Abstract
Background:
Atherothrombosis is becoming the leading cause of chronic morbidity in developing countries. This epidemiological transition will represent an unbearable socioeconomic burden in the near future. We investigated factors associated with 4‐year all‐cause mortality in a Latin American population at high risk.
Hypothesis:
Largely modifiable risk factors as well as polyvascular disease are the main predictors of 4‐year all‐cause and cardiovascular mortality in this Latin American cohort.
Methods:
We analyzed 1816 Latin American stable outpatients (62.3% men, mean age 67 years) with symptomatic atherothrombosis (87.1%) or with multiple risk factors only (12.9%), in the Reduction of Atherothrombosis for Continued Health registry.
Results:
Of patients with symptomatic atherothrombosis, 57.3% had coronary artery disease, 32% cerebrovascular disease, and 11.7% peripheral artery disease at baseline (9.1% polyvascular). The main risk factors were hypertension (76%), hypercholesterolemia (60%), and smoking (52.3%) in patients with established atherothrombosis; and hypertension (89.7%), diabetes (80.8%), and hypercholesterolemia (73.9%) in those with risk factors only. Four‐year all‐cause mortality steeply increased with none (6.8%), 1 (9.2%), 2 (15.5%), and 3 (29.2%) symptomatic arterial disease locations. In patients with only 1 location, cardiovascular mortality was significantly higher with peripheral artery disease (11.3%) than with cerebrovascular disease (6%) or coronary artery disease (5.1%). Significant baseline predictors of 4‐year all‐cause mortality were congestive heart failure (hazard ratio [HR]: 3.81), body mass index <20 (HR: 2.32), hypertension (HR: 1.84), polyvascular disease (HR: 1.69), and age ≥65 years (HR: 1.47), whereas statin use (HR: 0.49) and body mass index ≥30 (HR: 0.58) were associated with a reduced risk.
Conclusions:
Hypertension was the main modifiable risk factor for atherothrombosis and all‐cause mortality in this Latin American cohort. Nearly one‐third of the population with 3 symptomatic vascular‐disease locations died at 4‐year follow‐up.
The REACH registry is sponsored by sanofi, Bristol‐Myers Squibb, and the Waksman Foundation (Tokyo, Japan). The REACH registry is endorsed by the World Heart Federation. The REACH registry enforces a no‐ghostwriting policy. This manuscript was written and edited by the authors, who take full responsibility for its content. Dr. Cantú‐Brito and Dr. Chiquete wrote the first draft of this manuscript.
Dr. Cantú‐Brito has received research grants from sanofi, Ferrer Grupo and Bayer, as well as speaker honoraria from sanofi. Dr. Chiquete has received research grants from sanofi and Ferrer Grupo, as well as speaker honoraria from Novartis. Dr. Ruiz‐Sandoval has received research grants from sanofi, Boehringer Ingelheim, and Ferrer Grupo. Dr. Gaxiola has received research grants from sanofi and Eli Lilly, as well as consultancy and speaker honoraria from Sanofi, Eli Lilly, Pfizer, AstraZeneca, and Abbott. Dr. Albuquerque has received research grants from sanofi, AstraZeneca, Servier, Roche, and BMS/Pfizer, as well as consultancy and speaker honoraria from sanofi and AstraZeneca. Dr. Corbalán has no relevant disclosures. Mrs. Ramos is an employee at sanofi, in the Clinical Research Department. Dr. Deepak L. Bhatt discloses the following relationships ‐ Advisory Board: Medscape Cardiology; Board of Directors: Boston VA Research Institute, Society of Chest Pain Centers; Chair: American Heart Association Get With The Guidelines Science Subcommittee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); Research Grants: Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: PLx Pharma, Takeda. Dr. Steg has received research grants from Servier; consultancy fees/honoraria from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo/Eli Lilly alliance, Eisai, GlaxoSmithKline, Medtronic, Merck Sharpe and Dohme, Pfizer, Roche, sanofi, Servier, and The Medicines Company; and has equity ownership in Aterovax.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Atherothrombosis is the main cause of chronic morbidity and mortality worldwide.1, 2 This complex condition, pragmatically classified as coronary artery disease (CAD), cerebrovascular disease (CVD), and peripheral artery disease (PAD) according to disease location, is now becoming an important health strain in low‐ to medium‐income nations.3, 4, 5, 6 For some Latin American countries, chronic vascular disease will become an unbearable burden in the near future.4, 5, 6 Economic limitations and lack of knowledge on the specific public health problems may represent the main barriers for effective prevention and treatment of atherothrombosis. Therefore, there is an urgent need to better recognize the factors that contribute to this growing public health problem in Latin America.
