Abstract
Hypertension represents a significant global public health concern, contributing to vascular and renal morbidity, cardiovascular mortality, and economic burden. The opportunity to influence clinical outcomes through hypertension management is therefore paramount. Despite adherence to multiple available medical therapies, a significant proportion of patients have persistent blood pressure elevation, a condition termed resistant hypertension. Recent recognition of the importance of the renal sympathetic and somatic nerves in modulating blood pressure and the development of a novel procedure that selectively removes these contributors to resistant hypertension represents an opportunity to provide clinically meaningful benefit across wide and varied patient populations. Early clinical evaluation with catheter‐based, selective renal sympathetic denervation in patients with resistant hypertension has mechanistically correlated sympathetic efferent denervation with decreased renal norepinephrine spillover and renin activity, increased renal plasma flow, and has demonstrated clinically significant, sustained reductions in blood pressure. The SYMPLICITY HTN‐3 Trial is a pivotal study designed as a prospective, randomized, masked procedure, single‐blind trial evaluating the safety and effectiveness of catheter‐based bilateral renal denervation for the treatment of uncontrolled hypertension despite compliance with at least 3 antihypertensive medications of different classes (at least one of which is a diuretic) at maximal tolerable doses. The primary effectiveness endpoint is measured as the change in office‐based systolic blood pressure from baseline to 6 months. This manuscript describes the design and methodology of a regulatory trial of selective renal denervation for the treatment of hypertension among patients who have failed pharmacologic therapy. Clin. Cardiol. 2012. doi: 10.1002/clc.22008
Dr. Kandzari receives research/grant support and consulting honoraria from Medtronic CardioVascular, Abbott Vascular and Boston Scientific; Dr. Bhatt receives honoraria from WebMD and research grants from Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, and The Medicines Company. Dr. Oparil receives research/grant support from Merck and Co., NHLBI, Novartis, and Takeda, honoraria from Daiichi Sankyo and Pfizer, and is a consultant for Bayer, Medtronic, Novartis, Pfizer, and Daiichi Sankyo. Dr. Rocha‐Singh is a consultant for Medtronic, Covidien, and Cardiosonic. Dr. Flack receives grant/research support from NIH, Daiichi Sankyo, sanofi aventis, and Novartis. He is on the Speaker Bureau for Novartis, Daiichi Sankyo, and Boehringer Ingleheim, and is a consultant for Glaxo‐Smith‐Kline, Novartis, NIH, Daiichi Sankyo, and Boehringer Ingleheim, Medtronic, and Back Beat Hypertension. Dr. Katzen is a consultant for Abbott, CRBard, Boston Scientific, WL Gore, and Medtronic. Dr. Massaro is a member of the Data Safety Monitoring Board and will no longer participate as a member of the SYMPLICITY HTN‐3 Steering Committee. Dr. Leon, Dr. O'Neill, and Dr. Esler have nothing to disclose. Dr. Negoita, Dr. Sobotka, and Craig Straley are employees of Medtronic, Inc. Dr. Bakris receives grant/clinical trial support (paid directly to University of Chicago) from Forest Laboratories, Medtronic, and Relapysa, and is a consultant to Takeda, Abbott, CVRx, Johnson & Johnson, Eli Lilly, and the Food and Drug Administration. Dr. Bakris is on the Speaker Bureau for Takeda, and the Boards of the National Kidney Foundation and the American Society of Hypertension. He is Editor for the American Journal of Nephrology and Associate Editor for Diabetes Care and Nephrology Dialysis and Transplantation.
Introduction
Hypertension represents a significant global public health concern, contributing to vascular and renal morbidity, cardiovascular mortality, and economic burden.1 As the most commonly diagnosed condition and largest contributor to mortality in industrialized countries, the increasing prevalence of hypertension exceeds more than 1 in 3 individuals, and the risk of cardiovascular mortality is related linearly with both systolic and diastolic pressures, doubling for every 20‐mm Hg and 10‐mm Hg increase in the systolic and diastolic blood pressure, respectively, above 115/75 mm Hg.2., 3. Despite advances in pharmacological therapies to safely and effectively achieve blood pressure control, the percentage of patients for whom guideline‐recommended measures are attained remains unacceptably low.4., 5., 6., 7. Use of an inappropriate pharmacologic strategy or inappropriate dosing of antihypertensive medications may account for lack of blood pressure control. Additionally, nonadherence and nonpersistence with prescribed polypharmacy, due in part to patient intolerance to the drug therapy, as well as treatment inertia on the part of health care providers are major contributors to lack of blood pressure goal achievement.3., 5., 6., 7., 8., 9., 10. Moreover, despite adherence to multiple available medical therapies, a significant proportion of patients have persistent blood pressure elevation, a condition termed resistant hypertension.2., 7., 11., 12. Accordingly, the recent recognition of the importance of the renal sympathetic and somatic nerves in modulating blood pressure, and the development of a novel procedure that selectively removes these contributors to resistant hypertension, represent an opportunity to provide clinically meaningful benefit across wide and varied patient populations. Herein we present the methodology of a regulatory trial of selective renal denervation for the treatment of hypertension in a subset of patients who have failed pharmacologic therapy.
