Abstract
The goals of optimal medical therapy in patients with stable angina pectoris are to reduce the risk of cardiovascular mortality and future cardiovascular events, improve exercise capacity, and enhance quality of life. Whereas myocardial revascularization is frequently employed in the management of patients with stable angina, a variety of pharmacologic interventions are recommended as part of optimal medical management. The use of short‐ and rapidly‐acting nitrates (eg, sublingual nitroglycerin spray and tablets) is at the core of the therapeutic armamentarium and should be integrated into optimal medical therapy for stable angina along with exercise therapy. The potential clinical implications from these observations are that prophylactic sublingual nitrates, when combined with cardiac rehabilitation, may allow the patient with angina to exercise to a greater functional capacity than without sublingual nitrates. Clin. Cardiol. 2012 DOI: 10.1002/clc.21993
William E. Boden: Advisory Board for Arbor Pharmaceuticals. Aloke V. Finn: Sponsored research agreement with Medtronic Vascular; Scientific Advisory Board for Medtronic Vascular; Advisory Board for Arbor Pharmaceuticals. Dharmesh Patel: Speakers Bureau for Novartis, Arbor Pharmaceuticals, Medtronic, Takeda Pharmaceuticals, Abbott, St Jude's Medical, Cleveland Heart Lab, Boston Heart Lab, Forrest Pharmaceuticals, Boehringer Ingelheim, and Jansen & Jansen. W. Frank Peacock: Advisory Board for Abbott, Alere, Arbor Pharmaceuticals, Lilly, and The Medicines Co; Received research grants from Alere, Brahms Comprehensive Research Associates, Electrocore, Novartis, The Medicines Co; Speakers Bureau for Abbott, Alere, and Electrocore; Ownership interest in EKR, Emergencies in Medicine, and Vital Sensors. Udho Thadani: Received consultation fee from Arbor Pharmaceuticals for interpretation of the study data and for writing this manuscript; Advisory Board for Arbor Pharmaceuticals; Consultant for Gilead Sciences, Astra Zeneca, Pfizer, Merck, Bristol‐Myers Squibb, Bayer, Forest Laboratories, and Servier; Speaker's program for Eli Lilly and Daiichi‐Sankyo and Gilead Sciences. Franklin H. Zimmerman: Advisory Board for Arbor Pharmaceuticals; Speakers Bureau for Boerhinger‐Ingelheim.
**At the time this manuscript was written, Dr Peacock's affiliation was: Department of Emergency Medicine, The Cleveland Clinic, Cleveland, Ohio.
Introduction
Coronary artery disease (CAD) is the leading cause of death and disability in the United States and the rest of the developed world,1., 2. with angina pectoris being a dominant expression clinically in the majority of patients.1 In the United States, the prevalence of angina is nearly 9 million individuals overall, with an additional 500 000 new cases diagnosed every year.1 Incompletely or inadequately treated angina also results in significant increases in indirect costs due to inability to work or loss of employment, decreased productivity in the workplace, and reduced quality of life. Often, the extent to which quality of life is diminished is dependent on angina frequency and severity3 and may present as worsened physical functioning, sleep quality, and emotional well‐being.4 The age‐adjusted prevalence of angina is higher in women than in men among Americans age 40–74 years, with an estimated prevalence of 5.5 million vs 4.7 million, respectively, based on data from 4 national cross‐sectional health‐examination studies.5 Age is also a factor in the prevalence of angina. According to estimates from the American Heart Association (AHA), half of patients with angina are age >65 years,5., 6. and the incidence of symptomatic CAD increases in an age‐dependent manner in men and women.5
Patients with angina often have multiple comorbid conditions, including hypertension, diabetes, chronic obstructive pulmonary disease (COPD), congestive heart failure, and chronic kidney disease.6., 7., 8. These comorbidities need to be managed aggressively along with CAD.
In selected high‐risk patients, or those with refractory angina whose symptoms are not controlled with medical therapy, percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) may be appropriate to improve prognosis and prevent ischemic complications.9 Patients with stable angina can be treated with a variety of options. These include medication with or without coronary revascularization (PCI or CABG) surgery. All approaches, regardless of the presence of comorbidities, require appropriate lifestyle modifications, such as a low‐fat diet and regular aerobic exercise.
Although PCI is a viable treatment modality for patients with stable angina with a high rate of initial procedural success and an acceptable level of risk,10 patients may continue to experience angina due to residual CAD despite initially successful intervention.11., 12., 13., 14. This may be due to either target‐vessel restenosis or new lesion progression in vessels that were not originally flow limited.5., 15. In patients who received stents for multivessel CAD, Serruys and colleagues observed that 21% still experienced angina 12 months later.12 Similar results were seen in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study, where 34% of patients who received PCI still had angina 1 year later.14
Resource utilization among patients with stable angina and CAD is substantial. Costs related to treatment and management of stable angina are often underestimated, as these patients may be classified only as having CAD. An analysis of the costs for medical utilization related to chronic angina showed yearly direct medical costs reached $1.9 billion and $33 billion when chronic angina or CAD‐related International Classification of Diseases, Ninth Revision (ICD‐9) codes were listed as the first diagnosis, respectively.16 The substantial impact of stable angina on the US healthcare system underscores the importance of optimal management.
Optimal Medical Therapy
Goals and Treatment Considerations for Optimal Medical Therapy
In patients with stable angina, the multifaceted goals of optimal medical therapy (OMT) are to reduce the risk of mortality and future cardiovascular events, improve exercise capacity, and enhance quality of life by reducing symptoms.10., 14., 17., 18., 19., 20., 21. A variety of interventions are recommended as part of OMT and include both lifestyle modification and aggressive pharmacologic secondary prevention.14., 21. Lifestyle interventions include regular physical activity, complete cessation of tobacco use and exposure to tobacco smoke, and diet modifications to improve blood pressure, blood glucose levels, and serum lipid levels, along with control of diabetes and weight management.22., 23. Pharmacologic treatments to reduce adverse clinical outcomes include aspirin, statins, and angiotensin‐converting enzyme (ACE) inhibitors, and/or angiotensin‐receptor blockers in those with reduced left ventricular function.17., 19., 22., 23. These prognosis‐modifying agents can be used in conjunction with medications used specifically to treat symptoms of stable angina, which include nitrates, β‐blockers, calcium channel blockers, and ranolazine, which may be prescribed as monotherapy or in various combinations.10., 17., 19., 22., 23. Adverse effects and limitations of current antianginal medications are listed in Table 1.
