV) antibiotics (ATBs) were administered during 6 weeks.
All patients gave their informed consent before the extraction procedure. Lead removal was performed surgically with local anesthesia (anesthesia and cardiovascular surgical services were available on site to provide support in the event of complications). Locking stylets (Liberator Universal Locking Stylet; Cook Medical Inc, Bloomington, IN) and polypropylene telescopic dilator sheaths (8.5‐Fr Byrd dilator sheaths; Cook Medical Inc) were used for percutaneous extraction of the system. Neither sole manual traction nor retrieval baskets or snares were used.
Patients with vegetations and clinical evidence of embolism underwent pulmonary perfusion‐ventilation scintigraphy for detection of pulmonary embolism after lead extraction.
Follow‐up after discharge was performed by medical visits to the center every 3 months or through telephone calls in case of patients living far away.
Statistical Analysis
Continuous variables were expressed as median and interquartile ranges (IQRs), and discrete variables were expressed by absolute values and percentages. Statistical data management was performed using the statistics pack SPSS version 11.0.1 (2001) (SPSS Inc, Chicago, IL). Statistical significance was established at P < 0.05.
Results
Baseline Characteristics and Clinical Presentation
Twenty‐one patients with IE associated with CIED were identified: 8 cases with vegetations ≥20 mm were included (Table 1). Median age was 65 years (IQR, 59.5–69.7 years); 75% were male. All patients had at least 1 risk factor for CIED infection/endocarditis: renal dysfunction, diabetes mellitus, other causes of immunosuppression, or device reimplantation. The clinical signs on admission were pocket erythema, secretion, ulcer, or pain (62%); febrile syndrome (75%); bacteriemia (50%); and hypotension, which was present in 1 patient (12%). The laboratory tests showed leukocytosis in 75% of cases (6 patients).
Table 1.
Study Participants
| Population (N = 21) | Vegetation ≥20 mm, n = 8 | Vegetation <20 mm, n = 13 | P Value | |
|---|---|---|---|---|
| Age, y, mean (range) | 65.3 (53–75) | 70.0 (37–92) | NS | |
| Sex, male, no. (%) | 6 (75) | 9 (69) | NS | |
| Basal disease, no. (%) | Previous device replacement | 3 (37) | 6 (46) | NS |
| Diabetesmellitus | 2 (25) | 4 (31) | NS | |
| Immunosuppression | 1 (12) | 2 (15) | NS | |
| Neoplasm | 2 (25) | 2 (15) | NS | |
| Chronic renal failure | 1 (12) | 1 (8) | NS | |
| Dispositive, no. (%) | Permanent pacemaker (VVI) | 2 (25) | 3 (23) | 0.925 |
| Permanent pacemaker (DDD) | 5 (62) | 8 (62) | 0.967 | |
| Implantable cardioverter defibrillator | 1 (12) | 2 (15) | 0.863 | |
| Implantation leads, median time from (range), mo | 12.5 (4–21) | 14.8 (3–26) | 0.941 | |
| Clinical evidence, no. (%) | Fever | 6 (75) | 10 (77) | 0.925 |
| Erosion or pocket infection | 5 (62) | 10 (77) | 0.502 | |
| Hypotension (systolic blood pressure <90 mm Hg) | 1 (12) | 0 (0) | NS | |
| Laboratory abnormalities, no. (%) | Leukocytosis | 6 (75) | 10 (77) | 0.925 |
| High erythrocyte sedimentation | 7 (87) | 11 (85) | 0.863 | |
| Elevated C‐reactive protein | 8 (100) | 12 (92) | 0.447 | |
| Cultures, no. (%) | Positive blood cultures | 7 (87) | 12 (92) | 0.732 |
| Positive lead | 5 (62) | 9 (69) | 0.765 | |
| Echocardiography, no. (%) | Positive transthoracic | 5 (62) | 5 (38) | 0.308 |
Abbreviations: NS, not significant.
Preoperative characteristics of patients with infective endocarditis associated with cardiovascular implantable electronic devices. Continuous variables are expressed as median (range), and discrete variables are expressed by absolute values and percentages. Statistical significance was established at P < 0.05.
