Abstract
Background:
Takotsubo cardiomyopathy (TTC) presents clinically as an acute coronary syndrome. It is characterized by transient left ventricular wall dyskinesis‐akinesis, without significant epicardial coronary lesions. Late gadolinium enhancement (LGE) sequences on cardiac magnetic resonance (CMR) allow to clarify the pathophysiology in patients with chest pain, elevated troponin, and normal epicardial coronary arteries; in patients with TTC, previous studies have shown absence of LGE.
Hypothesis:
Early CMR in Takotsubo patients could show a morphological pattern of LGE improving clinical diagnosis.
Methods:
Between January 2005 and January 2007, 8 consecutive patients with TTC criteria underwent CMR within the first 3 days of admission. Cine, T2‐weighted, and LGE images were acquired. Patient follow‐up included clinical exam and imaging techniques: echocardiogram on days 3, 7, 30, and 60, and CMR at 3 months.
Results:
Six patients had experienced a previous stressful situation. No significant lesions were found on coronary angiography, and wall motion improvement was noted at 15 (7–30) days. Median EFs at admission and recovery were 46.5% and 65%, respectively. Dyskinesis was midapical in 6 cases, apical in 1 case, and mid‐ventricular in 1 case. Late gadolinium enhancement showed mild hyperenhancement in areas of abnormal wall motion, whereas normal segments had no contrast enhancement. On follow‐up CMR, wall motion was normal without late enhancement.
Conclusions:
Early CMR in TTC demonstrates a special morphological pattern of late gadolinium uptake that might correspond to localized inflammation and edema in the affected area, suggesting diffuse microcirculation damage rather than epicardial vessel involvement. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
See Editorial on Page 145
Introduction
Takotsubo cardiomyopathy (TTC) was described long ago in Japan, and received this name due to the shape of the left ventricle (LV), which is similar to a traditional pot used by fishermen for catching octopi. It is an emerging clinical entity, characterized by transient LV dysfunction with symptoms mimicking an acute myocardial infarction but without significant coronary lesions on angiography; it may be preceded by emotional or physical stress. Although the pathophysiology remains unclear, the explanation that appears more robust is myocardial stunning mediated by direct catecholamine effect.1 Other suggested hypotheses are epicardial or microcirculatory vasospasm, LV outflow obstruction, or plaque rupture with spontaneous fibrinolysis.2, 3, 4, 5, 6, 7
Prior studies have shown the usefulness of cardiac magnetic resonance (CMR) with contrast to differentiate small infarcts from myocarditis or TTC.8, 9, 10 In TTC, although many cases have elevated troponins as a sign of myocardial damage, sequences of late gadolinium enhancement are notably negative. In this study we describe 8 patients with findings consistent with TTC, who on early CMR (ie, immediately after cardiac catheterization) exhibited a unique morphological pattern of late gadolinium uptake that has not been described in previous series.
Methods
Population and Study Protocol
From January 2005 to January 2007, 8 consecutive patients with a diagnosis of TTC were evaluated with CMR performed immediately after cardiac catheterization. Diagnosis was made based on the following criteria: (1) transient hypokinesis, akinesis, or dyskinesis in the LV apical or midventricular segments; regional wall‐motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger; (2) the absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiogram (ECG) abnormalities (ST‐segment elevation and/or T‐wave inversion) or modest elevation in cardiac troponin; and (4) the absence of pheochromocytoma and myocarditis.1 Patients were followed with a clinical exam and imaging techniques consisting of an echocardiogram at 3, 7, 15, 30, and 60 days to assess the precise moment of LV wall‐motion recovery. Finally, follow‐up with CMR was performed at 90 days after admission.
Imaging Techniques
Protocol and Assessment With Cardiac Magnetic Resonance:
CMR images were acquired on a 1.5T scanner (Signa CVi‐HDx, GE Healthcare, Waukesha, WI) with a dedicated cardiac coil. The protocol included balanced steady‐state free precession gradient‐echo images (cine), T2‐weighted images (T2), and LGE inversion recovery images (10' after intravenous administration of 0.2 mmol/kg of gadopentetate dimeglumine contrast [(Magnevist; Schering, Berlin, Germany]). All sequences were acquired in short‐axis, 2‐, 3‐, and 4‐chamber views. Cine images were used to evaluate LV systolic function. Visual analysis of myocardial LGE was performed, as well as T2 high signal and wall‐motion assessment of the 17 LV myocardial segments.