The worldwide Reduction of Atherothrombosis for Continued Health (REACH) study is a registry of stable outpatients with a wide spectrum of atherothrombosis, from asymptomatic individuals at risk to patients with established arterial disease (CAD, CVD, and PAD).7, 8, 9, 10 This registry represents an important dataset to analyze the factors responsible for atherothrombotic complications and mortality in the contemporary real‐practice world. We sought to investigate the profile of risk factors and characteristics associated with long‐term all‐cause mortality in the Latin American cohort of the REACH registry.
Methods
The methods of the REACH registry have been published in detail elsewhere.7, 8 In brief, the protocol was submitted to institutional review boards of each participating center. Signed informed consent was required for all patients. Patients were enrolled between 2003 and 2004 and followed up until 2008. Inclusion criteria were age ≥45 years and ≥1 of the following 4 criteria7, 8: (1) Any combination of ≥3 atherosclerosis risk factors (type 1 or type 2 diabetes mellitus [DM], diabetic nephropathy, ankle/brachial index [ABI] <.9, documented carotid plaques by ultrasonography, documented asymptomatic carotid stenosis ≥70%, systolic blood pressure ≥150 mm Hg despite therapy for ≥3 months, hypercholesterolemia, current smoking status >15 cigarettes per day, and male age ≥65 years or female age ≥70 years), (2) documented CAD, (3) documented CVD, or (4) documented PAD. Therefore, all persons comprising the REACH cohort were diseased or at high risk, either because they were asymptomatic with ≥3 cardiovascular risk factors or because they had ≥1 symptomatic documented occlusive vascular disease. As a result, some traditional risk factors are more frequent among asymptomatics at high risk as compared with patients with documented atherothrombosis. Polyvascular disease was defined as coexistent symptomatic (clinically recognized) arterial disease in 2 or 3 locations (CAD, CVD, and/or PAD) in each patient.7, 8, 9
Documentation of symptomatic atherothrombosis required an expert report and confirmed clinical records of CAD, CVD, or PAD. Documented CAD consisted of ≥1 of the following criteria: history of stable or unstable angina, history of percutaneous coronary intervention, history of coronary artery bypass grafting or previous myocardial infarction (MI). Documented CVD consisted of a history of ischemic stroke or transient ischemic attack (TIA). Documented PAD consisted of current intermittent claudication with ABI <0.9 and/or a history of intermittent claudication together with a previous intervention, such as angioplasty, stenting, atherectomy, peripheral arterial bypass grafting, or other vascular interventions, including amputations. Diabetes was defined as any history of DM or current DM (diagnosed by ≥2 fasting blood glucose measures >126 mg/dL) treated with medication, lifestyle changes, or both.7, 8, 9
Information was collected in a standardized clinical research form at baseline with annual follow‐up at 1, 2, 3, and 4 years.9 The participating physicians registered ischemic events, hospitalizations, invasive procedures, and deaths based on official clinical records. Cardiovascular death included fatal stroke, fatal MI, and deaths related with other cardiovascular events (eg, deaths of cardiac origin, sudden death, pulmonary embolism, death after vascular operations or vascular procedures, amputation, death after a visceral or limb infarction, and any other death that cannot be definitely ascribed to a nonvascular cause). Any MI or stroke followed by death within 28 days was considered fatal MI or fatal stroke. No endpoint adjudication committee confirmed the outcome events. All clinical events during the follow‐up relied upon the investigator's imputation. Different combinations of prespecified study outcomes were modeled from the registered events.7, 8, 9, 10 For the purpose of the present report, cardiovascular and all‐cause case fatality rates are analyzed.
Statistical Analysis
Demographic data are presented as simple frequencies. Pearson χ 2 test or Fisher exact test (as appropriate) were used to compare the frequencies of nominal variables distributed between 2 groups, or to evaluate the distribution homogeneity of these variables in ≥3 groups. Age is expressed as mean and interquartile range, as it followed a non‐normal distribution. Student t test was used to compare continuous variables of normal distribution, between 2 groups. Multivariate analyses were constructed by using the Cox proportional hazards model, to find independent variables predicting 4‐year all‐cause mortality. For this analysis, relevant independent covariables with ≥98% registration rate were chosen to enter the model by a first‐step univariate analyses if P< 0.1, but those variables potentially involved in prediction of 4‐year mortality (mainly traditional risk factors such as DM or smoking habit) were also included for adjustment. Only significant predictors in the final multivariate model are described. Multivariate hazard ratios (HR) and their respective 95% confidence intervals (CI) are provided. All Pvalues calculated were 2‐tailed and considered significant when P< 0.05. SPSS version 17.0 (SPSS Inc., Chicago, IL) was used in all calculations in this report.