Table 1.
| β‐Blockers | Nitrates | Calcium Channel Blockers | Ranolazine | |
|---|---|---|---|---|
| Side effects | Hypotension | Hypotension | Hypotension | Dizziness |
| Syncope | Syncope | Flushing | Headache | |
| Sexual dysfunction | Headache | Dizziness | Constipation | |
| Fatigue | Tolerance | Edema | Nausea | |
| Depression | Fatigue | |||
| Precautions/ contraindications | Bradycardia | LV outflow tract obstruction | Bradycardia* | Use with QT‐ prolonging drugs |
| AV conduction problems | Erectile dysfunction (concomitant use of PDE5 inhibitors) | AV conduction problems* | Significant liver disease | |
| Sick sinus syndrome | Sick sinus syndrome* | Contraindicated with strong CYP3A4 inhibitors (ketoconazole, clarithromycin, or nelfinavir) and CYP3A inducers (rifampin, phenobarbital) | ||
| Peripheral vascular disease | Heart failure* | |||
| COPD | LV dysfunction* |
Abbreviations: AV, atrioventricular; COPD, chronic obstructive pulmonary disease; CYP3A, cytochrome P450 3A; CYP3A4, cytochrome P450 3A4; LV, left ventricular; PDE5, phosphodiesterases.
Non‐dihyropyridines (diltiazem and verapamil)
Recent guidelines from the European Society of Cardiology recommend considering a lower threshold for implementing therapy for hypertension (130/85 mm Hg) in patients with CAD, including those with angina.10 Patients with diabetes should be treated with a target blood‐pressure goal of <130/80 mm Hg. In addition, as diabetes alone is a strong risk factor for cardiac disease, glycemic control may be important.10 However, specific targets for hemoglobin A1c (HbA1c) remain unclear,10 and recent data challenge whether lower glycated hemoglobin values clinically benefit macrovascular disease.24., 25. For example, results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial showed no significant reduction in macrovascular complications in patients with type 2 diabetes who had received intensive therapy (targeted HbA1c level <6%) compared with standard therapy (targeted HbA1c level 7%–7.9%).25 In addition, patients receiving intensive therapy had increased mortality and a higher rate of hypoglycemia than patients receiving standard therapy.25 The decision to initiate intensive glucose management in an individual patient should therefore factor in the duration of diabetes, the presence of preexisting macrovascular disease or significant comorbidities, and hypoglycemic unawareness.24
Outcomes of Optimal Medical Therapy
Over the past 40 years, coronary artery revascularization, including PCI with stents or percutaneous transluminal coronary angioplasty (PTCA), has been compared with pharmacologic interventions for the treatment of stable angina. Earlier studies generally showed that revascularization was more effective than antianginal drug therapy. However, medical therapy for patients with stable angina has since improved, and now β‐blockers, antiplatelet agents, ACE inhibitors, and lipid‐lowering therapies are available and used routinely.14., 17. Randomized trials comparing PCI or CABG with conservative medical treatment have also shown that there was no significant difference between the 2 treatment strategies in terms of mortality, cardiac death, or fatal or nonfatal myocardial infarction (MI).19., 26., 27., 28., 29.
Recent trials support the viability of initial management of stable angina using OMT. The COURAGE trial was designed to assess whether PCI combined with intensive medical therapy and lifestyle management (defined as OMT) was superior to OMT alone in reducing the risk of cardiovascular events.14 The trial at 50 US and Canadian medical centers enrolled 2287 patients with evidence of myocardial ischemia and significant CAD.14 The results of COURAGE, published in 2007, determined that, as an initial management strategy, PCI combined with OMT did not reduce the risk of death and nonfatal MI compared with OMT alone during follow‐up of 2.5–7 years.14 The rates of subsequent revascularization were significantly lower in the PCI group compared with OMT alone. However, there was a substantial reduction in angina in both groups, with 74% and 72% of patients in the PCI and OMT groups, respectively, being angina‐free at 5 years.14
In 2009, the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI‐2D) study assessed optimal treatment of patients with comorbid diabetes (mean duration: 10.4 y) and stable angina.30 In BARI‐2D, 2368 patients were assigned to undergo either revascularization (PCI or CABG) combined with intensive OMT, or OMT alone along with insulin‐sensitizing drugs or insulin therapy. The 5‐year rates of survival did not significantly differ between the revascularization + OMT group (88%) and the OMT‐only group (88%).30
Based on the results of these large prospective, randomized trials and other observational data, the American College of Cardiology (ACC) and AHA guidelines on management of patients with chronic stable angina emphasize the initial use of medical therapy.18., 23. However, a challenge remains in that many patients with stable CAD often undergo PCI prior to a trial of OMT, despite guidelines supporting the use of medical therapy and landmark studies demonstrating little incremental clinical benefit of initial PCI/CABG over OMT.
Nitrates in Optimal Medical Therapy
Benefits of Nitrate Therapy
Nitroglycerin has multiple beneficial cardiovascular effects, including epicardial coronary vasodilation, decreased coronary vascular resistance, enhanced coronary collateral flow, decreased likelihood of coronary steal, increased venous capacitance, and decreased preload, which, in the aggregate, result in reduced myocardial oxygen consumption and increased exercise capacity.31 Nitrates are an important component of OMT and complement or supplement other antianginal agents.32 The antianginal effects of nitroglycerin have been known since 1879.33 For more than 3 decades, studies have demonstrated the efficacy of prophylactic nitrate use to increase exercise tolerance in patients with CAD.34., 35., 36.
Use of Short‐ and Long‐Acting Nitrates
Nitrates can be either short‐ or long‐acting and can be administered as a sublingual tablet, capsule, spray, patch, or ointment (Table 2). Clinical considerations influencing usage with the different preparations include time to onset of action, duration of activity, stability/potency, symptomatic relief vs prophylaxis, potential for resistance, development of tolerance, convenience, compliance, cost, patient preference, and concomitant medications.