Microbiology
Blood cultures were positive in 7 patients and lead cultures were positive in 62%. Coagulase‐negative staphylococcus and Staphylococcus aureus were the most common infective organisms, isolated in 67% of blood cultures (Figure 1).
Figure 1.
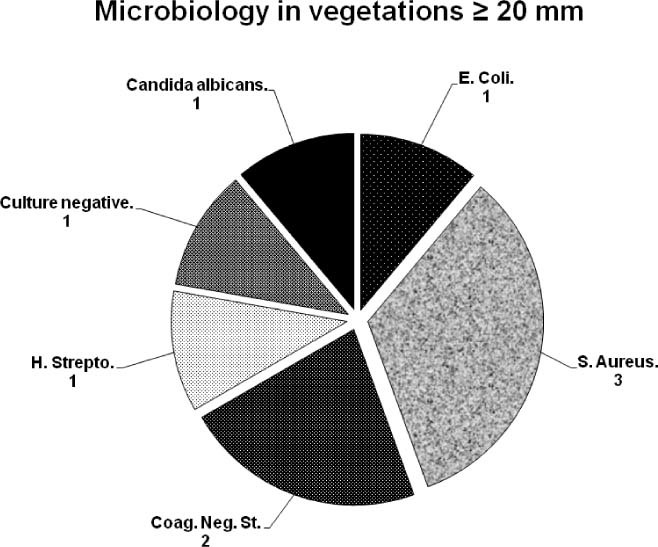
Microbiology data. Infectious organisms isolated from blood cultures. Abbreviations: S. Aureus, Staphylococcus aureus; Coag. Neg. St., coagulase‐negative staphylococcus; H. Strepto: beta hemolytic streptococcus; E. Coli: Escherichia coli.
Echocardiography
All patients underwent a TTE study, which was positive in 62% of cases. Vegetations were located on the right atrial lead in 4 patients and on the ventricular lead in 4 patients; 25% had compromised the atrial endocardium and tricuspid valve (Figure 2). Vegetation size ranged from 20 to 28 mm in the largest longitudinal diameter; the median diameter was 22 mm (IQR, 2,25 mm). Seventy‐five percent of vegetations were sessile. Tricuspid regurgitation (TR) after extraction occurred in 3 patients but was not severe in any patient (Table 2).
Figure 2.
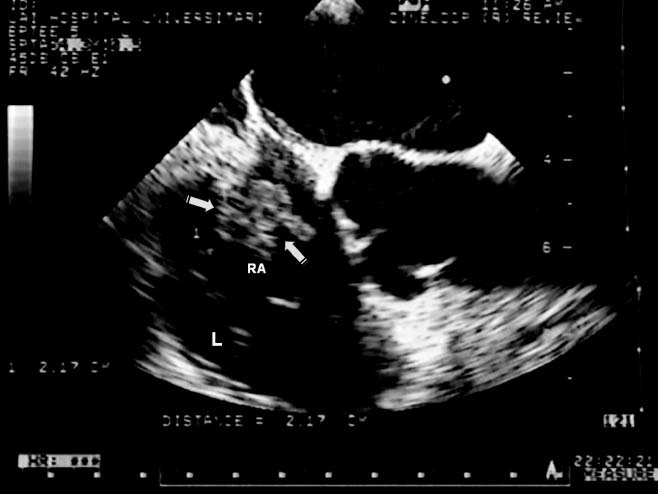
Vegetation and transesophageal echocardiography (TEE). Large vegetation on a right atrial lead seen with TEE. The largest longitudinal diameter is 22 mm (arrow). Abbreviations: RA, right atrial; L, leads.
Table 2.
Echocardiographic Findings in 8 Patients With Infective Endocarditis With Cardiovascular Implantable Electronic Devices
| Finding | No. (%) |
|---|---|
| Positive Vegetation | |
| TTE | 5/8 (62) |
| TEE | 8/8 (100) |
| Location of vegetation | |
| Auricular leads | 4/6 (67) |
| Ventricular leads | 4/8 (50) |
| Endocardium or tricuspid valve | 2/8 (25) |
| Both (lead and endocardium) | 2/8 (25) |
| Mobility of vegetation | |
| Sessile | 6/8 (75) |
| Pedunculated | 2/8 (25) |
| Size of vegetation, mm | 22, 2.25 |
Abbreviations: TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Continuous variables are expressed as median (interquartile range), and discrete variables are expressed as absolute values and percentages.