Results
We studied 1816 stable outpatients (mean age: 67 years, interquartile range: 59.3–74.5 years) who comprised the Latin American cohort of the REACH registry. There were 1131 (62.3%) men and 685 (37.7%) women. As expected, men were significantly younger than women (mean age 66.4 years vs 68.0 years, respectively; P = 0.001). A total of 1582 (87.1%) patients corresponded to the symptomatic group with established ischemic arterial disease (either CAD, CVD, PAD, or a combination of these) and 234 (12.9%) to patients with multiple risk factors only (Table 1). Among patients with established ischemic arterial disease (9.1% with polyvascular disease), 1041 (57.3%) had CAD, 582 (32%) had CVD, and 213 (11.7%) had PAD (Table 1). A total of 1532 (74.4%) patients had documented symptomatic atherothrombosis in 1 vascular territory (either CAD, CVD, or PAD), 206 (11.3%) had 2 vascular disease locations and 24 (1.3%) had 3. Of the patients with 2 vascular disease locations, the combination of CAD+CVD was the most frequent, followed by CAD+PAD and CVD+PAD (6.3%, 3.5%, and 1.5%, respectively).
Table 1.
Main Baseline Characteristics of the Latin American REACH Cohort (n = 1816)a
| Baseline Characteristics | Asymptomatic Patients With Multiple Risk Factors [n = 234] | Patients With Established Vascular Disease | ||
|---|---|---|---|---|
| CAD [n = 1041] | CVD [n = 582] | PAD [n = 213] | ||
| Men, % | 41.0 | 72.3 | 56.7 | 63.4 |
| Age, mean (SD), y | 66.0 (10.3) | 66.2 (9.9) | 69.0 (10.2) | 69.1 (10.5) |
| DM, % | 80.8 | 37.2 | 37.5 | 56.8 |
| Hypertension, % | 89.7 | 75.3 | 77.7 | 81.7 |
| CHF, % | 4.7 | 11.0 | 6.9 | 6.6 |
| Hypercholesterolemia, % | 73.9 | 69.2 | 48.5 | 51.2 |
| BMI ≥30, % | 38.5 | 22.1 | 22.3 | 14.6 |
| WHtR ≥0.60, % | 65.0 | 42.7 | 45.0 | 34.7 |
| AF, % | 3.4 | 6.9 | 9.6 | 4.4 |
| Past or current smoking status, % | 37.2 | 57.0 | 43.3 | 65.6 |
| Current smoking status, % | 14.6 | 7.6 | 7.1 | 16.0 |
| ABI <0.9, % | 6.5% | 12.6 | 12.4 | 87.0 |
| Statin therapy, % | 67.9 | 75.0 | 47.8 | 46.9 |
| Aspirin therapy, % | 62.0 | 87.0 | 67.5 | 77.0 |
| Other antiplatelet therapy, % | 9.9 | 30.6 | 38.4 | 32.9 |
| Oral anticoagulant therapy, % | 1.7 | 6.4 | 14.4 | 10.0 |
| Diuretic therapy, % | 50.0 | 34.9 | 35.3 | 40.6 |
| ACEI therapy, % | 47.0 | 45.7 | 39.2 | 34.8 |
| ARB therapy, % | 29.7 | 20.2 | 20.6 | 21.4 |
| Insulin therapy, % | 18.1 | 9.8 | 9.0 | 23.5 |
Abbreviations: ABI, ankle/brachial index; ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cerebrovascular disease; DM, diabetes mellitus; PAD, peripheral artery disease; SD, standard deviation; WHtR, waist‐to‐height ratio.
Patients may not add up to 1816 because some individuals with established vascular disease have ≥1 identified vasculopathy location.
In asymptomatic patients without established vasculopathy, hypertension (89.7%), DM (80.8%), and hypercholesterolemia (73.9%) were the main risk factors (Table 1). Among the symptomatic patients, hypertension (76%), hypercholesterolemia (60%), and smoking habit (52.3%) were the most frequent traditional risk factors (Table 1). Obesity, as defined by a body mass index (BMI) ≥30, was not as prevalent as could be expected (Table 1); however, the waist‐to‐height ratio ≥0.60, a measure of central adiposity, was a common characteristic. As can be expected given the REACH inclusion criteria, many risk factors were more prevalent among asymptomatic patients without established vascular disease than in subjects with clinically recognized ischemic arterial disease.
