Abstract
Background:
Data on the effect of revascularization on outcome in patients with high‐risk non–ST‐segment elevation acute coronary syndrome (NSTEACS) and significant comorbidities are scarce. Recently, a simple comorbidity index (SCI) including 5 comorbidities (renal failure, dementia, peripheral artery disease, heart failure, and prior myocardial infarction [MI]) has shown to be a useful tool for risk stratification. Nevertheless, therapeutic implications have not been derived.
Hypothesis:
We sought to evaluate the prognostic effect attributable to revascularization in NSTEACS according the SCI score.
Methods:
We included 1017 consecutive patients with NSTEACS. The effect of revascularization on a combined end point of all‐cause mortality or nonfatal MI was evaluated by Cox regression according to SCI categories.
Results:
A total of 560 (55.1%), 236 (23.2%), and 221 (21.7%) patients showed 0, 1, and ≥2 points according to the SCI, respectively. Coronary angiography was performed in 725 patients (71.5%), and 450 patients (44.3%) underwent revascularization. During a median follow‐up of 16 months (interquartile range, 12–36 months), 305 (30%) patients experienced the combined end point (202 deaths [19.9%] and 170 MIs [16.7%]). In multivariate analysis, a differential prognostic effect of revascularization was observed comparing SCI ≥2 vs 0 (P for interaction = 0.008). Thus, revascularization was associated with a greater prognostic benefit in patients with SCI ≥2 (hazard ratio [HR]: 0.51, 95% confidence interval [CI]: 0.29–0.89), P = 0.018), whereas no significant benefit was observed in those with 0 and 1 point (HR: 1.31, 95% CI: 0.88–1.94, P = 0.171 and HR: 1.11, 95% CI: 0.70–1.76, P = 0.651, respectively).
Conclusions:
In NSTEACS, the SCI score appears to be a useful tool for identifying a subset of patients with a significant long‐term death/MI risk reduction attributable to revascularization. © 2011 Wiley Periodicals, Inc.
This study was supported by a grant from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, RED HERACLES (FEDER) RD06/0009/1001 (Madrid, Spain). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Despite current guidelines recommending an early revascularization invasive strategy (RIS) in high‐risk non–ST‐segment elevation acute coronary syndrome (NSTEACS), randomized trials comparing the impact of a conservative or selective invasive strategy (CS) versus an RIS have shown conflicting results, especially regarding major end points (death and myocardial infarction [MI]).1, 2, 3, 4 In contrast, data from large NSTEACS registries generally indicate that an RIS is associated with a reduction of major outcomes.5 Several reasons have been postulated to explain these discrepancies, such as differences in methodology and the definition of outcomes measures, or most importantly, differences in patient characteristics,6 especially regarding comorbidity burden.
Patients included in clinical trials are highly selected, and their risk profiles differ substantially from that of patients seen in daily clinical practice.6, 7, 8, 9 Thus, the exclusion of comorbid and older patients from cardiovascular trials10 involves not only the loss to a great extent of vulnerable patients presenting with NSTEACS, but also inadequacy of randomized data to clinical decision making. In this regard, data from observational studies11 and post hoc analyses indicate that these high‐risk patients seem to benefit most from an RIS.12, 13, 14, 15
Recently, a simple comorbidity index (SCI) integrating frequent comorbidities observed in patients with NSTEACS has shown to be simpler and proved to be as accurate as the more complex comorbidity indices16, 17, 18 for NSTEACS risk stratification.19 We speculate that in patients with high‐risk NSTEACS, SCI classification could be a useful tool for identifying a subgroup of patients with a greater benefit when an RIS is applied. The aim of the present study was to investigate the prognostic effect of in‐hospital revascularization in high‐risk NSTEACS patients according to an SCI index on the risk of a long‐term composite end points of death or MI.
Methods
Patient Population
We prospectively included 1017 consecutive patients admitted to our hospital with high‐risk NSTEACS, defined as chest pain in the last 24 hours with elevated troponin and/or ST‐segment depression from October 1, 2002 to October 1, 2008. The type of treatment strategy (RIS vs CS) was left to the discretion of the cardiologist in charge of the patient, but by institutional policy according to current guidelines20, 21 an RIS was strongly recommended. A detailed medical history, vital signs, 12‐lead electrocardiogram, and routine lab measurements were obtained on admission. Troponin‐I was measured at the patient's arrival and serially every 8 to 12 hours. Coronary angiography was performed during the index hospitalization at a mean of 96 ± 48 hours after patient admission. The indication for revascularization was based on angiographic results.