Table 2.
Common Forms of Nitrates Used for Antiangina Therapy38
| Compound | Route | Usual Dose (Daily Unless Mentioned) | Onset of Action, min | Duration |
|---|---|---|---|---|
| Nitroglycerin | ||||
| Sublingual | 0.3–0.6 mg, up to 1.5 mg as needed | 2–5 | 10–30 min | |
| Spray/mist/aerosol | 0.4 mg, 1–2 sprays as needed, up to 3 doses 5 min apart | 2–5 | 10–30 min | |
| Ointment 2% | 7.5–40 mg (6 × 6 in. or 15 × 15 cm) | 20–60 | 3–8 h | |
| Transdermal patch | 0.2–0.8 mg/h q24 h; remove at night for 12 h | 60–120 | 8–12 ha | |
| Isosorbide dinitrate | Oralb | 5–80 mg, 2–3× daily | 30–60 | 8 h |
| Isosorbide mononitrate | Oral | 20 mg twice daily, 7 h apart | 30–60 | 12–14 h |
| Isosorbide mononitrate SR | Oral | 120–240 mg daily, given 1× daily | 30–60 | 12 h |
Abbreviations: q24 h, every 24 hours; SR, sustained release.
This table was published in Drugs for the Heart, 4th ed.38; used with permission.
Requires 8–10 hours of nitroglycerin‐free recovery period because of tolerance.
Also available in sublingual form.
Short‐acting nitrates are often given to relieve acute anginal pain and can also be used prophylactically to improve exercise tolerance and prevent exercise‐induced ischemia.34., 35., 36. In a study of patients with chronic stable angina, prophylactic treatment with sublingual nitroglycerin metered spray resulted in a dose‐dependent increase in time to onset of angina, ST‐segment depression, and exercise duration.36 This approach to treatment may also be particularly appropriate for patients with predictable angina precipitated by exertion or specific activities.19 Patients with suspected CAD who are being discharged from the emergency department or those recovering after inpatient hospitalization should also be considered for angina treatment with short‐acting nitrates.
Short‐acting nitrates may also be used to supplement long‐acting nitrates when patients experience acute attacks. Long‐acting nitrates, either as monotherapy or in combination with β‐blockers or calcium channel blockers, are often used to prevent or reduce the frequency of angina in patients with CAD.19., 20., 37., 38. These long‐acting agents may be used to extend the duration of action of the short‐acting forms.
Nitrate Tolerance and Rebound Angina
Tolerance and cross‐tolerance with nitrates are known to occur, and tolerance may develop quickly—usually within 12–24 hours—therefore, a nitrate‐free period of 10–12 hours per day or a low nitrate level at night is generally recommended.38., 39., 40. It should be noted that this approach carries a risk for increased angina frequency during nitrate‐free periods, limiting the ability for a continuous therapeutic effect.41., 42., 43., 44. In addition, an increase in angina at rest has been observed during nitrate‐free periods with transdermal nitrate formulations, as has a decrease in exercise duration prior to retreatment (often referred to as time‐zero effect).41., 42. However, neither an increase in nocturnal angina nor the time‐zero effect have been observed with orally administered long‐acting nitrates.40
The exact mechanism underlying the development of tolerance is not fully understood.45., 46. In vivo and in vitro studies have indicated that sulfhydryl groups are depleted following long‐term nitrate therapy. In addition, impaired nitroglycerin bioconversion, reduced bioavailability of nitric oxide and guanosine phosphate production, activation of the vasoconstrictor renin‐angiotensin‐aldosterone system and sympathetic nervous system in response to nitrate‐induced vasodilation, and increased levels of free oxygen radicals have also been implicated in the development of nitrate tolerance.46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65., 66. However, the use of concomitant sulfhydryl donors, ACE inhibitors, angiotensin‐receptor blockers, diuretics, arginine, carvedilol, and oxygen free radical scavengers has not demonstrated robust evidence in preventing the development of tolerance.40., 46., 51. Therefore, implementing a nitrate‐free period or a low nitrate level at night may offer the most reliable means to maintain effectiveness.38., 39., 40.
Contraindications to Nitrate Use
Both short‐ and long‐acting nitrate formulations are contraindicated in patients with allergic reactions to organic nitrates.43., 44. Concomitant use with phosphodiesterase inhibitors for erectile dysfunction—sildenafil, tadalafil, and vardenafil—is also contraindicated, given the potential for amplified vasodilation resulting in hypotension.43., 44., 67., 68., 69. All forms of nitroglycerin should also be used cautiously during the early days of acute MI.43., 44. In the event that treatment is initiated under this condition, particular attention must be paid to hemodynamic monitoring and clinical status.43., 44. Long‐acting nitrates should also be used cautiously in patients with congestive heart failure.43
Like any medication with vasodilatory effects, even small doses of short‐ and long‐acting nitrates can cause dizziness or hypotension, especially when patients are in an upright posture.43., 44., 70. This risk may be particularly magnified in elderly patients due to natural declines in autonomic nervous system regulation,71 in patients who are volume‐depleted (eg, diuretic therapy), or in patients who have low systolic blood pressure (ie, <90 mm Hg).43., 44. Nitroglycerin‐induced hypotension can also be accompanied by paradoxical bradycardia and increased angina pectoris.43., 44. Importantly, nitrates can aggravate angina from hypertrophic cardiomyopathy.43., 44. This may be particularly true regarding use of long‐acting nitrates in the elderly.43
Barriers to Nitrate Use
Many physicians use sublingual nitroglycerin solely for short‐acting relief of angina. Physicians are concerned about the development of tolerance and tachyphylaxis and the related concern that patients will not have a sustained benefit from nitroglycerin. Finally, with the increasing availability of percutaneous interventions, physicians tend to perceive medical therapy as less effective compared with invasive procedures.72 Patients also may be reluctant to take their prescribed nitrate. As a consequence, they often prefer to down‐regulate activity or exertion to avoid angina rather than use nitroglycerin as a preventive measure to alleviate angina. In other cases, patients do not understand that nitrates can be used for symptom prophylaxis.32
Cardiac Rehabilitation
Secondary prevention is an essential component of CAD management and includes multifaceted strategic interventions intended to reduce modifiable risk factors for cardiovascular disease.73 The goal of cardiac rehabilitation is to stabilize, slow, or reverse the progression of CAD, enhance the patient's understanding of the disease through education, promote improved dietary habits through nutritional counseling, and improve quality of life.73., 74.