Renal Denervation as Therapy for Hypertension
Elevated sympathetic nervous system activity has been identified as a common pathophysiologic denominator to disease conditions including hypertension,13., 14. heart failure,15 sleep disturbances,16 metabolic syndrome and glycemic control,17., 18., 19. and chronic kidney disease.20 Hyperstimulation of the renal efferent nerves mediates hypertension by promoting sodium retention, decreasing renal blood flow and glomerular filtration, and increasing renin release, thus promoting activation of the renin‐angiotensin‐aldosterone neurohormonal cascade.21 Similarly, renal somatic afferent nerve activity, via direct hypothalamic signaling, may mediate pleiotropic effects not directly related to systemic hypertension.
Recognition of the renal nerves as a potential target for the treatment of hypertension has inspired clinical investigation of the mechanistic relationship between renal nerve activation and high blood pressure and examination of potential therapeutic opportunities related to selective renal denervation in patients with resistant hypertension.21 Early clinical investigation with surgical sympathectomy demonstrated generally positive albeit inconsistent reductions in blood pressure and improved survival compared to medical treatment.22., 23., 24. However, predictable adverse effects related to the nonselectivity of the procedure, including severe orthostatic hypotension and impaired bowel/bladder activity, in addition to the invasiveness of the procedure, led to its dismissal as a therapeutic option. Sympathetic neural activity, assessed by measurements of plasma norepinephrine, norepinephrine spillover, vascular resistance, and muscle sympathetic nerve activity, has been correlated with the severity of hypertension and its resolution in patients following nephrectomy20., 25., 26. and catheter‐based selective renal sympathetic and somatic denervation.27–29 In the presence of renal denervation, homeostatic mechanisms regulating electrolytes, volume status, and adrenaline‐mediated stress responses are preserved,27 consistent with historical confirmation that the transplanted denervated human kidney maintains electrolyte and volume homeostasis.
The novel approach of selective renal denervation may address an important mechanism underlying treatment‐resistant hypertension. Moreover, the opportunity to develop a treatment strategy not dependent on patient or physician commitment to lifelong polypharmacy has enormous potential benefit.28 This trial addresses the first critical question, whether renal sympathetic and somatic denervation plays a clinically important role in the treatment of patients with resistant hypertension failing polypharmacy.
Clinical Trials With Catheter‐Based Renal Denervation
Early clinical evaluation with a catheter‐based approach to selective renal sympathetic denervation in patients with resistant hypertension, has mechanistically correlated sympathetic efferent denervation with decreased renal norepinephrine spillover, halving of renin activity and increased renal plasma flow, and suggests reduced central sympathetic drive through demonstration of reduced total body norepinephrine spillover and muscle sympathetic nerve activity.27 Selective renal denervation has also demonstrated clinically significant sustained reductions in blood pressure.29., 30., 31. In a pooled analysis of initial pilot studies (SYMPLICITY HTN‐1 First‐in‐Human and additional phase I studies), limitations in sample size and follow‐up notwithstanding, office‐based systolic and diastolic blood pressures were substantially reduced from baseline measurements and were sustained through 2 years (Table 1). A reduction in systolic blood pressure of at least 10 mm Hg was achieved in 92% of patients.
Table 1.