The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was approved by the local ethics committee. Written informed consent was obtained from all patients.
Simple Comorbidity Index
The SCI was constructed using 5 variables that emerged as the most important and independent comorbid factors predicting mortality from the multivariate analysis.19 One or 2 points were then assigned to each variable according to the weight of their hazard ratio (HR) (1 point if the HR = 1–2 and 2 points if the HR = 2–3 points). Severe renal dysfunction (defined as a glomerular filtration rate [GFR] <20 mL/min/1.73m2), dementia (defined as a chronic cognitive defect determined using information from a physician and a validated questionnaire performed by a trained nurse), prior history of heart failure, or peripheral artery disease were worth 2 points. Moderate renal failure (defined as GFR 20–50 mL/min/1.73m2) or prior MI were assigned 1 point.
End Points and Follow‐Up
The end point of the present study was a composite of death for any cause or nonfatal MI, whichever occurred first. MI was defined as an elevation of cardiac necrosis markers (troponin I or creatine kinase‐MB) with typical chest pain and/or ST‐segment deviation, or elevation of creatine kinase‐MB at 3 times its normal value after coronary intervention or 5 times after coronary surgery.22 Clinical follow‐up was performed during routine ambulatory visits in the outpatient clinic.
Statistical Analysis
Continuous variables were expressed as mean ±1 standard deviation or median (interquartile range [IRQ]) when appropriate. Discrete variables were presented as percentages. Cumulative risk for death or nonfatal MI during follow‐up was estimated using the Kaplan‐Meier method and the log‐rank test. Kaplan‐Meier curves were built according to the SCI and were stratified in relation to revascularization procedures.
Owing to the observational nature of this study, a propensity score for undergoing coronary angiography was created that aimed to minimize the inherent referral bias.23 We created a multivariable logistic regression model to identify those variables associated with undergoing a coronary angiography (a necessary step prior to revascularization). A propensity score was created using all variables yielding a P < 0.25 in univariate analysis. The final model included: age, gender, dyslipidemia, ST‐segment depression, troponin‐I elevation, Killip > I, ability of physical activity, anemia (defined as hemoglobin <12 g/dL for women or <13 g/dL for men), white blood cell count >10 × 103, left ventricular dysfunction (left ventricular ejection fraction ≤50%), recurrent angina, and previous history of ischemic heart disease, known coronary stenosis >50%, percutaneous coronary intervention, chronic obstructive pulmonary disease, renal failure, peripheral artery disease, and heart failure. The most important predictors of performing a coronariography ranked in order of importance were: age (R 2 = 67%), estimated GFR (R 2 = 7.3%), known coronary stenosis >50% (R 2 = 6.1%), ST‐segment depression (R 2 = 4.1%), hemoglobin (R 2 = 4%), troponin‐I elevation (R 2 = 2.5%), and Killip > I (R 2 = 2.4%). The area under the receiver operating characteristic curve of the resulting model was 0.837, indicating an excellent discriminative ability.
The association between SCI and the time to the combined end point of death or nonfatal MI was assessed using a Cox proportional hazard regression model. A parsimonious, highly predictive model was derived using backward step‐down selection. The proportionality assumption for the hazard function over time was tested by means of the Schoenfeld residuals. For the final Cox regression model we included the propensity score, the interaction among SCI points, and revascularization procedures during index hospitalization. The model's discriminative accuracy was assessed by the Harrell's C statistic, whereas its calibration was tested by the Gronnesby and Borgan test.24 A 2‐tailed P value of <0.05 was considered statistically significant. An additional Cox regression analysis adapted for competing events was performed for the combined end point of MI/cardiovascular death. All analyses were performed using Stata 11 statistical software (StataCorp, College Station, TX)
Results
Basic demographic and clinical characteristics, revascularization procedures, and pharmacologic treatment at discharge are summarized in the Table 1. The prevalence of SCI comorbidities were: 249 (25%), 218 (21%), 93 (9%), 74 (7.4%), 27 (2.7%), and 14 (1.4%) for previous MI, moderate renal failure, peripheral artery disease, heart failure, severe renal failure, and dementia, respectively. The distribution of the patient population among SCI points was 560 (55.1%), 236 (23.2%), 91 (8.9%), and 130 (12.8%) for 0, 1, 2, and >2 points, respectively.
