Abstract
Background:
The pathogenetic role of vascular endothelial growth factor (VEGF) in malignant pericardial effusion and diagnostic value of pericardial VEGF levels to discriminate malignant from benign pericardial effusions are uncertain.
Hypothesis:
We hypothesized that pericardial VEGF levels would be higher in malignant than benign pericardial effusion and that VEGF would be a useful marker for the diagnosis of malignant pericardial effusion.
Methods:
Using an enzyme‐linked immunosorbent assay, we assessed pericardial and serum VEGF levels in patients with malignant pericardial effusion (n = 19), in patients with nonmalignant pericardial effusion (n = 30), and for control, in patients without pericardial disease (n = 26).
Results:
Vascular endothelial growth factor pericardial levels in malignant pericardial effusion (13 593.8 ± 22 410.24 pg/mL) were significantly higher compared with VEGF in nonmalignant effusion (610.63 ± 1289.08 pg/mL; P = 0.001) and pericardial fluid (5.5 ± 15.97 pg/mL; P < 0.001). In serum, VEGF was significantly higher in patients with nonmalignant pericardial effusion (188.3 ± 240.35 pg/mL) compared with patients with malignant pericardial effusion (67.52 ± 125.77 pg/mL; P = 0.024) and coronary artery disease patients (29.13 ± 76.26 pg/mL; P < 0.001). Pericardial VEGF levels were significantly higher than matched serum levels only in patients with malignant pericardial effusion (P = 0.023). Pericardial VEGF levels ≥2385 pg/mL had 75% sensitivity and 90% specificity for the recognition of malignant pericardial effusion in patients with breast or lung cancer.
Conclusions:
Vascular endothelial growth factor levels in pericardial effusion are markedly elevated in patients with malignant pericardial effusion, indicating abundant local release within the pericardial cavity. It is thus possible that VEGF participates in the pathogenesis of malignant pericardial effusion. Measurement of VEGF in pericardial effusion offers potential as a diagnostic tool to discriminate malignant from benign effusions in patients with breast or lung cancer.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Vascular endothelial growth factor (VEGF) is an important mediator of angiogenesis and vascular permeability.1 Vascular endothelial growth factor is produced by nearly all cells of normal tissue, but also many tumor cells overexpress this cytokine.1., 2., 3., 4., 5., 6. For its angiogenic and permeability‐inducing properties, VEGF has been implicated in various pathological conditions, including tumor angiogenesis.1., 7. Several studies have also reported that VEGF might play an etiological role in the formation of malignant pleural effusion and malignant ascites,8., 9., 10., 11., 12., 13., 14. but no data exist concerning the diagnostic and pathogenetic role of VEGF in malignant pericardial effusion.
Pericardial effusions are present in a variety of pathologic conditions, including infectious, malignant, autoimmune, and metabolic diseases. Correct diagnosis and effective treatment are crucial in reducing morbidity and mortality from pericardial disease. Of particular therapeutic and prognostic importance is the definite differentiation of malignant pericardial effusion from benign conditions. Even in patients with underlying malignant disease, pericardial effusion may be benign (eg, due to radiation) or malignant, with important implications for prognosis. However, establishing the etiology of pericardial effusion is often challenging and difficult to assess on the basis of clinical assessment only. Therefore, evaluation of pericardial effusion, accurate diagnosis of the underlying disease, and adequate therapy are of great interest. Vascular endothelial growth factor levels in pericardial effusion of different etiology have not been assessed previously.