Recommendations and Benefits of Cardiac Rehabilitation
Cardiac‐rehabilitation programs include aerobic exercise and resistance training, diet and nutrition counseling, weight control, lipid management, blood‐pressure monitoring, diabetes management, and smoking cessation, as well as medication education75; these interventions mesh with interventions recommended as part of OMT. The ACC and AHA guidelines for the management of patients with chronic stable angina identify exercise therapy as a core element of cardiac rehabilitation.22 Thirty to 60 minutes of moderate‐intensity aerobic activity (eg, brisk walking) are recommended at least 5 days (ideally 7 days) per week.23 This exercise should be supplemented by increased daily activities, such as walking breaks during work or gardening. Expanding physical activity to include resistance training on 2 days per week may be reasonable.23 Medically supervised cardiac rehabilitation is recommended for at‐risk patients, such as those who have experienced recent acute coronary syndrome, revascularization, or stable angina.22., 23. It is important to assess risk with a physical activity history and to perform an exercise test to guide the exercise prescription.23., 76.
To further define the effects of exercise‐based cardiac rehabilitation in patients with coronary heart disease, a systematic review and meta‐analysis of randomized, controlled clinical trials was performed.74 In the meta‐analysis, a total of 48 trials enrolling 8940 patients found that compared with usual care, cardiac rehabilitation was associated with reduced all‐cause mortality and cardiac mortality, whereas there were no differences in the rates of nonfatal MI and revascularization. Table 3 compares the effects of exercise‐based cardiac rehabilitation on study endpoints.73., 74.
Table 3.
| Outcome | Mean Difference, % | 95% CI | P Value |
|---|---|---|---|
| Total mortality | −20 | −7% to −32% | 0.005 |
| Cardiac mortality | −26 | −10% to −29% | 0.002 |
| Nonfatal MI | −21 | −43% to 9% | 0.150 |
| CABG | −13 | −35% to 16% | 0.400 |
| PTCA | −19 | −51% to 34% | 0.400 |
Abbreviations: CABG, coronary artery bypass grafting; CI, confidence interval; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Copyright Wolters Kluwer Health. Adapted with permission from Leon et al.73
In a prospective study designed to compare regular exercise vs PCI for patients with stable CAD, 101 male patients were randomized to either 12 months of exercise training (20 minutes daily exercise) or PCI. Exercise training resulted in a significantly higher event‐free survival rate (Figure 1) and increased maximal oxygen uptake. The improvements in event‐free survival and increased exercise capacity occurred at lower costs due to reduced rehospitalizations and repeat revascularizations.77
Figure 1.
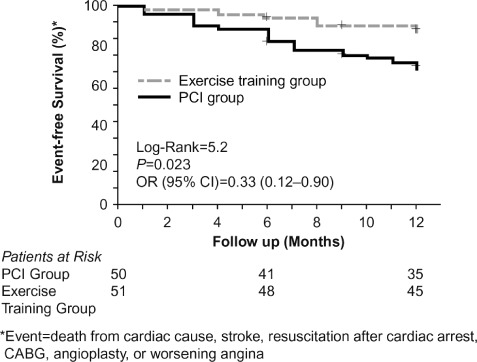
Event‐free survival after 12 months, exercise training group vs PCI group. Event = death from cardiac cause, stroke, resuscitation after cardiac arrest, CABG, or worsening angina. Abbreviations: CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention. Copyright Wolters Kluwer Health. Adapted with permission from Hambrecht et al.77
Factors Influencing Patient Participation in Cardiac Rehabilitation
Psychological and social factors that negatively influence mental health are relatively common among patients receiving cardiac rehabilitation. These factors include depression, anger, anxiety, and social isolation related to their condition.73 Depression and low perceived social support (LPSS) after MI are associated with higher morbidity and mortality. The Enhanced Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial assessed whether mortality and recurrent infarction are reduced by treatment of depression and LPSS with cognitive behavior therapy, supplemented with antidepressant therapy when indicated.78 Cognitive behavior therapy was initiated at a median of 17 days after MI for an average of 11 individual sessions throughout 6 months, plus group therapy when feasible.78 Patients in the ENRICHD trial who received these interventions achieved significant improvements in both depression and LPSS; however, they did not demonstrate a significant benefit in terms of mortality and recurrent infarction.78
Despite evidence demonstrating that exercise training for patients with CAD increases exercise capacity, reduces ischemia, and delays or eliminates symptoms of angina, exercise training is underutilized. Evidence indicates that only approximately 20% of eligible patients are referred to cardiac‐rehabilitation programs. A number of factors are implicated in this underutilization, including the healthcare perception that exercise may have little clinical benefit, poor provider training in exercise therapeutics, poor financial reimbursement for providers, and the absence of advocates for exercise training.