Summary of SYMPLICITY HTN‐1 and HTN‐2 Clinical Trials
| HTN‐1 [N = 153] | HTN‐2 [N = 106] | |
|---|---|---|
| Trial design | Multicenter, prospective, open‐label (pooled analysis of first‐in‐man and phase I studies) | Multicenter, open‐label, randomized trial; n = 52 randomized to immediate RDN, n = 54 controls |
| Patient age (mean ± SD), y | 57 ± 11 | 58 ± 12 |
| No. antihypertensive medications | 5.1 ± 1.5 | 5.2 ± 1.5 (RDN), 5.3 ± 1.8 (control) |
| Antihypertensive use by class at baseline | ||
| ACE inhibitor/ARB | 91% | 95% |
| β‐blocker | 82% | 75% |
| CCB | 75% | 81% |
| Diuretic | 95% | 89% |
| Mean baseline blood pressure (mean ± SD), mm Hg | 176/98 ± 17/14 | 178/96 ± 18/16 (RDN), 178/97 ± 17/16 (control) |
| Reduction in blood pressure | −25/−11 (at 6 months; n = 86), −32/−14 (at 24 months; n = 18) | −32/−12 (RDN group at 6 months; n = 49),a +1/0 (control group at 6 months; n = 51) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; CCB, calcium channel blocker; RDN, renal denervation; SD = standard deviation.
P < 0.0001 for difference between RDN and control systolic and diastolic blood pressure reductions.
Renal denervation treatment was delivered without acute complications in 149 (97%) patients. One renal artery dissection on placement of the treatment catheter prior to radiofrequency energy delivery occurred requiring stenting in 1 patient, and 3 patients developed a vascular access site complication. Moreover, no acute or chronic adverse hemodynamic events, electrolyte abnormalities, or decline in renal function were observed.
Favorable safety and efficacy results from preliminary studies of patients with uncontrolled hypertension led to the conduct of the open‐label randomized SYMPLICITY HTN‐2 trial in Europe and Australia.31 Enrollment criteria included an office‐based systolic blood pressure at least 160 mm Hg despite treatment with 3 or more antihypertensive medications. Following an initial qualification screening phase, patients were randomized to immediate renal denervation or a control group. Both groups were maintained on their antihypertensive medications. The primary end point was reduction in office blood pressure at 6 months. Secondary end points included 24‐hour ambulatory blood pressure at 6 months postprocedure, procedural safety, and assessment of cardiovascular events and renal function. No significant differences between cohorts were identified at baseline. Primary results are represented in Table 1. By 6 months, 84% of patients treated with sympathetic denervation experienced a systolic blood pressure reduction exceeding 10 mm Hg, and more than 80% had an office blood pressure <160 mm Hg. No device‐related procedural complications were observed, and renal imaging at 6 months did not identify any renal abnormalities directly attributed to denervation or requiring therapy.
SYMPLICITY HTN‐3 Trial Design
Design and Study Population
The SYMPLICITY HTN‐3 Trial is a regulatory study designed as a prospective, randomized, masked procedure, single‐blind trial evaluating the safety and effectiveness of catheter‐based bilateral renal denervation for the treatment of uncontrolled hypertension despite compliance with at least 3 antihypertensive medications of different classes (at least 1 of which is a diuretic) at maximal tolerable doses (www.clinicaltrials.gov identifier NCT01418261). The primary effectiveness end point is measured as the change in office‐based systolic blood pressure (SBP) from baseline to 6 months (Table 2). A major secondary effectiveness analysis is the change in average 24‐hour SBP by ambulatory blood pressure monitoring (ABPM) from baseline to 6 months. The primary safety end point is the incidence of major adverse events (MAE) defined as the composite incidence of events detailed in Table 2 or a new renal artery stenosis >70% confirmed by angiography within 6 months of randomization. The trial will randomize 530 patients in a single‐blinded, masked‐controlled, 2:1 treatment design at approximately 90 research sites within the United States. The trial tests the hypothesis that interruption of renal sympathetic nerves in patients with resistant hypertension will result in significant blood pressure lowering and goal achievement.
Table 2.
Primary Effectiveness and Safety End Points for the SYMPLICITY HTN‐3 Trial
| Effectiveness end point |
| Change in office SBP from baseline to 6 months postrandomization |
| Safety end point (MAE) |
| Through 1 month postrandomization, composite of: |
| All‐cause mortality |
| End‐stage renal disease (eGFR< 15 mL/min/m2 or need for renal replacement therapy) |
| Significant embolic event resulting in end‐organ damage (eg, kidney/bowel infarct, lower extremity ulceration or gangrene, or doubling of serum creatinine) |
| Renal artery perforation requiring intervention |
| Renal artery dissection requiring intervention |
| Vascular complications (eg, clinically significant groin hematoma, arteriovenous fistula, pseudoaneurysm) requiring surgical repair, interventional procedure, thrombin injection, or blood transfusion (requiring more than 2 units of packed red blood cells within any 24‐hour period during the first 7 days postrandomization) |
| Hospitalization for hypertensive crisis not related to confirmed nonadherence with medications, |
| Or |
| New renal artery stenosis >70%, confirmed by angiography within 6 months of randomization |
Abbreviations: eGFR, estimated glomerular filtration rate; MAE, major adverse events; SBP, systolic blood pressure.