At 4‐year follow‐up, the frequency of polyvascular disease in survivors increased to 13.6%. Among all patients, fatal or nonfatal stroke, and fatal or nonfatal MI occurred at similar rates: 4.9% vs 4.0%, respectively. But acute ischemic events in patients with a single symptomatic atherothrombosis at baseline (either CAD, CVD, or PAD) occurred in a congruent pattern with respect to the affected vascular bed at study entry, so that fatal and nonfatal 4‐year ischemic coronary events were more common among patients having CAD at baseline, whereas stroke and TIA were more frequently observed among subjects with documented CVD at study entry (Table 2). Importantly, patients with PAD had a similar risk for developing nonfatal coronary or cerebrovascular events, but the risk of fatal stroke doubled in this group.
Table 2.
Fatal and Nonfatal Coronary and Cerebrovascular Ischemic Events Among Asymptomatic Patients With Multiple Risk Factors, or Patients With 1 Established Arterial Vascular Disease at Baseline (n = 1586)
| Vasculopathy at Baseline | 4‐Year Nonfatal Ischemic Events | 4‐Year Fatal Ischemic Events | |||
|---|---|---|---|---|---|
| AMI | Strokea | TIAa | AMI | Stroke | |
| Asymptomatics at risk (n = 234), % | 1.7 | 2.1 | 0.4 | 1.3 | 0.4 |
| CAD (n = 839), % | 2.0 | 1.4 | 1.1 | 1.5 | 0.4 |
| CVD (n = 416), % | 1.7 | 9.9 | 3.8 | 2.2 | 1.4 |
| PAD (n = 97), % | 2.1 | 4.1 | 1.0 | 2.1 | 2.1 |
Abbreviations: AMI, acute myocardial infarction; CAD, coronary artery disease; CVD, cerebrovascular disease; PAD, peripheral artery disease; TIA, transient ischemic attack. Patients with ≥1 vascular disease location were not analyzed here.
P < 0.05 for homogeneity among vasculopathy classes at baseline.
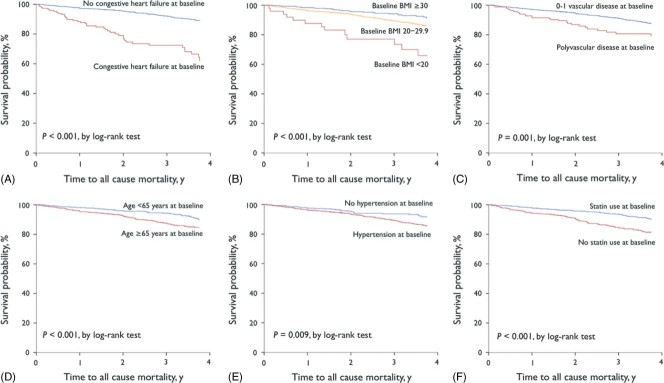
The 4‐year all‐cause fatality rate was 9.9% (n = 179), without a significant difference between symptomatic patients with established ischemic arterial disease and individuals with multiple risk factors only (10.3% vs 6.8%, respectively; P = 0.097). The 4‐year cardiovascular case fatality rate was 6.5% (n = 118, 65.9% of total deaths), also without differences between symptomatic patients and asymptomatics with multiple risk factors (6.9% vs 3.8%, respectively; P = 0.078). However, the number of symptomatic arterial diseases at baseline had a direct relationship with all‐cause and cardiovascular 4‐year case fatality rate, so that mortality increased directly with the number of symptomatic arterial disease locations (Figure 1). Moreover, increasing mortality rate was observed in CAD, CVD, and PAD patients, in this order. After multivariate analysis by the Cox proportional hazards model on baseline risk factors associated with 4‐year all‐cause mortality, significant predictors were congestive heart failure (CHF), BMI <20, polyvascular disease, hypertension, and age ≥65 years; whereas statin use and BMI ≥30 were inversely associated with this risk (Table 3 and Figure 2). Notably, hypertension was highly frequent in patients age ≥65 years (81.4%), with CHF (79.6%) and with PAD (81.7%); but less frequent in patients with BMI <20 (75.5%). At 4‐year follow‐up, the main global REACH outcome of cardiovascular mortality/MI/stroke occurred in 11.8% (n = 215) of the Latin American population; and notably, hypertension and BMI cutoffs were no longer predictors in a multivariate model constructed for this composite outcome, though CHF, polyvascular disease, advanced age, and lack of statin use were highly significant predictors (Table 3).
Figure 1.
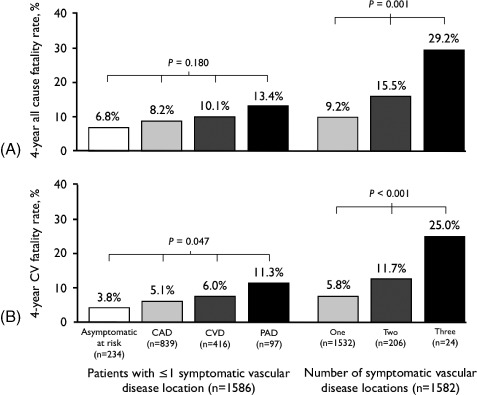
Four‐year (A) all‐cause and (B) cardiovascular case fatality rate according to vascular disease status. Abbreviations: CAD, coronary artery disease; CV, cardiovascular disease; CVD, cerebrovascular disease; PAD, peripheral artery disease.