Role of Nitroglycerin in Exercise Tolerance
Relatively few randomized, double‐blind, placebo‐controlled studies have addressed the effectiveness of prophylactic use of nitroglycerin in increasing or improving exercise tolerance, and studies thus far have been limited to the spray formulation. Kimchi and colleagues studied the efficacy of nitroglycerin oral spray in 20 patients with angina in a randomized, crossover trial employing treadmill exercise testing.34 Nitroglycerin spray delayed the onset of angina during exercise in 13 patients (P < 0.001) and prevented pain entirely in 7 patients. Exercise‐induced ST‐segment depression was abolished or delayed (P < 0.001), and maximal‐exercise–induced ST‐segment depression also decreased (P < 0.001).34 In addition, nitroglycerin spray increased the duration of time to onset of angina symptoms during the treadmill test by 31% (P < 0.001), indicating that nitroglycerin spray is an effective prophylactic agent for exercise‐induced angina.34
A double‐blind, randomized, placebo‐controlled crossover study of 3 doses (0.2 mg, 0.4 mg, and 0.8 mg) of lingual nitroglycerin spray was conducted in 20 patients with chronic, stable, exercise‐induced angina.79 Patients completed initial exercise testing and then participated in 3 exercise tests, ≥90 minutes apart. After the initial test, but prior to each subsequent test, patients received a dose of nitroglycerin spray and then began an exercise test 5 minutes after the medication was administered. Each dose of nitroglycerin spray increased time to the onset of angina and time to development of moderate angina during exercise. The authors concluded that nitroglycerin spray was an effective means to administer nitrates prophylactically for exercise‐induced angina.79
Lastly, a double‐blind, randomized, placebo‐controlled crossover study was conducted to determine the effectiveness of a metered nitroglycerin spray in patients with exercise‐induced angina.36 Fifty‐one patients received 0.2‐mg, 0.4‐mg, 0.8‐mg, and 1.6‐mg single doses of a sublingual metered nitroglycerin spray or placebo. The time to onset of moderate angina was significantly delayed in all active treatment groups (0.2 mg, 0.4 mg, 0.8 mg, and 1.6 mg nitroglycerin spray) compared with placebo (Figure 2, Table 4). In addition, a linear dose‐related increase in time to onset of angina was observed.36
Figure 2.
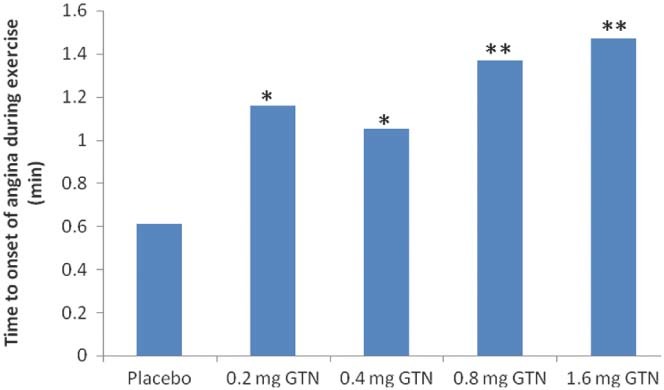
Adjusted mean change in time to onset of angina. Investigational ETT − control ETT; sublingual nitroglycerin spray vs placebo. Abbreviations: ETT, exercise tolerance time; GTN, glyceryl trinitrate. ∗︁ P < 0.05. ∗︁∗︁ P < 0.001. Adapted from Wittig and Beuscher.36
Table 4.
Exercise Tolerance Time Following Administration of GTN Spray in Time to Onset of Angina (min)a
Abbreviations: ETT, exercise tolerance time; GTN, glyceryl trinitrate.
Investigational ETT − control ETT; sublingual nitroglycerin spray vs placebo.
P < 0.05.
p < 0.001.
From Wittig and Beuscher.36
Collectively, these findings indicate that prophylactic sublingual nitrates, as part of cardiac rehabilitation, may allow patients with angina to exercise to a greater functional capacity than a treatment plan without nitrates.
Additional Considerations
There is ongoing debate regarding the best clinical use of short‐ vs long‐acting nitrates. Some physicians recommend short‐acting nitrates if angina occurs a few times a week and long‐acting nitrates if angina is more frequent. In patients who have angina with exertion, long‐acting nitrates are sometimes preferred to prevent symptoms throughout the day. However, it is generally well known that continuous treatment with organic nitrates can lead to the development of tolerance, which in turn can lead to a loss of clinical efficacy. It is therefore not clinically feasible to provide continuous antianginal prophylaxis with any of the currently available long‐acting nitrates.
In contrast, short‐acting nitrates can be used effectively to prevent symptoms before activities that provoke angina, but patients must remember to carry the medication in the event that prophylactic treatment is needed. Short‐acting nitroglycerin is available either as a tablet or a spray. Although tablets are less expensive, the spray may offer greater convenience and ease of administration and has a long shelf life.
There is a critical need to educate both physicians and patients about the use of nitrates and cardiac rehabilitation as secondary prevention in patients with CAD. Healthcare professionals should both consider and utilize nitrates as a prophylactic part of the exercise prescription. Nitrates should also be considered as part of a discharge medication program with the goal of decreasing angina readmission rates and mortality. As previously discussed, recurrent bouts of angina are debilitating clinically and increase both direct and indirect healthcare costs. As such, the dialogue between the patient and physician is critically important. Patients need confidence that they can return to normal daily activities without fear of anginal pain or compromised quality of life. As a matter of public health policy, performance measures should be implemented to track prospectively the impact of nitrate usage and cardiac‐rehabilitation prescriptions on long‐term patient outcomes.
Of note, more recent literature has introduced the term stable ischemic heart disease (SIHD).80., 81. This description encompasses patients with stable angina as well as those with stable CAD, whether symptomatic or asymptomatic. Based on the current use of this terminology and the active SIHD committee associated with the ACC and AHA,82 it is likely that future recommendations and trials including patients with stable angina will fall under the spectrum of SIHD management.
Nonpharmacologic Interventions for Refractory Angina
As a final consideration, the ACC and AHA guidelines recommend several alternative therapies to manage refractory angina in patients for whom cardiac revascularization is not an option.83 For example, following enhanced external counterpulsation, increased time to exercise‐induced ischemia and improved symptoms have been observed.84., 85., 86. Laser transmyocardial revascularization, an emerging technique to treat more severe refractory angina, could improve myocardial revascularization through the creation of transmural endomyocardial channels.83 Spinal‐cord stimulation has also been shown to provide analgesia.83 However, additional follow‐up studies demonstrating the long‐term benefits of these techniques are needed.83
Conclusion
Optimal medical therapy for patients with stable angina consists of disease‐modifying agents and symptomatic treatment for angina. Although treatment with rapid‐ and short‐acting nitrates is often underappreciated therapeutically by clinicians, these medications are a pivotal component of OMT and should be integrated into secondary prevention measures. Exercise therapy, as part of a structured cardiac‐rehabilitation program, should also be considered a central component of secondary prevention in patients with chronic stable angina. Here, acute nitrate administration could fulfill a key role in achieving patient outcomes, given the demonstrated improvement in exercise tolerance with prophylactic use. Greater patient and physician understanding of the importance of OMT, the role of short‐acting nitrate therapy, and the impact of regular exercise can collectively improve symptomatic outcomes and increase quality of life for patients with stable CAD.