Table 3 details trial enrollment criteria. Initial patient recruitment will identify individuals (1) with an average office SBP ≥160 mm Hg, and (2) receiving a stable antihypertensive treatment regimen (ie, without change in dose or medication) and including the maximum tolerated dose of at least 3 medications of different classes, of which 1 must be a diuretic, for at least 2 weeks prior to enrollment. A full dose of antihypertensive medication must be documented as the highest dose per product labeling or treatment guidelines, or highest tolerated or appropriate dose per the investigator's best judgment.
Table 3.
Enrollment Criteria for the SYMPLICITY HTN‐3 Trial
| Inclusion criteria |
| Age ≥18 and ≤80 years at time of randomization |
| Stable medication regimen including full tolerated doses of 3 or more antihypertensive medications of different classes, including a diuretic (with no changes for a minimum of 2 weeks prior to screening) and no expected changes for at least 6 months |
| Office SBP ≥160 mm Hg based on an average of 3 blood pressure readings measured at both an initial and a confirmatory screening visit |
| Written informed consent |
| Exclusion criteria |
| Renal artery anatomy ineligible for treatment including: |
| Main renal arteries with <4 mm diameter or with <20 mm treatable length |
| Multiple renal arteries where the main renal artery is estimated to supply <75% of the kidney |
| Renal artery stenosis (>50%) or renal artery aneurysm in either renal artery |
| History of prior renal artery intervention including balloon angioplasty or stenting |
| eGFR of <45 mL/min/1.73 m2 |
| >1 in‐patient hospitalization for a hypertensive crisis within the past year |
| ABPM 24 hour average SBP <135 mm Hg |
| ≥1 episode(s) of orthostatic hypotension (reduction of SBP of ≥20 mm Hg or DBP of ≥10 mm Hg within 3 minutes of standing) coupled with symptoms within the past year or during the screening process |
| Pregnant, nursing, or planning to be pregnant |
| Chronic oxygen support or mechanical ventilation (eg, tracheostomy) required other than nocturnal respiratory support for sleep apnea |
| History of or currently have any of the following medical conditions: |
| Primary pulmonary hypertension |
| Type 1 diabetes mellitus |
| Severe cardiac valve stenosis for which a significant reduction of blood pressure is contraindicated |
| Myocardial infarction, unstable angina pectoris, syncope, or a cerebrovascular accident within 6 months of the screening period |
| History of pheochromocytoma, Cushing's disease, coarctation of the aorta, hyperthyroidism, or hyperparathyroidism |
| Any condition that would prohibit or interfere with ability to obtain an accurate blood pressure measurement using the protocol‐specified automatic blood pressure monitor (eg, arm diameter too large for the cuff, arrhythmia that interferes with automatic monitor's pulse sensing and prohibits an accurate measurement) |
| Any serious medical condition that may adversely affect the safety of the participant or the study (eg, patients with clinically significant peripheral vascular disease, abdominal aortic aneurysm, bleeding disorders such as thrombocytopenia, hemophilia, or significant anemia) |
| Scheduled or planned surgery or cardiovascular intervention in the next 6 months |
| Any known, unresolved history of drug use or alcohol dependency, lacks the ability to comprehend or follow instructions, or would be unlikely or unable to comply with study follow‐up requirements |
| Currently enrolled in another investigational drug or device trial |
Abbreviations: ABPM, ambulatory blood pressure monitoring; DPB, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Following institutional ethics approval, potentially eligible subjects will provide informed consent and begin a screening period, including at least 2 weeks of home blood pressure recording and confirmation that medications were taken daily. If medications are altered after enrollment but prior to randomization, a subject will either be excluded or must reinitiate the screening process after at least 2 weeks stabilization on the revised medical regimen. Study staff blinded to the patients' treatment allocation will perform all office‐based blood pressure measurements through 6 months using an automatic Omron blood pressure monitor. Patients will be seated comfortably for 5 minutes with feet flat on the ground prior to taking blood pressure measurements. Measurements will be taken using the same arm (identified at screening) for each visit. Office‐based blood pressures are determined by the average of 3 sitting blood pressure measurements taken 1 minute apart. If the lowest and highest SBP values are more than 15 mm Hg apart, additional readings will be performed. The last 3 consecutive consistent readings will be recorded. If SBP values are more than 20 mm Hg apart after 6 measurements, the patient will be excluded from the trial.