Table 1.
Baseline Characteristics According to Simple Comorbidity Index Categories
| All, n = 1017 | SCI = 0, n = 560 | SCI = 1, n = 236 | SCI ≥ 2, n = 221 | P for Trend | |
|---|---|---|---|---|---|
| Age, y | 68 ± 13 | 64 ± 12 | 71.3 ± 13 | 74.3 ± 10 | <0.001 |
| Male, n (%) | 668 (66) | 379 (67.7) | 148 (63) | 141 (64) | 0.212 |
| Hypertension, n (%) | 656 (65) | 329 (59) | 167 (71) | 162 (73.3) | <0.001 |
| Dyslipidemia, n (%) | 494 (49) | 271 (48.4) | 109 (46.2) | 115 (52) | 0.486 |
| Smokers, n (%) | 243 (24) | 177 (32) | 37 (15.7) | 29 (13.1) | <0.001 |
| Diabetes mellitus, n (%) | 404 (40) | 185 (33) | 85 (36) | 134 (61) | <0.001 |
| Family CHD, n (%) | 60 (6) | 49 (9) | 7 (3) | 4 (2) | <0.001 |
| Previous PCI, n (%) | 85 (8) | 17 (3) | 38 (16) | 30 (13.6) | <0.001 |
| Previous CABG, n (%) | 74 (7) | 13 (2.3) | 26 (11) | 35 (16) | <0.001 |
| Stroke, n (%) | 81 (8) | 33 (6) | 25 (11) | 23 (10) | 0.015 |
| Troponin elevation, n (%) | 692 (68) | 369 (66) | 166 (70) | 157 (71) | 0.122 |
| ST‐segment depression, n (%) | 384 (38) | 202 (36.1) | 92 (39) | 90 (41) | 0.202 |
| Killip > I, n (%) | 148 (14.5) | 28 (5) | 36 (15.3) | 84 (38) | <0.001 |
| LVEF, % | 60 ± 13 | 64 (10) | 57 (12.4) | 52 (14.5) | <0.001 |
| LVEF < 55%, n (%) | 164 (27.6) | 82 (14.6) | 84 (35.6) | 115 (52) | <0.001 |
| Creatinine, mg/dL | 1.21 ± 0.81 | 0.97 ± 0.19 | 1.26 ± 0.39 | 1.79 ± 1.52 | <0.001 |
| WBC > 10 × 103 cells/mL, n (%) | 316 (31) | 173 (31) | 65 (27.5) | 78 (35.5) | 0.369 |
| TIMI risk score | 2.93 ± 1.34 | 2.44 ± 1.18 | 3.34 ± 1.15 | 3.72 ± 1.39 | <0.001 |
| In‐hospital revascularization procedures | |||||
| In‐hospital coronariography, n (%) | 725 (71) | 467 (83.4) | 160 (67.8) | 98 (44.3) | <0.001 |
| In‐hospital PCI, n (%) | 359 (35) | 250 (44.6) | 74 (31.4) | 35 (15.8) | <0.001 |
| In‐hospital CABG, n (%) | 92 (9) | 53 (9.5) | 24 (10.2) | 15 (6.8) | 0.323 |
| Revascularization, n (%) | 450 (44) | 303 (54.1) | 97 (41.1) | 50 (22.6) | <0.001 |
| Pharmacologic treatment | |||||
| Aspirin, n (%) | 930 (91) | 498 (90) | 213 (93) | 185 (93) | 0.136 |
| Clopidogrel, n (%) | 448 (44) | 279 (50.4) | 96 (42) | 70 (35) | <0.001 |
| Statins, n (%) | 701 (68.9) | 390 (70.4) | 161 (70) | 135 (68) | 0.527 |
| β‐Blockers, n (%) | 795 (78) | 427 (77) | 187 (81.3) | 150 (75.4) | 0.995 |
| ACE inhibitors, n (%) | 666 (65) | 250 (45.1) | 96 (42) | 82 (41.2) | 0.295 |
Abbreviations: ACE, angiotensin‐converting enzyme; CABG, coronary artery bypass grafts; CHD, coronary heart disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SCI, simple comorbidity index; TIMI, thrombolysis in myocardial infarction; WBC, white blood cell.