Acknowledgements
Writing and editorial assistance was provided by David Christiansen, PhD, and Sabrina L. Maurer, PharmD, from Fishawack Communications and funded by Arbor Pharmaceuticals.
References
- 1. Roger VL, Go SA, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association [published corrections appear in Circulation. 2011;123:e240 and 2011;124:e426]. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . World Health Organization Fact Sheet No. 317: cardiovascular diseases (CVDs). http://www.who.int/mediacentre/factsheets/fs317/en/index.html. Published September 2011.. Accessed December 14, 2011.
- 3. Dougherty CM, Dewhurst T, Nichol WP, et al. Comparison of three quality of life instruments in stable angina pectoris: Seattle Angina Questionnaire, Short Form Health Survey (SF‐36), and Quality of Life Index‐Cardiac Version III. J Clin Epidemiol. 1998;51:569–575. [DOI] [PubMed] [Google Scholar]
- 4. Brorsson B, Bernstein SJ, Brook RH, et al. Quality of life of patients with chronic stable angina before and four years after coronary revascularisation compared with a normal population. Heart. 2002;87:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association [published corrections appear in Circulation. 2010;121:e260 and 2011;124:e425]. Circulation. 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 6. Mangoni AA. Cardiovascular drug therapy in elderly patients: specific age‐related pharmacokinetic, pharmacodynamic and therapeutic considerations. Drugs Aging. 2005;22:913–941. [DOI] [PubMed] [Google Scholar]
- 7. Daly C, Clemens F, Lopez‐Sendon JL, et al; Euro Heart Survey Investigators. The impact of guideline compliant medical therapy on clinical outcome in patients with stable angina: findings from the Euro Heart Survey of stable angina. Eur Heart J. 2006;27:1298–1304. [DOI] [PubMed] [Google Scholar]
- 8. Wilson SR, Scirica BM, Braunwald E, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double‐blind, placebo‐controlled MERLIN‐TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non‐ST‐Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol. 2009;53:1510–1516. [DOI] [PubMed] [Google Scholar]
- 9. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA Guidelines for the management of patients with unstable angina and non–ST‐segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) [published correction appears in Circulation. 2000;102:1739]. Circulation. 2000;102:1193–1209. [DOI] [PubMed] [Google Scholar]
- 10. Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary. The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381. [DOI] [PubMed] [Google Scholar]
- 11. Holubkov R, Laskey WK, Haviland A, et al; NHLBI Dynamic Registry Investigators. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J. 2002;144:826–833. [DOI] [PubMed] [Google Scholar]
- 12. Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary‐artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–1124. [DOI] [PubMed] [Google Scholar]
- 13. Serruys PW, Ong AT, van Herwerden LA, et al. Five‐year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–581. [DOI] [PubMed] [Google Scholar]
- 14. Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 15. Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 16. Javitz HS, Ward MM, Watson JB, et al. Cost of illness of chronic angina. Am J Manag Care. 2004;10(11 suppl):S358–S369. [PubMed] [Google Scholar]
- 17. Deedwania PC, Carbajal EV. Medical therapy versus myocardial revascularization in chronic coronary syndrome and stable angina. Am J Med. 2011;124:681–688. [DOI] [PubMed] [Google Scholar]
- 18. Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 Guideline Update for the Management of Patients with Chronic Stable Angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 2003;41:159–168. [DOI] [PubMed] [Google Scholar]
- 19. Thadani U. Current medical management of chronic stable angina. J Cardiovasc Pharmacol Ther. 2004;9(suppl 1):S11–S29. [DOI] [PubMed] [Google Scholar]
- 20. Thadani U. Medical therapy of stable angina pectoris. Cardiol Clin. 1991;9:73–87. [PubMed] [Google Scholar]
- 21. Boden WE, O'Rourke RA, Teo KK, et al. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial Veterans Affairs Cooperative Studies Program no. 424. Am Heart J. 2006;151: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 22. Fraker TD Jr, Fihn SD, Gibbons RJ, et al. 2007. Chronic Angina Focused Update of the ACC/AHA 2002 Guidelines for the Management of Patients with Chronic Stable Angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina [published correction appears in Circulation. 2007;116:e558]. Circulation. 2007;116: 2762–2772. [DOI] [PubMed] [Google Scholar]
- 23. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–2446. [DOI] [PubMed] [Google Scholar]
- 24. Terry T, Raravikar K, Chokrungvaranon N, et al. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2012;14:79–88. [DOI] [PubMed] [Google Scholar]
- 25. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chamberlain DA, Fox KA, Henderson RA, et al; RITA‐2 trial participants. Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA‐2) trial. Lancet. 1997;350:461–468. [PubMed] [Google Scholar]
- 27. Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta‐analysis. Circulation. 2005;111:2906–2912. [DOI] [PubMed] [Google Scholar]
- 28. Caracciolo EA, Davis KB, Sopko G, et al. Comparison of surgical and medical group survival in patients with left main coronary artery disease: long‐term CASS experience. Circulation. 1995;91:2335–2344. [DOI] [PubMed] [Google Scholar]
- 29. Peduzzi P, Kamina A, Detre K. Twenty‐two‐year follow‐up in the VA Cooperative Study of Coronary Artery Bypass Surgery for Stable Angina. Am J Cardiol. 1998;81:1393–1399. [DOI] [PubMed] [Google Scholar]