Echocardiography:
Echocardiograms were performed using a Vivid 3 echocardiography system (GE Healthcare) with a 3S transducer (1.5–3.6 MHz) and an iE33 echocardiography system (Philips Medical Systems, Bothell, WA) with S5‐1 and X3‐1 transducers. Images acquired were stored in digital format. Two patients with a poor window received a 1‐ml bolus of sulfur hexafluoride (SonoVue; Bracco SpA, Milan, Italy) echocardiographic contrast injected into a peripheral vein to improve the assessment of the LV endocardium.
Statistical Analysis
Statistical analysis was performed with STATA software, version 10.0 (StataCorp LP, College Station, TX). The data exhibited a nonparametric distribution; hence, they are expressed as medians and their respective interquartile intervals (P25–P75). The comparison of early and recovery ejection fractions was performed with the Wilcoxon test for paired samples. A 2‐tailed P value <0.05 was considered significant in all cases.
Results
Demographic, Clinical, and Laboratory Data
The 8 patients were women (median age 57.5 y, range 53–73 y) and were hospitalized due to TTC. Among them, 41% had coronary risk factors (hypertension in 50%, dyslipidemia in 62.5%, and smoking in 12.5%). At admission, all 8 patients (100%) had chest pain, 2 had dyspnea, and 2 had hypertension (25%). A previous stressful situation was identified in 6 patients (75%). The ECG exhibited ST‐segment elevation in 4 patients (50%) and negative T waves in all cases (100%). Three patients (37.5%) had signs of heart failure and 6 had elevated troponin I and creatinine kinase MB isoenzyme. No patient had significant coronary stenosis at cardiac catheterization.
Imaging Techniques
Echocardiography:
Echocardiography, angiographic ventriculography, and acute‐phase CRM showed typical wall‐motion abnormalities, characterized by akinesis/dyskinesis in the midapical segments in 6 patients, apical dyskinesis in 1 patient, and midventricular dyskinesis in 1 patient. Ejection fractions at admission and recovery estimated by echocardiography were 46% (range, 39.2%–53%) and 64% (range, 59.2%–71.7%), respectively (P = 0.008).
Cardiac Magnetic Resonance:
The CMR exams were performed within 72 hours of the beginning of symptoms in all patients; all had good‐quality balanced steady‐state free precession and LGE images for visual and quantitative assessment. In 3 patients, the T2‐weighted images were of suboptimal quality for edema quantification. The remaining 5 patients had a transmural area of high T2 signal intensity matching the location of hypokinesis. In 8 patients, LGE images showed mild signal enhancement in the segments with abnormal contractility, which was clearly different from the segments with no signal enhancement and normal contractility.
Imaging Techniques During Follow‐Up:
On echocardiography, improvement in LV wall motion occurred in average at 15 (range, 7–30) days from the beginning of symptoms. A follow‐up CMR at 3 months revealed a completely normal wall motion and absence of LGE in all cases, which is shown in the illustrations from patients 2 to 5 and 7 (See figures 1, 2, 3, 4, 5).
Figure 1.
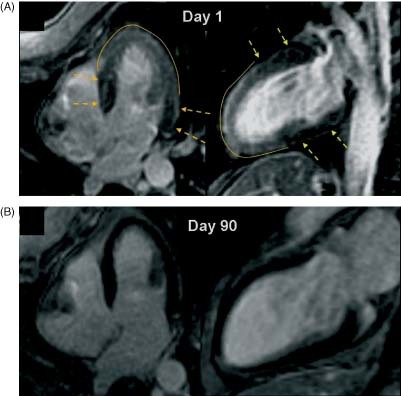
(A) CMR, LGE sequence, showing mild transmural gadolinium enhancement in the midapical region (day 1). Note a normal washout zone in segments with normal contractility (arrows). (B) CMR, LGE sequence that disappears completely in the follow‐up CMR (day 90). Abbreviations: CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Figure 2.
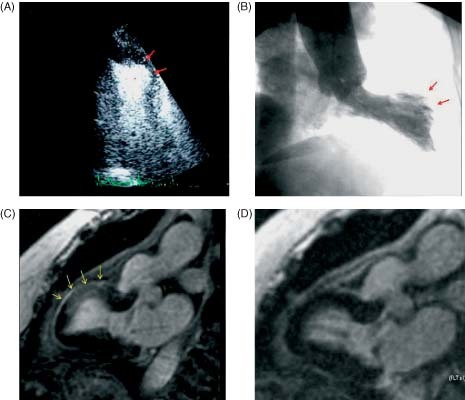
Patient with transient midventricular dyskinesis. (A) Contrast echocardiogram (systolic image), apical 2‐chamber view showing dyskinesis in the middle segment of the anterior wall (red arrows). (B) Angiographic ventriculography, showing similar findings to those described by echo (red arrows). (C) CMR, sequence of 2‐chamber LGE showing hyperintensity in the area of abnormal segmental wall motion (middle segment of the anterior wall) (arrows). (D) Complete normalization after 30 days. Abbreviations: CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Figure 3.