Data represent the baseline characteristics of the study sample, coronarographies/revascularization procedures performed during the index hospitalization, and pharmacologic treatment according to SCI categories .
Revascularization and Prognosis According to SCI
Coronary angiography was performed less frequently when moving from a lower to higher SCI score: 83.4%, 67.8%, 56%, and 36.2% for 0, 1, 2, and >2, respectively (P for trend <0.001). A similar trend was observed for revascularization procedures: 54.1%, 41.1%, 29.7%, and 17.7% for 0, 1, 2, and >2 points, respectively (P value for trend <0.001). Nevertheless, revascularization rates among patients who received a coronary angiography showed less marked differences across SCI scores (64.9, 60.6%, 52.9%, and 48.9%; P value for trend = 0.001, from SCI 0 to >2, respectively).
Patients with higher SCI points showed higher rates of death/MI at short‐ and long‐term follow‐up (Supplementary Table 1).
Differential Prognostic Effect of Revascularization According SCI
During a median follow‐up of 16 months (IQR: 12–36 months), 202 (19.9%) patients died and 170 (16.7%) suffered a MI. The composite end point (death/MI) was experienced by 305 (30%) patients.
In the entire study group, revascularization was associated with lower rates of the composite end point (1.239 vs 2.239 per 10 person‐year of follow‐up, P < 0.001). Nevertheless, the prognostic impact of revascularization was not homogeneous along the SCI distribution. Compared to the nonrevascularized patients, revascularized patients with 0 and 1 points did not show lower rates of the composite end point (1.017 vs 1.021 per 10 person‐year of follow‐up, P = 0.587 and 1.812 vs 2.345 per 10 person‐year of follow‐up, P = 0.497, respectively). In contrast, in those patients with 2 and >2 points, revascularization was associated with a significant decrease of the occurrence of the combined end point (0.711 vs 3.415 per 10 person‐year of follow‐up, P = 0.002 and 2.684 vs 7.042 per 10 person‐year of follow‐up, P = 0.028). Because patients with 2 and >2 points exhibited a prognostic benefit attributable to revascularization, they where collapsed in one SCI category (≥2). Figures 1 and 2 display similar cumulative risk of the composite end point death/MI for patients with SCI = 0 and 1 across revascularization status. Conversely, Figure 3 illustrates how revascularized patients with SCI ≥2 points showed a lower risk of the composite end point, with noticeable differences observed after the first months. This differential effect was observed not only for the incidence of MI but also for the cumulative risk of all‐cause mortality (Figure 4).
Figure 1.
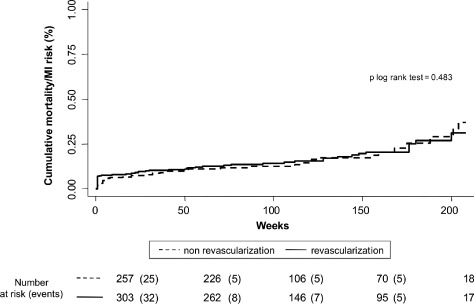
Prognostic impact of revascularization on combined end point in patients with simple comorbidity index score = 0 points. Abbreviation: MI, myocardial infarction.
Figure 2.
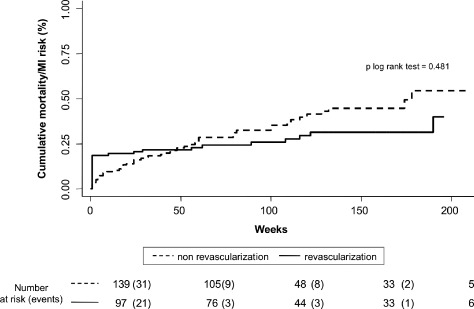
Prognostic impact of revascularization on combined end point in patients with simple comorbidity index score = 1 index. Abbreviation: MI, myocardial infarction.