Treatment
All patients started empirical broad‐spectrum IV ATB treatment within 6 hours after admission, once blood cultures had been taken; 50% patients switched to cephalothin according to bacteriologic sensitivity.
Lead extraction was performed by 2 operators. For percutaneous removal of the system, locking stylets (in all leads) and polypropylene telescopic dilator sheaths (in leads implanted more than 6 months ago) were used. Median time until removal of an infected device was 2,5 days (IQR, 2–3 days). The most common type of lead model extracted was ventricular passive fixation (7 ventricular leads, 6 atrial leads, and 1 ventricular ICD lead). Extraction was completed in all patients with vegetation ≥20 mm. In turn, in the group of patients with vegetations <20 mm, the success rate was 91% (not statistically significant).
Reimplantation of a New Device
The new device was reimplanted after completion of the ATB treatment. No patient required a transient pacemaker. Seven patients (87%) underwent reimplantation of a new device during hospitalization (1 patient refused reimplantation) using transvenous lead insertion with implantation of a generator on the opposite side of the infected pocket. Median time to reimplantation was 42 days (IQR, 42–43.2 days).
Outcomes
All leads (n = 14) were completely removed. Two patients had minor complications of percutaneous lead extraction such as pocket hematoma. None of the patients experienced vein laceration, hemothorax, or severe TR. Following removal, 2 patients had symptoms compatible with pulmonary embolism without hemodynamic collapse (minor complications), confirmed by ventilation‐perfusion scans showing defects in pulmonary perfusion (Figure 3). No patients with vegetations <20 mm developed symptoms compatible with pulmonary embolism or other major complication (only 2 patients had pocket hematomas).
Figure 3.
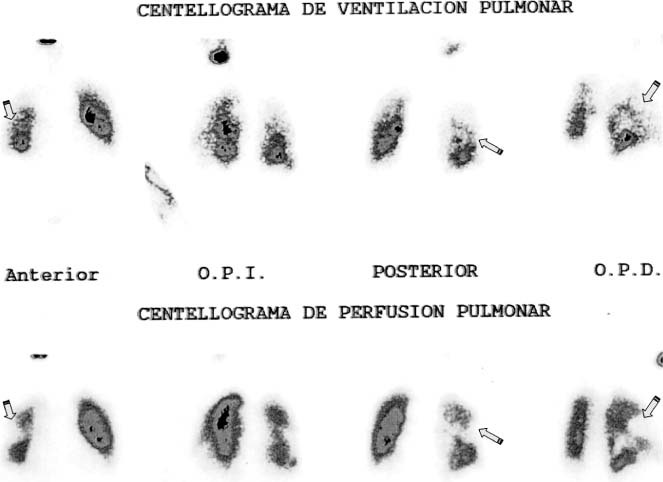
Pulmonary embolism. Ventilation‐perfusion scan of 1 patient who presented with dyspnea after percutaneous pacemaker lead extraction with perfusion defects compatible with embolism (arrows).
Median hospital stay was 45 days (IQR, 44–46.2 days). Follow‐up was carried out in 8 patients. After discharge, patients were evaluated by medical visits every 3 months or telephone calls in case of patients living far away. Median follow‐up was 612 days (IQR, 608.7–615.2 days). During follow‐up, no patient had clinical indicators of relapsing infection or other complications. None of the patients studied presented severe TR or died during follow‐up. The same results were noted on follow‐up of patients with <20‐mm vegetations.