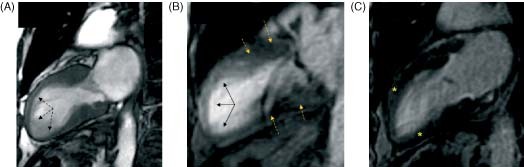
Patient with transient midapical dyskinesia. (A) CMR, SSFP sequence. During systole, midapical LV dyskinesia is observed (dotted arrows). (B) LGE image showing mild transmural gadolinium enhancement in the midapical region (arrows), while no enhancement is seen at the basal region (dotted arrows). (C) CMR, IR sequence. Normal images are observed at 30 days (asterisk). Abbreviations: CMR, cardiac magnetic resonance; IR, inversion recovery; LGE, late gadolinium enhancement; LV, left ventricular; SSFP, steady‐state free precession.
Figure 4.
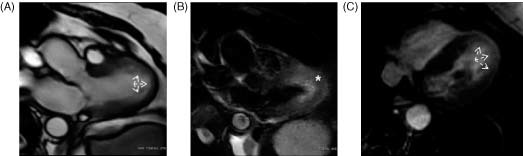
Patient with transient midapical dyskinesis. (A) CMR, SSFP sequence (3‐chamber view during systole). Note apical expansion (arrows). (B) T2‐weighted images revealing midapical edema (asterisk). (C) CMR, IR sequence (4‐chamber view). Note the presence of LGE at the affected region. Abbreviations: CMR, cardiac magnetic resonance; IR, inversion recovery; LGE, late gadolinium enhancement; SSFP, steady‐state free precession.
Figure 5.

Patient with transient apical dyskinesia. CMR, LGE images. (A) Image during the acute phase showing delayed myocardial enhancement at the apical segments. (B) Normalization at 3 months (asterisk). Abbreviations: CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Discussion
The TTC syndrome presents with marked and reversible LV dysfunction, often triggered by an emotional or physical stress. The signs and symptoms mimic an acute myocardial infarction with ECG changes, chest pain, and increase in serum markers, albeit without significant coronary artery lesions on angiography.1 Its pathophysiology remains unknown. It is unclear whether “myocardial stunning” is related to a direct catecholamine‐mediated effect on the myocardium or whether the main mechanism affects the coronary circulation via vasospasm or spontaneous lysis of a clot after a plaque accident.2, 3, 4, 5, 6, 7
Contrast CMR allows characterization of myocardial tissue,11 therefore allowing to retrospectively infer the pathophysiological mechanism involved in acute coronary syndromes with normal coronary arteries. As an example, the lesion of an epicardial artery is expressed on CMR by transmural or nontransmural typically subendocardial late enhancement; whereas, in cases of myocarditis, there is subepicardial or intramyocardial gadolinium uptake.12, 13, 14 Conversely, the finding of LGE as a sign of myocardial damage is very rarely seen on CMR of patients with TTC. Late gadolinium enhancement has only been found in isolated cases of TTC; an example of this is the large series reported by Sharkey et al, who among 22 patients described only 1 with LGE.15 Of note, LGE was absent in patients who had elevated serum markers of myocardial damage (ie, troponins). Such absence of LGE could be explained because imaging studies were not performed early enough or by the spatial resolution of CMR, which was insufficient to detect small myocardial lesions that were, however, detected by the markers of necrosis. Also, most reports in the literature do not clarify exactly when CMR was performed. In a case report, Bruder described a takotsubo cardiomyopathy occurring during a dobutamine‐stress CMR.16 In that case, LGE sequences were performed very early on, and the images obtained immediately after the event as well as those obtained the third day are identical to the images of patients included in our study. Similarly, follow‐up images showed complete disappearance of the initial findings. Hence, the delay in performing the study markedly affects the ability to obtain positive data, and a CMR performed early after the event may reveal a typical pattern of gadolinium uptake in this group of patients.
To our knowledge, this is the first prospective study that has analyzed late gadolinium uptake during the acute phase in a consecutive series of patients with TTC. It shows that when CMR is performed within 72 hours of admission, a characteristic pattern of mild transmural LGE is observed, located in the LV region with the wall‐motion abnormality. Such findings could be related to a slower gadolinium washout determined by the interstitial edema and inflammation, and perhaps very small areas of necrosis. As days go by, resorption of edema occurs, contractility improves, and late‐enhancement images become normal. Our findings show that CMR performed very early on may detect a typical and repeated pattern of LGE in patients with TTC. Hence, if the test is available and the patient is hemodynamically stable, it would be interesting to perform CMR early.