Methods
Study Population
A total of 75 consecutive patients with pericardial effusion of different etiology undergoing pericardiocentesis and pericardioscopy‐guided pericardial or epicardial biopsy for therapeutic and/or diagnostic reasons were included in this study after approval by the local ethics committee. The etiologic diagnosis of malignant and nonmalignant pericardial effusion followed the criteria defined by the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology.15 In brief, the diagnosis of malignant pericardial effusion was either based on the presence of malignant cells in pericardial fluid cytology and/or histologic evidence of malignant infiltration in pericardioscopy‐guided pericardial or epicardial biopsy specimens. The diagnosis of nonmalignant pericardial effusion comprised autoreactive and viral pericardial effusion. Patients with autoreactive pericardial effusion met the following criteria: (1) increased number of lymphocytes/mononuclear cells >5000/mm3 or the presence of antimyocardial antibodies in pericardial fluid; (2) inflammation in epicardial biopsies by ≥14 cells/mm2; (3) exclusion of active viral infection in pericardial effusion and epicardial biopsies (no virus isolation, negative polymerase chain reaction [PCR] for major cardiotropic viruses, no immunoglobulin M titer against cardiotropic viruses in the pericardial effusion); (4) exclusion of tuberculosis, Borrelia burgdorferi, Chlamydia pneumoniae, and other bacterial infections by PCR and/or cultures; (5) absent neoplastic infiltration in pericardial fluid and biopsy specimens; (6) exclusion of systemic, metabolic disorders and uremia. Viral pericarditis was diagnosed by the presence of viral genome (parvovirus B19, influenza A/B, cytomegalovirus, enterovirus, adenovirus, herpes simplex virus, Epstein‐Barr virus) detected by PCR in pericardial fluid and/or peri‐/epicardial biopsies. For extraction of DNA/RNA from pericardial effusion and peri‐/epicardial biopsies, the QIAamp Blood Mini Kit and the QIAamp Tissue Kit (Qiagen, Hilden, Germany) were used. Conditions for PCR and primers have been described elsewhere.16 For comparison, pericardial fluid of patients with coronary artery disease (CAD), which was obtained immediately after incision of the pericardium during coronary artery bypass graft surgery, was used. None of these patients had a history or clinical evidence of pericardial disease or malignancy.
Sampling of Pericardial Effusion, Pericardial Fluid, Peri‐/Epicardial Biopsies, and Serum
All pericardial effusion, pericardial fluid, and serum samples were immediately transferred into chilled sterile tubes containing a proteinase inhibitor cocktail (Complete; Roche, Penzberg, Germany) and subsequently stored at −80°C until analysis. Pericardial effusion and pericardial fluid samples for cell analysis (leukocyte counts, cytology) were drawn into potassium‐ethylene diamine tetraacetic acid–containing tubes at room temperature; leukocytes were counted within 1–3 hours by fluorescence‐activated cell sorting analysis, and cytology smears and spins were prepared immediately. Cytology examination of the pericardial effusion was performed by the same 2 independent experts for the entire study population.
For pericardioscopy, a flexible endoscope (AF 1101 Bl; Karl Storz, Tuttlingen, Germany) was introduced into the pericardial space through a 16‐F introductory sheath and up to 8 peri‐ and/or epicardial biopsies were taken under direct eye control through the working channel of the pericardioscope. The epicardial and pericardial biopsies were fixed and processed in the usual manner, embedded in paraffin and cut into 4‐mm serial sections by microtome, and then stained with hematoxylin‐eosin for routine histology and Ziehl‐Neelsen stain for mycobacteria. Pathohistology examination was performed by the same 2 independent experts for the entire study population.
Immunoassay for Vascular Endothelial Growth Factor
Vascular endothelial growth factor levels in pericardial effusion and serum were measured blinded to any clinical information with a commercially available sandwich enzyme‐linked immunosorbent assay (Quantikine Colorimetric Sandwich; R&D Systems) according to the manufacturer's guidelines.
In brief, after standard procedures, all samples were pipetted into the wells of the microtiter plates, specific horseradish peroxidase‐linked polyclonal antibodies were added, and immunoreactive levels of soluble VEGF165 were determined. The detection limit for VEGF165 was 9 pg/mL, and both interassay and intra‐assay coefficient of variation were <10%.
Statistical Analysis
Values below the detection limit were assumed to be zero for statistical analysis. All P values <0.05 were considered statistically significant. The software package SigmaPlot, version 11.0, was used for statistical analysis. Comparison of VEGF levels between the 3 groups was performed by Kruskal‐Wallis test. In case of significant differences between the groups, closed testing principle was applied to compare 2 groups by use of the Mann‐Whitney U test. Categorical parameters were compared using the χ 2 or Fisher exact test if appropriate. To evaluate the diagnostic utility of VEGF measurement in pericardial fluid to discriminate between malignant pericardial effusion and nonmalignant pericardial effusion, receiver operating characteristic curves and area under the curve (AUC) were calculated.