Discussion
According to various studies, IE associated with CIED is an uncommon complication.2, 6, 7, 8, 9, 10, 11 The most efficient treatment is the complete removal of the device and IV ATB for 6 weeks.2, 7, 12 Antibiotic (ATB) treatment alone or partial removal of the device has proved to be ineffective, with a high incidence of relapse and death.9, 13, 14, 15 Two different techniques have been used for leads extraction: open heart and transvenous extraction. The open surgery with cardiopulmonary bypass has higher complication rates and is more invasive than the transvenous approach.16 Transvenous removal by using locking stylets and dilator sheaths can be performed as an alternative to sternotomy.2, 3, 12, 13 In different studies, transvenous lead extraction was associated with minimal morbidity and mortality.17, 18 The incidence of major complications reported by different series of registries is variable, ranging from 0.4% to 3.3%, and can generally be attributed to patient risk factors and operator experience.3, 18, 19, 20, 21, 22
One factor associated with increased risk of complications was the time from implantation. Fibrous scar around the body of the lead from the subclavian vein to the right ventricle is a frequent finding. Some authors have described intense endocardial fibrosis encasing ICD leads, making then adhere strongly to the adjacent structures.19, 20, 21, 22 Byrd et al. reported an increased risk of failed or partial extraction with longer time from implantation, doubling the risk at 3 years.17, 18, 19, 20, 21, 22, 23, 24, 25 A shorter dwell time significantly improved lead extraction success. However, it is important to note that severe scarring has also been reported to occur in shorter periods such as 4 months.4 Removal of such leads can be a significantly complex procedure requiring tools and techniques that allow freeing the lead at fibrotic binding sites. In our study, the median time from implantation was 12.5 months (range, 4–21), and locking stylets (in all leads) and polypropylene telescopic dilator sheaths (in leads implanted more than 6 months ago) were used for transvenous extraction of the system. Other risk factors that portend difficult lead extraction and complication included young patients, female patients, and ICD leads.3 When analyzing the effectiveness and complications of extraction in our series, we found that 2 patients developed pocket hematoma (not requiring intervention), and extraction of the leads was complete in all patients. No patient experienced cardiac, hemothorax, subclavian vein laceration, or severe TR. Other authors have reported transvenous extraction success rates of 93% and 97% and major complication in <2%.18, 25 The following factors might be associated with a high improved lead extraction success and low complication rate: short time from implantation, low‐risk patients, extraction techniques, and experience of operators.
Another scenario occurs in cases of patients with IE associated with CIED and large vegetations. Historically, patients with vegetations >10 mm have been managed with open surgery because of potential pulmonary embolism and hemodynamic compromise. At present, management of patients with IE associated with CIED and large vegetations is under review.
Vegetation size is a controversial issue, because some authors believe that transvenous removal of leads with vegetations of up to 10 mm is possible. The study by Klug et al14 was the first in presenting a group of patients undergoing transvenous lead removal for large vegetations (vegetations >10 mm); 40% had pulmonary embolism but not associated with increased risk of mortality. They concluded that the technique of removal seemed to be safe in patients with vegetation >1 cm. Meier‐Ewert et al26 reported the successful transvenous removal in 9 patients with large vegetations (diameter, 10–38 mm). Pulmonary embolism occurred in 5 patients (55%), but survival and length of hospitalization were not influenced by this complication. Ruttmann et al27 described the safe and highly effective procedure in patients with vegetations >10 mm (mean vegetation, 1.78 ± 0.6 cm). They recommended that patients with vegetations larger than 2.5 cm should undergo open surgery lead removal. In turn, Sohail et al28 reported that no patient had a clinically significant pulmonary embolism (median diameter, 11 mm; IQR, 8–20 mm). In the study by Grammes et al29 it was shown that percutaneous lead extraction with vegetations of all sizes was possible and seemingly appropriate (range, 0.2–4 cm; mean diameter, 1.6 cm). They did not find that vegetations had a significant effect on procedure‐related short‐term mortality.22, 26, 27, 28, 29
However, there is still uncertainty concerning larger vegetations.2 In our study, only patients with IE associated with CIED and vegetations ≥20 mm (between 20 and 28 mm; median diameter, 22 mm) were selected. Experiences with vegetations larger than 20 mm have rarely been reported (isolated cases).7, 26, 29, 30 Patients with vegetation <20 mm were not included, as they were in other studies with large vegetations.