Figure 3.
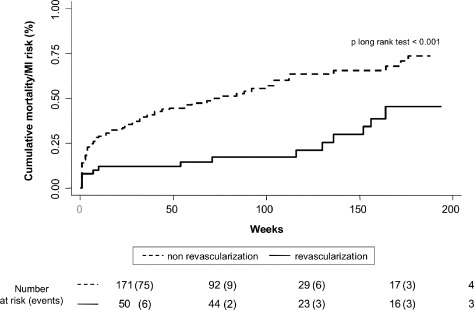
Prognostic impact of revascularization on combined end point in patients with simple comorbidity index score ≥2. Abbreviation: MI, myocardial infarction.
Figure 4.
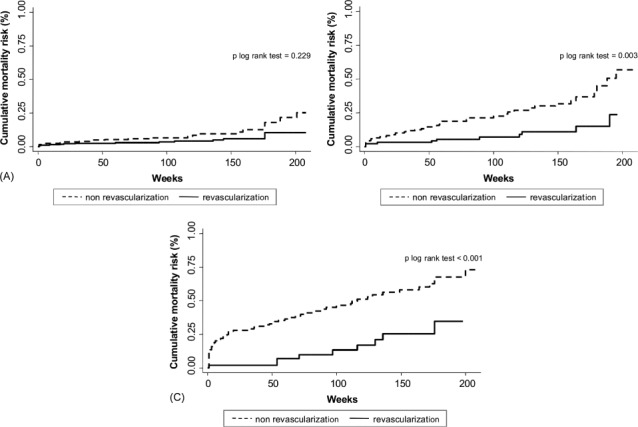
Prognostic impact of revascularization on mortality in patients with 0 (A), 1 (B), and ≥2 (C) points according to the simple comorbidity index. Abbreviation: MI, myocardial infarction.
In a multivariate analysis adjusted for a propensity score to receive a coronary angiography during hospitalization, this differential prognostic effect of coronary revascularization remained significant for SCI ≥2 (P value for interaction = 0.006). Thus, patients with SCI ≥2 displayed a significant and notorious risk reduction when revascularized (HR: 0.51, 95% CI: 0.29–0.89, P = 0.018). Conversely, in those with 0 and 1 point, no significant benefit attributable to revascularization was observed (HR: 1.31, 95% CI: 0.88–1.94, P = 0.171 and HR: 1.11, 95% CI: 0.70–1.76, P = 0.651, respectively). Harrell's C statistic of the multivariate model was 0.710, and the Gronnesby and Borgan test was 0.604.
This differential prognostic benefit attributable to revascularization persisted in those with SCI ≥2 in 3 additional sensitivity analyses: (1) forcing in the multivariate model pharmacological treatment such as aspirin, clopidogrel, β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and statins as binary variables (HR: 0.55, 95% CI: 0.31–0.96, P for interaction = 0.002); (2) excluding periprocedural MI (n = 33) from the composite end point (HR: 0.45, 95% CI: 0.25–0.81, P for interaction = 0.043); and (3) for the composite endpoint MI/cardiovascular death (n = 244), adjusting for noncardiac death as a competing event (HR: 0.41, 95% CI: 0.21–0.81, P for interaction = 0.007).
Comparison With TIMI Risk Score. Therapeutic Implications
Patients with higher SCI categories showed higher mean thrombolysis in myocardial infarction (TIMI) risk points (Table 1), but the concordance index was 18% (95% CI: 15–20, P < 0.001), indicating a significant although weak agreement between both scores. Respecting therapeutic implication of the TIMI risk score, intermediate and high‐TIMI risk categories showed a significantly favorable prognostic benefit effect attributable to revascularization compared to the low‐risk group in a univariate setting (HR: 0.53, 95% CI: 0.39–0.73 and HR: 0.52, 95% CI: 0.31–0.88 for intermediate and high‐TIMI risk groups, respectively) as is shown in Supplementary Table 2. In the multivariate model including SCI groups as covariate, revascularization differential effect did not persist for TIMI risk groups but did for SCI groups (Supplementary Table 2).