Results
Vascular Endothelial Growth Factor Levels in Pericardial Effusion, Pericardial Fluid, and Serum
A total of 75 consecutively enrolled patients were included in this study, of which pericardial effusion, pericardial fluid for CAD patients, and serum was available. The group with malignant pericardial effusion comprised 19 patients, and the group with nonmalignant pericardial effusion, 30 patients. The nonmalignant pericardial effusions were from 20 patients with autoreactive and 10 patients with viral pericardial effusion. The group with CAD comprised 26 patients. Table 1 depicts the demographic characteristics of study patients. The underlying malignant diseases in patients with malignant pericardial effusion were rather heterogeneous with various solid tumors or cancer of unknown primary (Table 2). In 4 out of 19 (21%) patients, the pericardial effusion was the first evidence of malignancy.
Table 1.
Demographic Characteristics of Study Patients
| Variable | All Patients, n = 75 | Malignant Pericardial Effusion, n = 19 | Nonmalignant Pericardial Effusion, n = 30 | Pericardial Fluid, n = 26 | P Valuea |
| M/F | 45/30 | 9/10 | 15/15 | 21/5b,c, b, b,c, c | <0.05 |
| Age, y | 64.1 ± 10.6 | 60.1 ± 8.9 | 61.1 ± 11.1 | 70.6 ± 8.3b,c, b, b,c, c | <0.05 |
| CAD, n (%) | 27 (36) | 0 | 1 (3) | 26 (100)b,c, b, b,c, c | <0.05 |
| Creatinine, mg/dL | 1.06 ± 0.77 | 0.82 ± 0.22 | 1.05 ± 1.01 | 1.29 ± 0.6b,c, b, b,c, c | <0.05 |
| Leukocytes, g/L | 9.55 ± 7.46 | 12.66 ± 13.11c | 8.76 ± 3.1 | 7.61 ± 1.98b | <0.05 |
| Protein, g/dL | 64.75 ± 15.03 | 65.22 ± 8.76 | 60.17 ± 19.55 | 72.06 ± 6.63 | NS |
| CRP, mg/L | 48.56 ± 86.97 | 81.59 ± 76.77c | 54.39 ± 106.61 | 3.25 ± 4.47b | <0.05 |
Abbreviations: ANOVA, analysis of variance; CAD, coronary artery disease; CRP, C‐reactive protein; F, female; M, male; mPE, malignant pericardial effusion; nPE, nonmalignant pericardial effusion; NS, not significant. Values are mean ± SD, or no. of patients (%).
ANOVA/χ 2 test.
P < 0.05 vs mPE.
P < 0.05 vs nPE.
Table 2.
Diagnoses of Patients With Malignant and Nonmalignant Pericardial Effusion
| Diagnosis | No. of Patients | F/M |
| Malignant pericardial effusion | 19 | 10/9 |
| Lung cancer | 8 | 3/5 |
| Pleural mesothelioma | 1 | 0/1 |
| Breast carcinoma | 3 | 3/0 |
| Gastrointestinal carcinoma | 3 | 2/1 |
| Renal cell carcinoma | 1 | 1/0 |
| Testicular carcinoma | 1 | 0/1 |
| Cancer of unknown primary | 2 | 1/1 |
| Nonmalignant pericardial effusion | 30 | 15/15 |
| Autoreactive | 20 | 12/8 |
| Viral | 10 | 3/7 |
Abbreviations: F, female; M, male.
Vascular endothelial growth factor levels in malignant pericardial effusion (13 593.8 ± 22 410.24 pg/mL) were significantly higher compared with VEGF in nonmalignant effusion (610.63 ± 1289.08 pg/mL; P = 0.001) and pericardial fluid (5.5 ± 15.97 pg/mL; P < 0.001) (Figure 1A). In patients without previous diagnosis of cancer (n = 4), pericardial VEGF levels were 21 758.7 ± 41 122.7 pg/mL. In serum, VEGF was significantly higher in patients with nonmalignant pericardial effusion (188.3 ± 240.35 pg/mL) compared with patients with malignant pericardial effusion (67.52 ± 125.77 pg/mL; P = 0.024) and CAD patients (29.13 ± 76.26 pg/mL; P < 0.001). Serum VEGF levels did not differ significantly between patients with malignant pericardial effusion and CAD patients (P = 0.393) (Figure 1B). Pericardial VEGF levels were significantly higher than matched serum levels only in patients with malignant pericardial effusion (P = 0.023 for malignant pericardial effusion, P = 0.123 for nonmalignant pericardial effusion, and P = 0.331 for CAD patients), indicating an increased local production within the pericardial cavity.