Table 3.
Multivariate Analyses on Baseline Factors Associated With 4‐Year Outcomes in Latin American Patients of the REACH Registry (n = 1816)
| Predictor | HR (95% CI) | P Value |
|---|---|---|
| All‐cause mortality | ||
| CHF | 3.810 (2.622–5.537) | <0.001 |
| BMI <20 | 2.322 (1.239–4.352) | 0.009 |
| Polyvascular diseasea | 1.690 (1.083–2.635) | 0.021 |
| Hypertension at baseline | 1.843 (1.171–2.899) | 0.008 |
| Age ≥65 y | 1.468 (1.034–2.083) | 0.033 |
| Statin use at baseline | 0.495 (0.362–0.678) | <0.001 |
| BMI ≥30 | 0.578 (0.373–0.895) | 0.014 |
| Cardiovascular mortality/MI/stroke | ||
| CHF | 2.506 (1.713–3.664) | <0.001 |
| Polyvascular diseasea | 2.043 (1.389–3.007) | <0.001 |
| Age ≥65 y | 1.617 (1.183–2.210) | 0.003 |
| Statin use at baseline | 0.470 (0.354–0.624) | <0.001 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; MI, myocardial infarction; WHtR, waist‐to‐height ratio.
Polyvascular disease = 2 to 3 vascular disease locations. Only statistically significant risk factors are included in the table, but the model was fully adjusted for baseline characteristics such as sex, DM, AF, past or current smoking habit, WHtR >60, and antiplatelet or anticoagulant therapy. Variables with ≥98% registration rate were included in the model.
Figure 2.
Kaplan‐Meier estimates of the 4‐year survival in Latin American stable outpatients of the REACH registry (n = 1816), according to (A) congestive heart failure, (B) BMI, (C) number of vascular‐disease locations, (D) age, (E) hypertension, and (F) statin use at baseline. Abbreviations: BMI, body mass index; REACH, Reduction of Atherothrombosis for Continued Health.
Discussion
In the present study, the frequency of polyvascular disease at baseline was 9%, increasing to 14% at 4‐year follow‐up (a 50% relative increase). The 4‐year fatal and nonfatal acute arterial ischemic events were mostly recurrences within a clinically recognized arteriopathy location at baseline. The group of patients with documented CVD at study entry had the highest proportion of new ischemic events, followed by PAD and CAD patients; but cardiovascular mortality was higher in patients with PAD. Recurrent stroke was more common than recurrent acute MI; nonetheless, patients with PAD at baseline had the same risk as CAD patients for developing fatal or nonfatal acute MI, and an intermediate risk for acute ischemic stroke, between patients with CAD and those with documented CVD at baseline. The present results are in line with the global REACH registry9, 11, 12, 13, 14 and other cohorts15, 16 in that PAD confers the highest risk of fatal cardiovascular events when compared with CAD and CVD. This is a very frequent vascular disease in Latin American populations with multiple risk factors8, 9, 10, 17 that may be overlooked, and, consequently, undertreated.18, 19 When significant occlusion in lower‐limb arteries has occurred, it is highly possible that other vascular beds are already affected.20, 21, 22 All these findings support the notion that atherosclerosis is a generalized inflammatory disease that affects some vascular regions to a greater extent than others, and that PAD represents an advanced clinical form of the atherothrombosis continuum.11, 12, 13, 14, 15, 16, 17, 18, 19
Clinical Implications
This study provides important figures for epidemiological comparisons and confirms that modifiable risk factors are responsible for a great proportion of the health burden in Latin America. The number of arterial disease locations has been recognized as a strong predictor of adverse outcomes in the REACH registry9, 10, 11, 12 and other cohorts.23, 24, 25, 26 This concept should be clearly approached when establishing preventive measures at the population level. Clinical trials and practice guidelines should stratify patients based on this characteristic, to allow identification of individuals who would experience the greatest benefit with particular therapeutic strategies.27, 28 Our findings also have relevant implications in designing clinical trials and regional recommendations aimed to prevent cardiovascular events and mortality. Classification of risk, and as a result, the magnitude of the intervention, are strongly influenced by identification of significant vascular disease.9 Therefore, the present data have the potential of influencing the prevention practices in Latin America.