Discussion
The main finding of the present study was that high‐risk NSTEACS patients with greater comorbidity, defined as SCI ≥2, benefit significantly in terms of reduction of long‐term mortality and/or reinfarction attributable to revascularization during index hospitalization despite adjustments for several potential confounders. Results also remained consistent in additional sensitivity analysis indicating the strength of the present results, even adjusting for TIMI risk score. SCI appears to be a simple tool for tailoring the expected benefit when an RIS is applied.
Invasive Strategy vs Conservative Strategy
Despite current recommendations for an RIS in high‐risk NSTEACS,25 evidence derived from clinical trials reveals heterogeneous and insufficient results when an RIS and a CS are compared. Regarding major end points, early trials did not demonstrate a superiority of an RIS over a CS or even inferiority.6 Nevertheless, the use of antithrombotic therapies was not optimal, the frequency of complications was unacceptably high, and coronary stents were not or only anecdotically used. In more recent trials and meta‐analyses the prognostic discrepancies still persist.1, 2, 3, 4, 6, 26, 27, 28 Several reasons, such as differences in methodology and protocols, have been proposed to explain these conflicting results.6, 26, 27, 28
Randomized Studies vs Observational Studies. Different risk scenarios
Strikingly, the rate of deaths and MI reported in observational studies is much higher than in controlled studies.4, 5 There may be various reasons for this circumstance, but the most plausible one is the difference in baseline risk profile of patients included in these studies. Patients with a high risk such as the elderly, patients with significant cardiovascular comorbidities, or those with hemodynamic complications are commonly under‐represented or excluded in randomized trials.6, 10, 29
Several observational studies have shown that an RIS is associated with a lower risk of in‐hospital and long‐term major events,5, 6, 7, 11 with risk reductions greater than those observed in randomized trials. The prognostic impact of hemodynamic instability, age, and vascular and extravascular comorbidity has been previously reported,8, 9, 11, 12, 13, 14, 15 and data derived from observational studies indicate that an RIS is beneficial in terms of major event reduction in the elderly.11, 12 Similarly, several post hoc analyses from randomized trials indicate that the benefit of an RIS is greater in patients with high baseline risk.13, 14
SCI: An Easy Tool for Quantifying Comorbidity and the Expected Benefit From an RIS
In this study we found that a non‐negligible 21.7% of patients showed ≥2 independent comorbid factors according to the SCI. These results are congruous with current large registries.5, 6, 7, 8
Conversely, to other comorbidity and risk stratification scores, the advantages of SCI are based on simplicity and excellent prognosis accuracy in a nonselected population with high‐risk NSTEACS19 that allows its easy application in daily clinical practice. In addition to prognostic implication, we reported that the SCI may be a useful tool for tailoring therapy. In contrast to a common clinician belief, patients with a high comorbidity burden appear to be the subgroup where the application of an RIS appears to have a dramatic effect on prognosis. Based on the present results, clinical utility of the SCI score is reinforced by the fact that the SCI score, and not TIMI risk score, provides independent information for selecting those who benefit from an RIS. Thus, our results would be in accordance with the aphorism: the higher the risk, the higher benefit. In this regard, patients with previous heart failure, severe renal failure, or dementia, conditions that are normally associated high frailty and prohibitive risk of cardiovascular and noncardiovascular complications, exhibit per se SCI ≥2. Consonantly, patients presenting at least 2 of the most frequent comorbidities included in the SCI (prior MI, moderate renal failure and peripheral artery disease) would identify the rest of the patients with a substantial benefit when revascularized.
Explaining a Paradox: The Higher the Risk, the Lower the Adherence to Recommendations
The greater burden of coronary atherosclerosis expected in patients with cardiovascular comorbidities (renal failure, peripheral artery disease, prior MI, or heart failure) and the greater vulnerability to intercurrent processes associated with comorbidities may explain why revascularization exhibits a greater benefit in this subgroup of patients.