Figure 1.
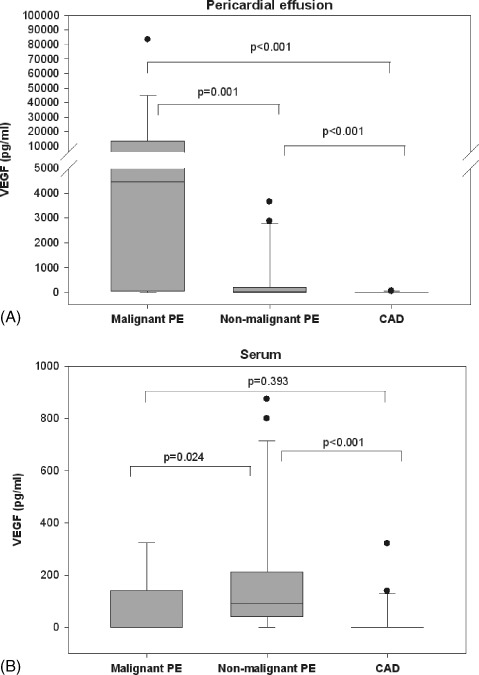
VEGF levels measured in pericardial effusion/pericardial fluid (A) and serum (B) from patients with malignant pericardial effusion, nonmalignant pericardial effusion, and CAD. The median values and upper and lower quartiles are depicted as box plots. Whiskers indicate the fifth and 90th percentiles. Abbreviations: CAD, coronary artery disease; PE, pericardial effusion; VEGF, vascular endothelial growth factor.
Receiver‐Operating Characteristic Analysis of Vascular Endothelial Growth Factor in Pericardial Effusion
Biomarkers in pericardial effusion may help to clarify the underlying etiology of the pericardial effusion and thus permit the initiation of specific therapies. To evaluate the diagnostic utility of pericardial VEGF measurement to differentiate between malignant and benign effusion, receiver operating characteristic curve and AUC were calculated. Pericardial VEGF yielded an AUC of 0.77 (95% confidence interval [CI]: 0.63‐0.92) in the heterogeneous group with malignant pericardial effusion. If we investigated patients with underlying breast cancer and lung cancer (including pleural mesothelioma), pericardial VEGF yielded a higher AUC of 0.84 (95% CI: 0.67‐1.0) (Figure 2). By this model, pericardial VEGF levels ≥2385 pg/mL had 75% sensitivity and 90% specificity for the recognition of malignant pericardial effusion. In the rather small group of only 4 patients without previous diagnosis of cancer, pericardial VEGF levels yielded a lower AUC of 0.74 (95% CI: 0.33‐1.0).
Figure 2.
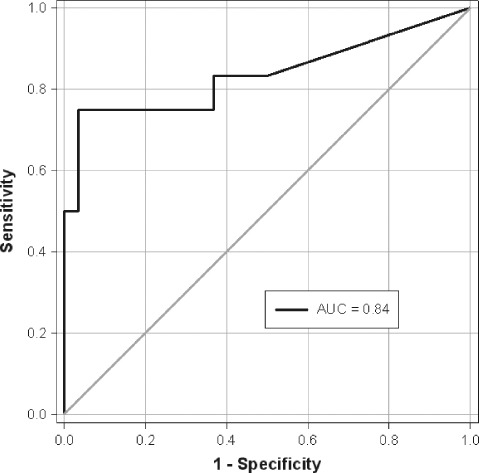
ROC curve and AUC of pericardial VEGF levels to discriminate malignant from nonmalignant pericardial effusion in patients with lung or breast carcinoma. Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic; VEGF, vascular endothelial growth factor.