Here, hypertension was the most important modifiable factor associated with risk profile, symptomatic atherothrombosis, and mortality. This confirms that hypertension is a major burden in Latin America that is associated with both ischemic events and mortality in patients with an already compromised vascular bed, possibly due to a suboptimal control.4, 29, 30 On the other hand, DM was not an independent predictor of mortality after multivariate analysis. We attribute this finding to the fact that DM is a strong risk factor for vascular disease, an overrepresented characteristic in this cohort, but with less contribution to all‐cause mortality when other strong covariables (such as CHF or polyvascular disease) are analyzed together. Another possible explanation may be the higher frequency of DM among asymptomatic individuals with multiple risk factors only, as compared with patients with documented symptomatic atherothrombosis. Nevertheless, the interaction of DM and hypertension control with symptomatic vascular disease on the risk of future ischemic events and mortality was not explored here, and it represents a topic that needs more study. A low BMI was associated with an increased mortality risk, whereas a high BMI was associated with an apparent survival advantage. This has also been observed in other REACH cohorts, but when waist circumference is used to define excessive adiposity instead of BMI, no survival benefit is identified.31 Body mass index reflects both lean and adipose tissue; therefore, it cannot be concluded that an excessive adiposity (the pathophysiological definition of obesity) is protective, based on BMI measures only.31 Better body‐composition indices are needed for redefinition of obesity in clinical grounds and for clarification of the so‐called obesity paradox.31, 32, 33, 34 A high BMI is an important risk factor for vascular disease in the first place,35 but adequate energy stores could be necessary to resist the chronic demands that result from certain vascular outcomes. In the present report, neither a low nor a high BMI were predictors of the composite outcome cardiovascular death/MI/stroke. Thus, it can be hypothesized that obesity is a risk factor for atherothrombotic disease, but when a vascular event has occurred, a high BMI (reflecting both adipose and lean mass) may be required to cope with energy demands in the terminal part of the continuum of vascular disease. The need for a normal body mass should be advised for the general population in order to avoid the complications associated with obesity.
Study Limitations
The main limitations of this study are the reduced sample size, as compared with the entire REACH cohort, and the lack of complete information for some characteristics at 4‐year follow‐up. Our study population is a very selected group at high cardiovascular risk that may not represent the whole Latin American population, especially the younger individuals in premorbid states. The main outcome events recorded during the follow‐up were not centrally adjudicated, which may represent a flaw especially in assigning the category of death.
An important characteristic of the worldwide REACH cohort was the overrepresentation of women in the asymptomatic group, whereas the male gender was more frequent in the cohort with established vascular disease.8, 9, 10, 11 This may reflect the natural consequence of selecting patients with multiple risk factors without documented vasculopathy—a more common feature of adult women as compared with men—and the high frequency of established vascular disease among adult males. This phenomenon has also been described in large population‐based cohorts such as the Framingham heart study and the World Health Organization Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) project.36, 37, 38 Although gender was not independently associated with 4‐year mortality in our study and the worldwide REACH cohort,8, 9, 10, 11 we cannot completely exclude a lower CV and all‐cause mortality among asymptomatic patients with multiple risk factors because the majority of them (59%) were women.
Conclusion
In conclusion, hypertension is the main modifiable risk factor associated with atherothrombosis and all‐cause mortality in this Latin American population at high risk. Congestive heart failure, polyvascular disease, advanced age, and lack of statin use were associated with high rates of the composite outcome of cardiovascular death, MI, or stroke.
REACH Investigators by Country
Chile: Acevedo M, Araya F, Barrientos N, Blanchi V, Chavez E, Corbalán R, Diaz R, Dighero H, Garayar B, Garjardo L, Garrido B, Gutierrez V, Hoppe A, Kauffmann R, Kramer A, Kunstmann S, Manriquez L, Srur EM, Tapia J, Varleta PE, Villar RA.
Panama: Arango AC, Arauz J, Benzadon A, Cabrera JR, Cantisano L, Castillo B, Chavez EH, Chen CH, Diaz E, Dina RE, Espinosa H, Hernandez D, Hernandez J, Lopez R, Madrid JL, Medine FP, Minchola JA, Mini M, Molina G, Motta J, Moya J, Ortiz ME, Parajeles A, Ramos CA, Rodriguez E, Saravia GL, Sprok JM, Tortos‐Guzman J, Urrutia V, Veras D, Vercauteren JP, Villa‐Garcin P, Vinocour M.