In our study, the real incidence of silent pulmonary embolism is unknown. Symptoms associated with pulmonary embolism were dyspnea, tachycardia, and tachypnea (2 patients). One patient was a 56‐year‐old woman with polymicrobial IE and a vegetation of 28 mm. The other patient was a 72‐year‐old man, under immunosuppressant treatment, with positive Escherichia coli blood cultures; TEE revealed a 23‐mm vegetation. Although there was a tendency for symptomatic pulmonary embolism in patients with vegetations larger than 20 mm (this was not statistically significant; P = 0.171), the morbidity and mortality and length of hospitalization were not influenced by this complication. Among the causes that explain the low morbidity of pulmonary embolism in patients with vegetations >20 mm are the characteristics of the embolic material. The vegetations are of colonies of microorganisms and leukocytes embedded in a matrix of fibrin and platelets with increased proteolytic activity, so it is more friable as compared to thrombotic embolus.1, 31 Another factor was low comorbidities of our patients.
Study Limitations
This study had several limitations. The main limitation was the small number of patients. The study also was retrospective. Further, only patients with clinical evidence of pulmonary embolism underwent pulmonary perfusion‐ventilation scan. The real incidence of silent pulmonary embolism is unknown and could have been underestimated. Most patients were referred from other centers, thus affecting the time of definite diagnosis and initiation of treatment with subsequent increase in morbidity and mortality. However, this fact was not observed.
Conclusion
Transvenous lead extraction in patients with large vegetations is a feasible technique. Among the factors associated with complete lead extraction success and the low complication rate, we found short time from implantation, low‐risk patients, extraction techniques, and experience of operators. There was a tendency toward symptomatic pulmonary embolism in patients with vegetations larger than 20 mm; however, the morbidity and mortality were not influenced by this complication in our series.
We agree with the consensus that this procedure is highly useful and that the selection of the removal techniques will depend not only on the size of vegetation, but also on prior cardiopulmonary conditions, concomitant cardiac surgery, atrial septal defect with risk of paradoxical embolism, center experience, cardiovascular surgical services available on site to provide support in the event of complications, and the possibility of complete removal of the device.
References
- 1. Robboy S, Harthorne J, Leinbach R, et al. Autopsy findings with permanent pervenous pacemakers. Circulation. 1969;39: 495–501. [DOI] [PubMed] [Google Scholar]
- 2. Baddour L, Epstein A, Erickson C, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. [DOI] [PubMed] [Google Scholar]
- 3. Field M, Jones S, Epstein L. How to select patients for lead extraction. Heart Rhythm. 2007;4:978–985. [DOI] [PubMed] [Google Scholar]
- 4. Wilkoff B, Love C, Byrd C. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm. 2009;6:1085–1104. [DOI] [PubMed] [Google Scholar]
- 5. Di Salvo G, Habib G, Pergola V, et al. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol. 2001;37:1069–1076. [DOI] [PubMed] [Google Scholar]
- 6. Catachin A, Murdock C, Athan E. Pacemaker infections: a 10‐year experience. Heart Lung Circ. 2007;16:434–439. [DOI] [PubMed] [Google Scholar]
- 7. Sohail M, Uslan D, Khan A, et al. Management and outcome of permanent pacemaker and implantable cardioverter‐defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859. [DOI] [PubMed] [Google Scholar]
- 8. Uslan D, Sohail M, St. Sauver J, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population‐based study. Arch Intern Med. 2007;167:669–675. [DOI] [PubMed] [Google Scholar]
- 9. Rundstrom H, Kennergren C, Andersson R, et al. Pacemaker endocarditis during 18 years in Goteborg. Scand J Infect Dis. 2004;36:674–679. [DOI] [PubMed] [Google Scholar]
- 10. Kleemann T, Becker T, Strauss M, et al. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace. 2010;12: 58–63. [DOI] [PubMed] [Google Scholar]