Discussion
Pericardial effusion is present in a variety of pathologic conditions, including infectious, malignant, autoimmune, and metabolic diseases. However, establishing the underlying cause of pericardial effusion remains challenging. With the application of standard clinical methods only, the etiological search is often inconclusive. Therefore, diagnostic tools, such as pericardial fluid and tissue analysis, are required to identify the definite cause in these cases. In this context, analysis of cytokines, inflammatory mediators, and serologic and immunologic markers may help to elucidate the underlying etiology of pericardial effusion. Particularly, the definite differentiation of malignant pericardial effusion from benign effusion, even in patients with underlying malignant disease, has important implications for therapy and prognosis.15 Vascular endothelial growth factor levels in pericardial effusion of different origin have not been assessed before.
Vascular endothelial growth factor has been implicated in the formation of malignant pleural effusion and malignant ascites.8., 9., 10., 11., 12., 13., 14. However, no data exist about VEGF in malignant pericardial effusion. In our study, we found markedly elevated VEGF levels in malignant pericardial effusion compared with nonmalignant pericardial effusion and pericardial fluid. In line with our data, high levels of VEGF have been found in malignant pleural effusion and malignant ascites, suggesting a possible etiologic role in the formation of malignant effusion.8., 12., 17., 18. Abundant local release of VEGF within a cavity, such as pericardial, pleural, or peritoneal, may promote fluid accumulation by enhancing vascular permeability.9., 11., 13., 14.
In 6 patients with malignant pericardial effusion, VEGF levels were only detected in pericardial effusion. At the same time, in malignant pericardial effusion patients with detectable concentrations in pericardial effusion and serum, VEGF levels were on average 85‐fold higher compared with blood levels. These results indicate a local production of VEGF within the pericardial cavity. Vascular endothelial growth factor may be produced by infiltrating inflammatory or tumor cells. Nagy et al demonstrated that tumor cells implanted in the peritoneal cavity of mice produce and secrete VEGF, resulting in hyperpermeability of the peritoneal microvasculature and accumulation of malignant ascites.9 However, further studies are warranted to clarify the pathogenetic role and exact source of VEGF within the pericardial cavity.
Biomarkers in pericardial effusion such as cytokines, autoantibodies, biochemical, inflammatory, or tumor markers may help to clarify the underlying etiology of the pericardial effusion facilitating the initiation of specific therapies.19., 20., 21., 22. The definite diagnosis of malignant pericardial effusion is established by positive cytologic examination of pericardial fluid. However, pericardial fluid cytology, though specific, has variable sensitivity, ranging from >90% to as low as 30%–50%.23., 24., 25. In this context, analysis of pericardial fluid, including tumor markers in pericardial effusion, is often ordered after therapeutic or diagnostic pericardiocentesis to diagnose malignant pericarditis.20., 21. Vascular endothelial growth factor has been ascribed a role as biomarker to differentiate malignant from benign conditions.8., 12., 26., 27., 28. In our study, in patients with underlying breast or lung cancer, pericardial VEGF levels ≥2385 pg/mL had 75% sensitivity and 90% specificity for the recognition of malignant pericardial effusion. Thus, pericardial VEGF may offer potential as a biomarker in differentiating malignant from benign pericardial effusion in patients with lung or breast cancer.
In conclusion, VEGF levels in pericardial effusion are markedly elevated in patients with malignant pericardial effusion, indicating abundant local release within the pericardial cavity. These results suggest that VEGF might play an important role in the pathogenesis of malignant pericardial effusion. Measurement of VEGF in pericardial effusion offers the potential as a diagnostic tool to discriminate malignant from benign effusions in patients with lung or breast cancer.
Acknowledgements
The authors thank the colleagues and nurses of the Department of Cardiovascular Surgery for their excellent cooperation. They also thank the staff of the cardioimmunological laboratory of the Department of Cardiology, University Hospital of Marburg for their valuable participation.