This study showed that although patients with comorbidity had a higher baseline risk profile, they were less likely to undergo coronary angiography and revascularization. This paradox is also observed in current national and international NSTEACS registries.30, 31, 32
Physicians, patients, and psychosocial contextual factors involved in clinical decision making largely influence diagnostic and therapeutic attitudes.33, 34 In this sense, it is undoubtedly that in the context of acute coronary syndromes, comorbidity, age, and gender play a crucial role in clinical decision making.6, 34
The results presented here should caution physicians about whether we are performing properly by being too conservative.
Limitations
This is an observational study from 2002 to 2008, in which the treatment strategy was left at the discretion of the physician, introducing a potential for selection bias that cannot be fully eliminated by adjusting for a propensity score. On the other hand, the medical treatment at discharge illustrates the suboptimal implementation of current guidelines for NSTEACS, which has already been observed in other registries.5 The mean time for coronarography was 96 hours and not 72 hours as suggested by current guidelines, a delay that could have some prognostic implications. In addition, the lack of assessment of systolic blood pressure and heart rate preclude to calculate GRACE (Global Registry of Acute Coronary Events) risk score and compare the prognostic ability and therapeutic implications of both scores. Finally, we assessed the presence of comorbid entities, but we did not take into account the severity, quality of life, functional impact, or frailty of the patients.
Conclusion
High‐risk NSTEACS patients with high comorbidity, expressed as SCI ≥2, display a significantly larger benefit from revascularization in terms of long‐term death/MI risk reduction over a long follow‐up period. Further randomized studies are needed to confirm these results. The inclusion criteria of further trials should reflect a real picture of patients seen in clinical practice. Furthermore, the impact of an RIS on other outcomes, such as quality of life in patients with high comorbidity, needs to be evaluated.
References
- 1. Wallentin L, Lagerqvist B, Husted S, et al. Outcome at 1 year after an invasive compared with a non‐invasive strategy in unstable coronary‐artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet. 2000;356:9–16. [DOI] [PubMed] [Google Scholar]
- 2. Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. [DOI] [PubMed] [Google Scholar]
- 3. Fox KA, Poole‐Wilson PA, Henderson RA, et al. Interventional versus conservative treatment for patients with unstable angina or non‐ST‐elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–751. [DOI] [PubMed] [Google Scholar]
- 4. de Winter RJ, Windhausen F, Cornel JH, et al. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med. 2005;353:1095–1104. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Roe MT, Peterson ED, et al. Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292:2096–2104. [DOI] [PubMed] [Google Scholar]
- 6. Nunez J, Sanchis J, Bodi V. Invasive revascularization strategy in non‐ST‐segment acute coronary syndromes. The debate continues. Med Clin Barc. 2009;133:717–723. [DOI] [PubMed] [Google Scholar]
- 7. Nunez J, Sanchis J, Nunez E, et al. Prognostic differences between routine invasive and conservative strategies for the management of high‐risk, non‐ST segment acute coronary syndromes: experience from two consecutive periods in a single center. Eur J Intern Med. 2007;18:409–416. [DOI] [PubMed] [Google Scholar]
- 8. Steg PG, Dabbous OH, Feldman LJ, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109:494–499. [DOI] [PubMed] [Google Scholar]
- 9. Nunez JE, Nunez E, Facila L, et al. Prognostic value of Charlson comorbidity index at 30 days and 1 year after acute myocardial infarction [in Spanish]. Rev Esp Cardiol. 2004;57:842–849. [PubMed] [Google Scholar]
- 10. Cherubini A, Oristrell J, Pla X, et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med. 2011;171:550–556. [DOI] [PubMed] [Google Scholar]
- 11. Bauer T, Koeth O, Junger C, et al. Effect of an invasive strategy on in‐hospital outcome in elderly patients with non‐ST‐elevation myocardial infarction. Eur Heart J. 2007;28:2873–2878. [DOI] [PubMed] [Google Scholar]