- 30. Frye RL, August P, Brooks MM, et al; BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kones R. Recent advances in the management of chronic stable angina II: anti‐ischemic therapy, options for refractory angina, risk factor reduction, and revascularization. Vasc Health Risk Manag. 2010;6:749–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graboys TB, Lown B. Cardiology patient page. Nitroglycerin: the “mini” wonder drug. Circulation. 2003;108:e78–e79. [DOI] [PubMed] [Google Scholar]
- 33. Murell W. Nitroglycerin as a remedy for angina pectoris. Lancet. 1879;113:642–646. [Google Scholar]
- 34. Kimchi A, Lee G, Amsterdam E, et al. Increased exercise tolerance after nitroglycerin oral spray: a new and effective therapeutic modality in angina pectoris. Circulation. 1983;67:124–127. [DOI] [PubMed] [Google Scholar]
- 35. Kolenda KD. Glycerol trinitrate and coronary heart disease: value of prophylactic administration within the scope of exercise therapy [article in German]. Fortschr Med. 1998;116:41–42. [PubMed] [Google Scholar]
- 36. Wittig T, Beuscher N. Increased physical performance following administration of glycerol trinitrate in spray form [article in German]. Fortschr Med. 1999;117:109–113. [Google Scholar]
- 37. Thadani U, Rodgers T. Side effects of using nitrates to treat angina. Expert Opin Drug Saf. 2006;5:667–674. [DOI] [PubMed] [Google Scholar]
- 38. Thadani U, Opie L. Nitrates. In: Opie L, ed. Drugs for the Heart. 4th ed. Philadelphia: WB Saunders; 1995:36–37. [Google Scholar]
- 39. Zimrin D, Reichek N, Bogin KT, et al. Antianginal effects of intravenous nitroglycerin over 24 hours. Circulation. 1988;77:1376–1384. [DOI] [PubMed] [Google Scholar]
- 40. Thadani U, Lipicky R. Short‐ and long‐acting nitrates for stable angina pectoris. In: Thadani U, Lipicky RJ, eds. Nitrates Updated: Current Use in Angina, Ischemia, Infarction, and Failure. Kluwer Academic Publications; (Springer; ); 1997:94–116. [Google Scholar]
- 41. DeMots H, Glasser SP. Intermittent transdermal nitroglycerin therapy in the treatment of chronic stable angina. J Am Coll Cardiol. 1989;13:786–795. [DOI] [PubMed] [Google Scholar]
- 42. Thadani U. Nitrate tolerance, rebound, and their clinical relevance in stable angina pectoris, unstable angina, and heart failure. Cardiovasc Drugs Ther. 1997;10:735–742. [DOI] [PubMed] [Google Scholar]
- 43. Nitro‐Dur [prescribing information] . Summit, NJ: Key Pharmaceuticals, Inc.; 2006.
- 44. Nitrolingual Pumpspray [prescribing information]. Raleigh, NC: Arbor Pharmaceuticals, Inc.; 2011.. [Google Scholar]
- 45. Thadani U. Nitrate therapy and the development of tolerance. Arch Fam Med. 1993;2:880–885. [DOI] [PubMed] [Google Scholar]
- 46. Thadani U, Whitsett T, Hamilton SF. Nitrate therapy for myocardial ischemic syndrome: current perspectives including tolerance. Curr Probl Cardiol. 1988;13:723–784. [DOI] [PubMed] [Google Scholar]
- 47. Axelsson KL, Andersson RG. Tolerance towards nitroglycerin, induced in vivo, is correlated to a reduced cGMP response and an alteration in cGMP turnover. Eur J Pharmacol. 1983;88:71–79. [DOI] [PubMed] [Google Scholar]
- 48. Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dakak N, Makhoul N, Flugelman MY, et al. Failure of captopril to prevent nitrate tolerance in congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1990;66:608–613. [DOI] [PubMed] [Google Scholar]
- 50. Dupuis J, Lalonde G, Lemieux R, et al. Tolerance to intravenous nitroglycerin in patients with congestive heart failure: role of increased intravascular volume, neurohumoral activation and lack of prevention with N‐acetylcysteine. J Am Coll Cardiol. 1990;16:923–931. [DOI] [PubMed] [Google Scholar]
- 51. Fung H, Bauer JA. Mechanism of nitrate tolerance. In: Thadani U, Opie LH, eds. Nitrates Updated: Current Use in Angina, Ischemia, Infarction, and Failure. Kluwer Academic Publications; (Springer; ); 1997:57–79. [Google Scholar]
- 52. Gori T, Burstein JM, Ahmed S, et al. Folic acid prevents nitroglycerin‐induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation. 2001;104:1119–1123. [DOI] [PubMed] [Google Scholar]
- 53. Ignarro LJ, Lippton H, Edwards JC, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S‐nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 54. Katz RJ, Levy WS, Buff L, et al. Prevention of nitrate tolerance with angiotension‐converting enzyme inhibitors. Circulation. 1991;83:1271–1277. [DOI] [PubMed] [Google Scholar]
- 55. Kobayashi A, Suzuki Y, Kamikawa T, et al. The effects of nitroglycerin on cyclic nucleotides in the coronary artery in vivo. Life Sci. 1980;27:1679–1685. [DOI] [PubMed] [Google Scholar]
- 56. Kukovetz WR, Holzmann S. Mechanism of nitrate‐induced vasodilation and tolerance. Z Kardiol. 1983;72(suppl 3):14–19. [PubMed] [Google Scholar]
- 57. Münzel T, Holtz J, Mülsch A, et al. Nitrate tolerance in epicardial arteries or in the venous system is not reversed by N‐acetylcysteine in vivo, but tolerance‐independent interactions exist. Circulation. 1989;79:188–197. [DOI] [PubMed] [Google Scholar]
- 58. Needleman P, Johnson EM Jr. Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973;184:709–715. [PubMed] [Google Scholar]
- 59. Parker JO, Parker JD, Caldwell RW, et al. The effect of supplemental L‐arginine on tolerance development during continuous transdermal nitroglycerin therapy. J Am Coll Cardiol. 2002;39:1199–1203. [DOI] [PubMed] [Google Scholar]
- 60. Sage PR, de la Lande IS, Stafford I, et al. Nitroglycerin tolerance in human vessels: evidence for impaired nitroglycerin bioconversion. Circulation. 2000;102:2810–2815. [DOI] [PubMed] [Google Scholar]
- 61. Torresi J, Horowitz JD, Dusting GJ. Prevention and reversal of tolerance to nitroglycerine with N‐acetylcysteine. J Cardiovasc Pharmacol. 1985;7:777–783. [DOI] [PubMed] [Google Scholar]