At a second visit, office SBP must be confirmed to be ≥160 mm Hg (Figure 1). During this screening period, 24‐hour ABPM will be performed to confirm the average 24‐hour blood pressure exceeds 135 mm Hg. Subjects meeting these criteria will then undergo renal angiography to determine anatomic eligibility (Table 3). Only patients fulfilling all clinical and anatomic requirements will be randomized (2 treatment:1 control); accordingly, it is estimated approximately 1060 will be enrolled to yield 530 patients for randomization. Randomization is accomplished at the time of the renal angiogram (Figure 1) using an interactive voice response system. Patients will be stratified by both study center and race (African American vs non‐African American).
Figure 1.
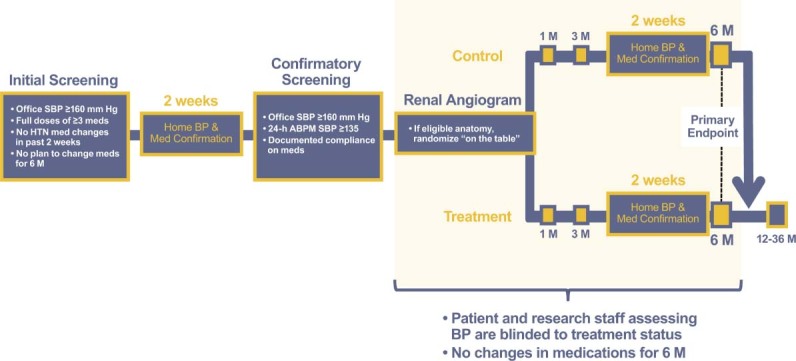
Trial design for the randomized SYMPLICITY HTN‐3 study. Following initial and confirmatory screening for eligibility, patients will undergo renal angiography to evaluate renal anatomy. Suitable patients will be randomized to receive the renal denervation procedure immediately or to the control group. Both groups will be maintained on their antihypertensive medical regimen. Patients and study staff assessing blood pressure (BP) are blinded throughout the study period until patients are unblinded after the 6‐month assessment. The primary effectiveness end point is the change in office‐based systolic blood pressure (SBP) at 6 months. After 6 months, control patients will be given the option to receive renal denervation if they remain eligible for the procedure. Abbreviations: ABPM, ambulatory blood pressure monitoring; HTN, hypertension.
Renal Denervation Procedure
To achieve effective renal denervation, a specifically designed 6F compatible catheter and radiofrequency generator/algorithm (Symplicity Renal Denervation System; Medtronic, Inc., Mountain View, CA) (Figure 2) were developed.28., 32. The catheter features a monopolar platinum‐iridium electrode at the distal tip of the catheter that is used in conjunction with a standard dispersive electrode. The platinum‐iridium electrode is radiopaque, thereby assisting in catheter positioning under fluoroscopic guidance. To minimize the thermal effects on the arterial wall, the design permits continuous blood flow enabling cooling of the renal artery intima throughout the treatment period. The renal denervation system received CE Mark approval in 2008 and is commercially available in selected countries, although not currently in the United States.
Figure 2.