Brazil: Albuquerque D, Atai de Jr L, Atie J, Avezum A, Bandeira F, Bodanese L, Brasileiro A, Carvalho AC, Dutra O, Esporcatte R, Esteves JP, Eugenio AM, Feitosa G, Fernandes J, Halpern A, Knobel M, Lopes C, Lyra R, Machado N, Maciel L, Marino R, Massaro A, Matos A, Mesquita E, Moreira M, Nagato YN, Nicolau JC, Nogueira PR, Pazin‐Filho A, Pires MT, Rocha A, Rocha R, Saraiva JF, Schmid H, Serro J, Tambascia M, Xavier SS, Yamamoto F, Yoshida WB, Zanella MT.
Mexico: Abundes A, Aguayo G, Alcocer MA, Alegría MA, Alexanderson G, Alpizar M, Amaya LE, Arauz A, Astorga A, Azpiri JR, Barinagarrementeria F, Benavides MA, Bernal E, Briseño HM, Caluo C, Cantú‐Brito C, Cedillo FR, Colorado H, Cortes J, Cossio J, De los Ríos MO, De Zatarain R, Díaz C, Eng L, Esparragoza J, Espinosa L, Espinoza C, Fernández P, García E, García R, Garza A, Gaxiola E, González FJ, Guerrero F, Herududez I, Kostine A, Leiva JL, Llamas‐López L, López B, López J, López M, Marcos A, Najar S, Nettel J, Ortiz F, Peralta R, Pérez JC, Ranero A, Rangel‐Guerra R, Rodríguez J, Rodríguez L, Rojas JA, Romero A, Rubio G, Ruiz JL, Sagastegui A, Talamas O, Tapia M, Toriz JL, Uribe A, Vázquez J, Velasco JA, Velasco EC, Velasco‐Sanchez RG, Villarreal J.
References
- 1. Franco M, Cooper RS, Bilal U, et al. Challenges and opportunities for cardiovascular disease prevention. Am J Med. 2011;124:95–102. [DOI] [PubMed] [Google Scholar]
- 2. Maher D, Waswa L, Baisley K, et al. Distribution of hyperglycaemia and related cardiovascular disease risk factors in low‐income countries: a cross‐sectional population‐based survey in rural Uganda. Int J Epidemiol. 2011;40:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ventura HO, Mehra MR. The growing burden of heart failure: the “syndemic” is reaching Latin America. Am Heart J. 2004;147:386–389. [DOI] [PubMed] [Google Scholar]
- 4. Rubinstein A, Alcocer L, Chagas A. High blood pressure in Latin America: a call to action. Ther Adv Cardiovasc Dis. 2009;3:259–285. [DOI] [PubMed] [Google Scholar]
- 5. Cuevas A, Alvarez V, Carrasco F. Epidemic of metabolic syndrome in Latin America. Curr Opin Endocrinol Diabetes Obes. 2011;18:134–138. [DOI] [PubMed] [Google Scholar]
- 6. Cantú‐Brito C, Ruiz‐Sandoval JL, Murillo‐Bonilla LM, et al. Acute care and one‐year outcome of Mexican patients with first‐ever acute ischemic stroke: the PREMIER study. Rev Neurol. 2010;51:641–649. [PubMed] [Google Scholar]
- 7. Ohman EM, Bhatt DL, Steg PG, et al. The Reduction of Atherothrombosis for Continued Health (REACH) registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events‐study design. Am Heart J. 2006;151:786.e1–786.e10. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 10. Gaxiola E, Eng‐Ceceña L, Ortiz‐Galvén F, et al; Reduction of Atherothrombosis for Continued Health Registry Investigators. Assessment of atherothrombosis and its treatment in Mexico: first‐year data of the REACH registry. Clin Cardiol. 2010;33:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steg PG, Bhatt DL, Wilson PW, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 12. Alberts MJ, Bhatt DL, Mas JL, et al. Three‐year follow‐up and event rates in the international Reduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meadows TA, Bhatt DL, Cannon CP, et al. Ethnic differences in cardiovascular risks and mortality in atherothrombotic disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) registry. Mayo Clin Proc. 2011;86:960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roussel R, Travert F, Pasquet B, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010;170:1892–1899. [DOI] [PubMed] [Google Scholar]
- 15. Murabito JM, Evans JC, Larson MG, et al. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study. Arch Intern Med. 2003;163: 1939–1942. [DOI] [PubMed] [Google Scholar]
- 16. Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. [DOI] [PubMed] [Google Scholar]
- 17. Canté‐Brito C, Chiquete E, Duarte‐Vega M, et al; Multicenter INDAGA Study Investigators. Abnormal ankle‐brachial index in the Mexican population with vascular risk [article in Spanish]. Rev Med Inst Mex Seguro Soc. 2011;49: 239–246. [PubMed] [Google Scholar]
- 18. Amudha K, Chee KH, Tan KS, et al. Prevalence of peripheral artery disease in urban high‐risk Malaysian patients. Int J Clin Pract. 2003;57:369–372. [PubMed] [Google Scholar]