- 62. Watanabe H, Kakihana M, Ohtsuka S, et al. Randomized, double‐blind, placebo‐controlled study of supplemental vitamin E on attenuation of the development of nitrate tolerance. Circulation. 1997;96:2545–2550. [DOI] [PubMed] [Google Scholar]
- 63. Watanabe H, Kakihana M, Ohtsuka S, et al. Randomized, double‐blind, placebo‐controlled study of carvedilol on the prevention of nitrate tolerance in patients with chronic heart failure. J Am Coll Cardiol. 1998;32:1194–1200. [DOI] [PubMed] [Google Scholar]
- 64. Watanabe H, Kakihana M, Ohtsuka S, et al. Randomized, double‐blind, placebo‐controlled study of the preventive effect of supplemental oral vitamin C on attenuation of development of nitrate tolerance. J Am Coll Cardiol. 1998;31:1323–1329. [DOI] [PubMed] [Google Scholar]
- 65. Watanabe H, Kakihana M, Ohtsuka S, et al. Preventive effects of carvedilol on nitrate tolerance—a randomized, double‐blind, placebo‐controlled comparative study between carvedilol and arotinolol. J Am Coll Cardiol. 1998;32:1201–1206. [DOI] [PubMed] [Google Scholar]
- 66. Packer M, Lee WH, Kessler PD, et al. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med. 1987;317:799–804. [DOI] [PubMed] [Google Scholar]
- 67. Cheitlin MD, Hutter AM Jr, Brindis RG, et al. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association [published correction appears in J Am Coll Cardiol. 1999;34:1850]. J Am Coll Cardiol. 1999;33:273–282. [DOI] [PubMed] [Google Scholar]
- 68. Webb DJ, Muirhead GJ, Wulff M, et al. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol. 2000;36:25–31. [DOI] [PubMed] [Google Scholar]
- 69. Kloner RA. Cardiovascular effects of the 3 phosphodiesterase‐5 inhibitors approved for the treatment of erectile dysfunction. Circulation. 2004;110:3149–3155. [DOI] [PubMed] [Google Scholar]
- 70. Muse (alprostadil) urethral suppository [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2011.. [Google Scholar]
- 71. Bourke E, Sowes J. The autonomic nervous system and blook pressure regulation in the elderly. In: Kuchel GA, Hof PR, eds. Autonomic Nervous System in Old Age. Vol 33. Interdiscipl Top Gerontol. 2004:44–52. [Google Scholar]
- 72. Carasso S, Markiewicz W. Medical treatment of patients with stable angina pectoris referred for coronary angiography: failure of treatment or failure to treat. Clin Cardiol. 2002;25:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association Scientific Statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American Association of Cardiovascular and Pulmonary Rehabilitation [published correction appears in Circulation. 2005;111:1717]. Circulation. 2005;111:369–376. [DOI] [PubMed] [Google Scholar]
- 74. Taylor RS, Brown A, Ebrahim S, et al. Exercise‐based rehabilitation for patients with coronary heart disease: systematic review and meta‐analysis of randomized controlled trials. Am J Med. 2004;116:682–692. [DOI] [PubMed] [Google Scholar]
- 75. Braverman DL. Cardiac rehabilitation: a contemporary review. Am J Phys Med Rehabil. 2011;90:599–611. [DOI] [PubMed] [Google Scholar]
- 76. Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354–2363. [DOI] [PubMed] [Google Scholar]
- 77. Hambrecht R, Walther C, Möbius‐Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109:1371–1378. [DOI] [PubMed] [Google Scholar]
- 78. Berkman LF, Blumenthal J, Burg M, et al; Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106–3116. [DOI] [PubMed] [Google Scholar]
- 79. Parker JO, Vankoughnett KA, Farrell B. Nitroglycerin lingual spray: clinical efficacy and dose‐response relation. Am J Cardiol. 1986;57:1–5. [DOI] [PubMed] [Google Scholar]
- 80. Beatty AL, Zhang MH, Ku IA, et al. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: data from the Heart and Soul Study. Atherosclerosis. 2012;220:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maron DJ, Stone GW, Berman DS, et al. Is cardiac catheterization necessary before initial management of patients with stable ischemic heart disease? Results from a Web‐based survey of cardiologists. Am Heart J. 2011;162:1034.e13–1043.e13. [DOI] [PubMed] [Google Scholar]
- 82. Kushner F. The New and Revised 2011. ACCF/AHA PCI and CABG Guidelines: A Revascularization Consensus Is at the Heart of the Matter. http://my.americanheart.org/professional/General/Revascularization‐Consensus‐is‐at‐the‐Heart‐of‐the‐Matter_UCM_433406_Article.jsp. Updated November 8, 2011. Accessed March 2, 2012.
- 83. Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 Guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107:149–158. [DOI] [PubMed] [Google Scholar]
- 84. Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST‐EECP): effect of EECP on exercise‐induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–1840. [DOI] [PubMed] [Google Scholar]
- 85. Barsness G, Feldman AM, Holmes DR Jr, et al. The International EECP Patient Registry (IEPR): design, methods, baseline characteristics, and acute results. Clin Cardiol. 2001;24:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lawson WE, Hui JC, Lang G. Treatment benefit in the enhanced external counterpulsation consortium. Cardiology. 2000;94:31–35. [DOI] [PubMed] [Google Scholar]
- 87. Lopressor (metoprolol tartrate) tablets, USP [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.. [Google Scholar]
- 88. Nitrostat (nitroglycerin sublingual) tablets, USP [prescribing information]. New York, NY: Pfizer; 2011.. [Google Scholar]
- 89. Cardizem (diltiazem hydrochloride) extended release tablets [prescribing information]. Chicago, IL: Abbott Laboratories; 2010.. [Google Scholar]
- 90. Ranexa (ranolazine) extended‐release tablets [prescribing information]. Foster City, CA: Gilead Sciences, Inc.; 2011.. [Google Scholar]