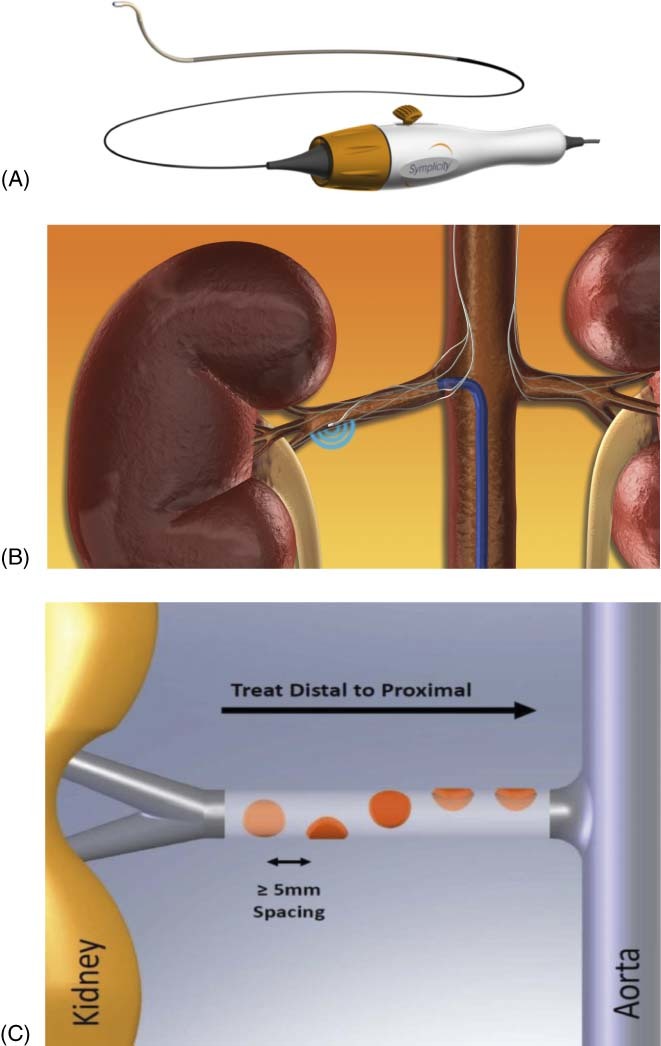
Renal sympathetic denervation using the Symplicity Renal Denervation System. (A) The Symplicity catheter is 6F compatible. (B) The catheter features an articulating tip with a radiopaque radiofrequency electrode. (C) Four to six 2‐minute treatments are delivered per artery. Figure adapted from Schlaich et al.32
Treatment involves approximately 4 to 6 applications according to an operator independent algorithm using low‐power (8 W) radiofrequency energy. Treatments are delivered in a helical fashion within the renal artery by rotation of the catheter and approximately 5 mm pullback between ablations. The generator provides the radiofrequency energy according to an automated algorithm designed uniquely for renal artery ablation, and the front panel displays information such as temperature, impedance, and treatment time. Preclinical evaluation has demonstrated that ablation produces distinct focal lesions that have no known clinically relevant late‐term sequelae to the vessel or kidney as noted in follow‐up angiograms in prior studies.31
Subjects will be blinded to randomization assignment by a combination of conscious sedation, sensory isolation (eg, blindfold and noise isolation), and lack of familiarity with the procedural details and duration. Procedural pain will be managed with opiates and sedatives. Control subjects will undergo screening renal angiography alone; however, all randomized patients will be hospitalized overnight postprocedure, with postprocedure monitoring practices as standard of care. All randomized patients (treatment and control groups) are managed identically following renal angiography to maintain the subject blinding.
Follow‐Up Assessments
Following randomization (and potential treatment), both patient groups will be maintained on the baseline antihypertensive medical regimen without changes for 6 months. Patients will remain blinded to their treatment group and will be followed at 1, 3, and 6 months postrandomization (Figure 1). Every effort will be made to maintain the initial antihypertensive treatment; nevertheless, if blood pressure lowering medication revisions are necessary due to a clinically important event, such changes will be permitted and documented. Defined as an escape medication change, alterations of antihypertensive medications are permissible in the presence of an adverse event or change in symptoms, SBP <115 mm Hg or increase in SBP >15 mm Hg above baseline. Designated study staff blinded to treatment status will be responsible for performance of blood pressure measurement according to protocol‐directives that include patient position and cuff size. In addition, prior to the 6‐month visit, subjects will be required to record daily home blood pressure measurements and medications for at least a 2‐week period. At 6 months, renal artery duplex imaging will be performed, and results will be assessed by an independent core laboratory. If a clinically significant stenosis (eg, renal artery to aorta peak systolic velocity ratio >3.5, or peak systolic velocity >200 cm/s with evidence of poststenotic turbulence) is identified by ultrasound, confirmatory angiography will be performed, and results will be compared with baseline imaging by an independent core laboratory blinded to treatment status.