Study Limitations
Several studies with CMR have demonstrated the presence of myocardial edema observed in T2 sequences.17 In our series, T2 sequences did not provide optimal‐quality images in 3 patients. Another limitation is that not all institutions have on‐site CMR, and referring these patients for the test may sometimes be contraindicated.
Conclusion
Early CMR in TTC demonstrates a special morphological pattern of late gadolinium uptake that might correspond to localized inflammation and edema in the affected area. Such findings contribute important information; hence, we suggest that CMR should not be delayed beyond 72 hours. Finally, the morphological appearance of this pattern suggests that the pathophysiology of this syndrome is related to diffuse damage of the myocardium and the microcirculation, rather than involvement of the coronary epicardial vessels.
References
- 1. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako‐Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 2. Angelini P. Transient left ventricular apical ballooning: a unifying pathophysiologic theory at the edge of Prinzmetal angina. Catheter Cardiovasc Interv. 2008;71:342–352. [DOI] [PubMed] [Google Scholar]
- 3. Sansen V, Holvoet G. Takotsubo cardiomyopathy presenting as multivessel coronary spasm syndrome: case report and review of the literature. Acta Cardiol. 2007;62:507–511. [DOI] [PubMed] [Google Scholar]
- 4. Kurisu S, Inoue I, Kawagoe T, et al. Prevalence of incidental coronary artery disease in tako‐tsubo cardiomyopathy. Coron Artery Dis. 2009;20:214–218. [DOI] [PubMed] [Google Scholar]
- 5. Ronderos R, Avegliano G, Dallasta G. Stress‐induced cardiomyopathy. Curr Cardiovasc Imaging Rep. 2009;2:332–342. [Google Scholar]
- 6. El Mahmoud R, Mansencal N, Pilliére R, et al. Prevalence and characteristics of left ventricular outflow tract obstruction in Tako‐Tsubo syndrome. Am Heart J. 2008;156:543–548. [DOI] [PubMed] [Google Scholar]
- 7. Chao T, Lindsay J, Collins S, et al. Can acute occlusion of the left anterior descending coronary artery produce a typical “takotsubo” left ventricular contraction pattern? Am J Cardiol. 2009;104:202–204. [DOI] [PubMed] [Google Scholar]
- 8. Haghi D, Fluechter S, Suselbeck T, et al. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2007;120:205–211. [DOI] [PubMed] [Google Scholar]
- 9. Gerbaud E, Montaudon M, Leroux L, et al. MRI for the diagnosis of left ventricular apical ballooning syndrome (LVABS). Eur Radiol. 2008;18:947–954. [DOI] [PubMed] [Google Scholar]
- 10. Eitel I, Behrendt F, Schindler K, et al. Differential diagnosis of suspected apical ballooning syndrome using contrast‐enhanced magnetic resonance imaging. Eur Heart J. 2008;29:2651–2659. [DOI] [PubMed] [Google Scholar]
- 11. McNamara MT, Higgins CB. Cardiovascular applications of magnetic resonance imaging. Magn Reson Imaging. 1984;2:167–183. [DOI] [PubMed] [Google Scholar]
- 12. Leurent G, Langella B, Boulmier D, et al. Contribution of cardiac MRI in the etiologic diagnosis of chest pain syndrome with a normal angiographic aspect of the coronary arteries [in French]. Ann Cardiol Angeiol (Paris). 2008;57:109–115. [DOI] [PubMed] [Google Scholar]
- 13. Assomull RG, Lyne JC, Keenan N, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. [DOI] [PubMed] [Google Scholar]
- 14. Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. [DOI] [PubMed] [Google Scholar]
- 15. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. [DOI] [PubMed] [Google Scholar]
- 16. Bruder O, Hunold P, Jochims M, et al. Reversible late gadolinium enhancement in a case of Takotsubo cardiomyopathy following high‐dose dobutamine stress MRI. Int J Cardiol. 2008;127:e22–e24. [DOI] [PubMed] [Google Scholar]
- 17. Abdel‐Aty H, Cocker M, Friedrich MG. Myocardial edema is a feature of Tako‐Tsubo cardiomyopathy and is related to the severity of systolic dysfunction: insights from T2‐weighted cardiovascular magnetic resonance. Int J Cardiol. 2009;132:291–293. [DOI] [PubMed] [Google Scholar]