References
- 1. Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 2005;109:227–241. [DOI] [PubMed] [Google Scholar]
- 2. Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86–91. [DOI] [PubMed] [Google Scholar]
- 3. Brown LF, Berse B, Jackman RW, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- 4. Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 5. Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. [DOI] [PubMed] [Google Scholar]
- 6. Maharaj AS, D'Amore PA. Roles for VEGF in the adult. Microvasc Res. 2007;74:100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. [DOI] [PubMed] [Google Scholar]
- 8. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178–187. [PubMed] [Google Scholar]
- 9. Nagy JA, Masse EM, Herzberg KT, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995;55:360–368. [PubMed] [Google Scholar]
- 10. Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998;58:2594–2600. [PubMed] [Google Scholar]
- 11. Yano S, Nokihara H, Hanibuchi M, et al. Model of malignant pleural effusion of human lung adenocarcinoma in SCID mice. Oncol Res. 1997;9:573–579. [PubMed] [Google Scholar]
- 12. Zebrowski BK, Liu W, Ramirez K, et al. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373–378. [DOI] [PubMed] [Google Scholar]
- 13. Yano S, Herbst RS, Shinohara H, et al. Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clin Cancer Res. 2000;6:957–965. [PubMed] [Google Scholar]
- 14. Yano S, Shinohara H, Herbst RS, et al. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol. 2000;157:1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary: the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610. [DOI] [PubMed] [Google Scholar]
- 16. Maisch B, Ristic AD, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone; the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J. 2002;23:1503–1508. [DOI] [PubMed] [Google Scholar]
- 17. Economidou F, Antoniou KM, Tzanakis N, et al. Angiogenic molecule Tie‐2 and VEGF in the pathogenesis of pleural effusions. Respir Med. 2008;102:774–779. [DOI] [PubMed] [Google Scholar]
- 18. Kalomenidis I, Kollintza A, Sigala I, et al. Angiopoietin‐2 levels are elevated in exudative pleural effusions. Chest. 2006;129:1259–1266. [DOI] [PubMed] [Google Scholar]
- 19. Burgess LJ. Biochemical analysis of pleural, peritoneal and pericardial effusions. Clin Chim Acta. 2004;343:61–84. [DOI] [PubMed] [Google Scholar]
- 20. Szturmowicz M, Tomkowski W, Fijalkowska A, et al. The role of carcinoembryonic antigen (CEA) and neuron‐specific enolase (NSE) evaluation in pericardial fluid for the recognition of malignant pericarditis. Int J Biol Markers. 1997;12:96–101. [DOI] [PubMed] [Google Scholar]
- 21. Szturmowicz M, Tomkowski W, Fijalkowska A, et al. Diagnostic utility of CYFRA 21‐1 and CEA assays in pericardial fluid for the recognition of neoplastic pericarditis. Int J Biol Markers. 2005;20:43–49. [PubMed] [Google Scholar]
- 22. Koh KK, Kim EJ, Cho CH, et al. Adenosine deaminase and carcinoembryonic antigen in pericardial effusion diagnosis, especially in suspected tuberculous pericarditis. Circulation. 1994;89:2728–2735. [DOI] [PubMed] [Google Scholar]
- 23. Krikorian JG, Hancock EW. Pericardiocentesis. Am J Med. 1978;65:808–814. [DOI] [PubMed] [Google Scholar]
- 24. Meyers DG, Meyers RE, Prendergast , TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111:1213–1221. [DOI] [PubMed] [Google Scholar]
- 25. Corey GR, Campbell PT, Van Trigt P, et al. Etiology of large pericardial effusions. Am J Med. 1993;95:209–213. [DOI] [PubMed] [Google Scholar]
- 26. Ziora D, Sielska‐Spytek E, Dworniczak S, et al. VEGF (vascular endothelial growth factor) concentration in serum and pleural fluid of patients with pleural malignancy and pleural tuberculosis [article in Polish]. Pneumonol Alergol Pol. 2002;70:458–467. [PubMed] [Google Scholar]
- 27. Dong WG, Sun XM, Yu BP, et al. Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol. 2003;9:2596–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nascimento I, Schaer R, Lemaire D, et al. Vascular endothelial growth factor (VEGF) levels as a tool to discriminate between malignant and nonmalignant ascites. APMIS. 2004;112: 585–587. [DOI] [PubMed] [Google Scholar]