- 12. Bach RG, Cannon CP, Weintraub WS, et al. The effect of routine, early invasive management on outcome for elderly patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2004;141:186–195. [DOI] [PubMed] [Google Scholar]
- 13. Januzzi JL, Cannon CP, Dibattiste PM, et al. Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes (The TACTICS‐TIMI 18 Trial). Am J Cardiol. 2002;90:1246–1249. [DOI] [PubMed] [Google Scholar]
- 14. Januzzi JL Jr, Buros J, Cannon CP. Peripheral arterial disease, acute coronary syndromes, and early invasive management: the TACTICS TIMI 18 trial. Clin Cardiol. 2005;28:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steg PG, Kerner A, Van de Werf F, et al. Global Registry of Acute Coronary Events (GRACE) Investigators. Impact of in‐hospital revascularization on survival in patients with non‐ST‐elevation acute coronary syndrome and congestive heart failure. Circulation. 2008;118:1163–1171. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17. Romano PS, Roos LL, Jollins JG. Adapting s clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. [DOI] [PubMed] [Google Scholar]
- 18. Sachdew M, Sun JL, Tsiatis AA, et al. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43:576–582. [DOI] [PubMed] [Google Scholar]
- 19. Sanchis J, Nunez J, Bodi V, et al. Influence of comorbid conditions on one‐year outcomes in non‐st‐segment elevation acute coronary syndrome. Mayo Clin Proc. 2011;86:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2002;23:1809–1840. [DOI] [PubMed] [Google Scholar]
- 21. Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes: acute coronary syndromes without persistent ST segment elevation; recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 2000;21: 1406–1432. [DOI] [PubMed] [Google Scholar]
- 22. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. [DOI] [PubMed] [Google Scholar]
- 23. Weitzen S, Lapane KL, Toledano AY, et al. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13: 841–853. [DOI] [PubMed] [Google Scholar]
- 24. May S, Hosmer DW. Hosmer and Lemeshow type goodness‐of‐fit statistics for the Cox proportional hazards model. In: Balakrishnana N, Rao CR, eds. Advances in Survival Analysis: Handbook of Statistics. Vol 23. Amsterdam, The Netherlands: Elsevier, North‐Holland; 2004:383–394. [Google Scholar]
- 25. Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. [DOI] [PubMed] [Google Scholar]
- 26. Qayyum R, Khalid MR, Adomaityte J, et al. Systematic review: comparing routine and selective invasive strategies for the acute coronary syndrome. Ann Intern Med. 2008;148:186–196. [DOI] [PubMed] [Google Scholar]
- 27. Fox KA, Clayton TC, Damman P, et al. Long‐term outcome of a routine versus selective invasive strategy in patients with non‐ST‐segment elevation acute coronary syndrome a meta‐analysis of individual patient data. J Am Coll Cardiol. 2010;55: 2435–2445. [DOI] [PubMed] [Google Scholar]
- 28. Sanchis J, Bodi V, Nunez J, et al. Conservative, true selective invasive, and routine invasive strategies in non–ST‐segment elevation acute coronary syndromes. J Am Coll Cardiol. 2010;56: 1609–1610. [DOI] [PubMed] [Google Scholar]
- 29. Bosch X, Delgado V, Verbal F, et al. Causes of ineligibility in randomized controlled trials and long‐term mortality in patients with non‐ST‐segment elevation acute coronary syndromes. Int J Cardiol. 2008;124:86–91. [DOI] [PubMed] [Google Scholar]
- 30. Ferreira‐Gonzalez I, Permanyer‐Miralda G, Marrugat J, et al. MASCARA (Manejo del Síndrome Coronario Agudo. Registro Actualizado) Study. General findings [in English, Spanish]. Rev Esp Cardiol. 2008;61:803–816. [PubMed] [Google Scholar]
- 31. Tricoci P, Peterson ED, Mulgund J, et al. Temporal trends in the use of early cardiac catheterization in patients with non‐ST‐segment elevation acute coronary syndrome (results from CRUSADE). Am J Cardiol. 2006;98:1172–1176. [DOI] [PubMed] [Google Scholar]
- 32. Fox KA, Anderson FA Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart. 2007;93:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tracy CS, Dantas GC, Moineddin R, et al. Contextual factors in clinical decision making: national survey of Canadian family physicians. Can Fam Physician. 2005;51:1106–1107. [PMC free article] [PubMed] [Google Scholar]
- 34. Elder AT. Which benchmarks for age discrimination in acute coronary syndromes? Age Ageing. 2005;34:4–5. [DOI] [PubMed] [Google Scholar]