- 19. Cacoub PP, Abola MT, Baumgartner I, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) registry. Atherosclerosis. 2009;204:e86–e92. [DOI] [PubMed] [Google Scholar]
- 20. Sen S, Lynch DR Jr, Kaltsas E, et al. Association of asymptomatic peripheral arterial disease with vascular events in patients with stroke or transient ischemic attack. Stroke. 2009;40: 3472–3477. [DOI] [PubMed] [Google Scholar]
- 21. Banerjee A, Fowkes FG, Rothwell PM. Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke. 2010;41:2102–2107. [DOI] [PubMed] [Google Scholar]
- 22. Vaartjes I, van Dis I, Grobbee DE, et al. The dynamics of mortality in follow‐up time after an acute myocardial infarction, lower extremity arterial disease and ischemic stroke. BMC Cardiovasc Disord. 2010;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meizels A, Zeitoun DM, Bataille V, et al; the Alliance Project. Impact of polyvascular disease on baseline characteristics, management and mortality in acute myocardial infarction. Arch Cardiovasc Dis. 2010;103:207–214. [DOI] [PubMed] [Google Scholar]
- 24. Sander D, Carolei A, Diehm C, et al. Challenges to the management of high‐risk stroke patients with multiple‐site occlusive vascular disease. Cerebrovasc Dis. 2011;31:315–321. [DOI] [PubMed] [Google Scholar]
- 25. Van Kuijk JP, Flu WJ, Galal W, et al. The influence of polyvascular disease on the obesity paradox in vascular surgery patients. J Vasc Surg. 2011;53:399–406. [DOI] [PubMed] [Google Scholar]
- 26. Van Kuijk JP, Flu WJ, Welten GM, et al. Long‐term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur Heart J. 2010;31:992–999. [DOI] [PubMed] [Google Scholar]
- 27. Ray KK, Seshasai SR, Erqou S, et al. Statins and all‐cause mortality in high‐risk primary prevention: a meta‐analysis of 11 randomized controlled trials involving 65 229 participants. Arch Intern Med. 2010;170:1024–1031. [DOI] [PubMed] [Google Scholar]
- 28. De Lorgeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin‐JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170:1032–1036. [DOI] [PubMed] [Google Scholar]
- 29. Hernández‐Hernández R, Silva H, Velasco M, et al. Hypertension in seven Latin American cities: the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study. J Hypertens. 2010;28:24–34. [DOI] [PubMed] [Google Scholar]
- 30. Baños‐González M, Cantú‐Brito C, Chiquete E, et al. Systolic blood pressure and functional outcome in patients with acute stroke: a Mexican registry of acute cerebrovascular disease (RENAMEVASC) [article in Spanish]. Arch Cardiol Mex. 2011;81:169–175. [PubMed] [Google Scholar]
- 31. Dallongeville J, Bhatt DL, Steg PG, et al. Relation between body mass index, waist circumference, and cardiovascular outcomes in 19 579 diabetic patients with established vascular disease: the REACH Registry. Eur J Cardiovasc Prev Rehabil. 2012;19:241–249. [DOI] [PubMed] [Google Scholar]
- 32. Chiquete E, Cantú‐Brito C, Villarreal‐Careaga J, et al. Obesity paradox and functional recovery in first‐ever acute ischemic stroke survivors: the PREMIER study [article in Spanish]. Rev Neurol. 2010;51:705–713. [PubMed] [Google Scholar]
- 33. Adamopoulos C, Meyer P, Desai RV, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity‐matched study. Eur J Heart Fail. 2011;13:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Myers J, Lata K, Chowdhury S, et al. The obesity paradox and weight loss. Am J Med. 2011;124:924–930. [DOI] [PubMed] [Google Scholar]
- 35. Whitlock G, Lewington S, et al; Prospective Studies Collaboration. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Margolis JR, Gillum RF, Feinleib M, et al. Community surveillance for coronary heart disease: the Framingham Cardiovascular Disease survey. Comparisons with the Framingham Heart Study and previous short‐term studies. Am J Cardiol. 1976;37:61–67. [DOI] [PubMed] [Google Scholar]
- 37. Wilson PW, Nam BH, Pencina M, et al. C‐reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165:2473–2478. [DOI] [PubMed] [Google Scholar]
- 38. Rautio A, Lundberg V, Messner T, et al. Favourable trends in the incidence and outcome of myocardial infarction in nondiabetic, but not in diabetic, subjects: findings from the MONICA myocardial infarction registry in northern Sweden in 1989–2000. J Intern Med. 2005;258:369–377. [DOI] [PubMed] [Google Scholar]