- 11. Vilacosta I, Sarria C, San Roman J, et al. Usefulness of transesophageal echocardiography for diagnosis of infected transvenous permanent pacemakers. Circulation. 1994;89:2684–2687. [DOI] [PubMed] [Google Scholar]
- 12. Vogt P, Sagdic K, Lachat M, et al. Surgical management of infected permanent transvenous pacemaker systems: ten year experience. J Card Surg. 1996;11:180–186. [DOI] [PubMed] [Google Scholar]
- 13. Del Rio A, Anguera I, Miro J, et al. Surgical treatment of pacemaker and defibrillator lead endocarditis: the impact of electrode lead extraction on outcome. Chest. 2003;124:1451–1459. [DOI] [PubMed] [Google Scholar]
- 14. Klug D, Lacroix D, Savoye C, et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation. 1997;95:2098–2107. [DOI] [PubMed] [Google Scholar]
- 15. Villamil Cajoto I, Rodriguez Framil M, Van den Eynde Collado A, et al. Permanent transvenous pacemaker infections: An analysis of 59 cases. Eur J Intern Med. 2007;18:484–488. [DOI] [PubMed] [Google Scholar]
- 16. Rusanov A, Spotnitz H. A 15‐year experience with permanent pacemaker and defibrillator lead and patch extractions. Ann Thorac Surg. 2010;89:44–50. [DOI] [PubMed] [Google Scholar]
- 17. Mathur G, Stables R, Heaven D, et al. Cardiac pacemaker lead extraction using conventional techniques: a single centre experience. Int J Cardiol. 2003;9:215–219. [DOI] [PubMed] [Google Scholar]
- 18. Jones IV S, Eckart R, Albert C, et al. Large, single‐center, single‐operator experience with transvenous lead extraction: Outcomes and changing indications. Heart Rhythm. 2008;5:520–525. [DOI] [PubMed] [Google Scholar]
- 19. Hauser R, Katsiyiannis W, Gornick C, et al. Deaths and cardiovascular injuries due to device‐assisted implantable cardioverter–defibrillator and pacemaker lead extraction. Europace. 2010;12:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith H, Fearnot N, Byrd C, et al. Five years experience with intravascular lead extraction. Pacing Clin Electrophysiol. 1994;17:2016–2020. [DOI] [PubMed] [Google Scholar]
- 21. Bracke F, Meijer A, Van Gelder L. Lead extraction for device related infections: a single‐centre experience. Europace. 2004;6: 243–247. [DOI] [PubMed] [Google Scholar]
- 22. Smith M, Love C. Extraction of transvenous pacing and ICD leads. Pacing Clin Electrophysiol. 2008;31:736–752. [DOI] [PubMed] [Google Scholar]
- 23. Le Franc P, Klug D, Jarwe M, et al. Extraction of endocardial implantable cardioverter‐defibrillator leads. Am J Cardiol. 1999;84:187–191. [DOI] [PubMed] [Google Scholar]
- 24. Wilkoff B, Byrd C, Love C, et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671–1676. [DOI] [PubMed] [Google Scholar]
- 25. Byrd C, Wilkoff B, Love C, et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994‐1996. Pacing Clin Electrophysiol. 1999;22:1348–1357. [DOI] [PubMed] [Google Scholar]
- 26. Meier‐Ewert H, Gray M, John R. Endocardial pacemaker or defibrillator leads with infected vegetations: a single‐center experience and consequences of transvenous extraction. Am Heart J. 2003;146:339–344. [DOI] [PubMed] [Google Scholar]
- 27. Ruttmann E, Hangler H, Kilo J, et al. Transvenous pacemaker lead removal is safe and effective even in large vegetations: an analysis of 53 cases of pacemaker lead endocarditis. Pacing Clin Electrophysiol. 2006;29:231–236. [DOI] [PubMed] [Google Scholar]
- 28. Sohail M, Uslan D, Khan A, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter‐defibrillator infection. Mayo Clin Proc. 2008;83:46–53. [DOI] [PubMed] [Google Scholar]
- 29. Grammes J, Schulze C, Al‐Bataineh M, et al. Percutaneous pacemaker and implantable cardioverter‐defibrillator lead extraction in 100 patients with intracardiac vegetations defined by transesophageal echocardiogram. J Am Coll Cardiol. 2010;55: 886–894. [DOI] [PubMed] [Google Scholar]
- 30. Calton R, Cameron D, Cusimano R, et al. Successful laser‐assisted removal of an infected ICD lead with a large vegetation. Pacing Clin Electrophysiol. 2006;29:910–913. [DOI] [PubMed] [Google Scholar]
- 31. Rupp M, Fey P, Heilmann C, et al. Characterization of the Importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter–associated infection in a rat model. J Infect Dis. 2001;183:1038–1042. [DOI] [PubMed] [Google Scholar]