After the 6‐month clinical follow‐up and all required testing is completed, patients randomized to the control group will be unblinded and have the option for renal denervation treatment, provided they continue to meet all inclusion and exclusion criteria. All patients independent of treatment assignment will be followed through 3 years following randomization. A trial steering committee has been charged with the responsibilities of trial oversight and conduct. An independent data safety monitoring board will provide scheduled review of events to ensure the health, safety, and welfare of patients. Additionally, a clinical events committee has been designated to adjudicate major adverse events.
Study End Points
The primary effectiveness end point is the change in office‐based SBP from baseline to 6 months (Table 2). The primary safety end point is the incidence of MAE, a patient‐level composite end point of the incidence of events detailed in Table 2 or a new renal artery stenosis >70% confirmed by angiography within 6 months of randomization. A major secondary effectiveness end point is the change in average 24‐hour systolic blood pressure by ABPM from baseline to 6 months. Other secondary efficacy end points evaluating blood pressure reduction at 6 months include the incidence of office SBP reductions of ≥10 mm Hg, ≥15 mm Hg, and ≥20 mm Hg; the incidence of achieving target office SBP (<140 mm Hg or <130 mm Hg for patients with diabetes or renal disease); escape medication changes; change in office diastolic blood pressure; and change in patient‐recorded home blood pressure. Additionally, changes in office systolic and diastolic blood pressure from baseline to 12, 18, 24, 30, and 36 months after renal denervation for all treated patients will be assessed as well the incidences of incremental reductions in office SBP (≥10 mm Hg, ≥15 mm Hg, and ≥20 mm Hg) at all follow‐up time points. Secondary safety end points include each of the components of MAE, chronic safety at 6 months postrandomization (eg, cardiovascular events, end‐stage renal disease or serum creatinine increase >50%, new renal artery stenosis >70% confirmed by angiography, hospitalization for hypertensive crisis) and change in renal function, as measured by serum creatinine and cystatin C, compared between groups from baseline to 6 months postrandomization.
Statistical Analysis Plan
All primary analyses of the effectiveness end points will be performed according to the intent‐to‐treat principle (all randomized patients) unless otherwise specified. Regarding the primary safety end point, a performance goal of 9.8% was derived based on meta‐analysis of trials involving other renal interventions with estimated adjustment for hypertensive crisis. Under the assumption the true MAE rate for the renal denervation arm is 6%, and using a 1‐sided 0.05 level of significance, a sample size of 316 renal denervation patients yields 80% power to show that the MAE rate is significantly lower than the performance goal. If at most 22 of the 316 (7%) renal denervation patients experience a MAE, the MAE rate will be declared statistically significantly lower than the performance goal. A supplementary nonpowered analysis on safety will compare the MAE rate between the treatment and control groups. Secondary safety endpoints—including each of the components of MAE listed in Table 2—will also be analyzed and reported.
Regarding the primary effectiveness end point, a reduction in office‐based SBP of ≥5 mm Hg is considered a clinically meaningful improvement.33 Specifically, a 5‐mm Hg reduction in SBP has been associated with a 14% decrease in stroke, a 9% decline in cardiovascular disease, and 7% reduction in mortality.33 Assuming a true difference between treatment means of 15 mm Hg with a 25 mm Hg standard deviation of SBP change per group, a sample size of 316 treatment and 158 control subjects provides >95% statistical power to demonstrate a >5‐mm Hg difference between treatment groups at a 1‐sided alpha level 0.025. To evaluate for consistency of results among subgroups of interest, exploratory subgroup analyses are prespecified, for example, according to race, diabetes, sex, age, and body mass index.
If the primary effectiveness end point is met, then the major secondary end point, the change in average 24‐hour SBP by ABPM from baseline to 6 months, will be tested. Assuming a true difference between treatment means of 7 mm Hg with a 18 mm Hg standard deviation of SBP change per group, a sample size of 316 treatment and 158 control subjects provides >80% statistical power to demonstrate a >2 mm Hg difference between cohorts at a 1‐sided α level 0.025. To account for approximately 10% rate of premature withdrawal or failure to obtain the primary end point measure, 530 patients will be randomized (2 treatment:1 control).
Baseline characteristics and secondary end points will also be analyzed and compared between the 2 groups. Unless otherwise specified, continuous variables will be compared between groups using a 2‐sample t test, and categorical variables will be compared using the Fisher exact test.
Summary: Potential Clinical Impact of Renal Denervation
The progressive nature of hypertension from asymptomatic status to end‐organ failure is well recognized. Both thrombotic and embolic stroke are recognized companions of chronic hypertension, in addition to systolic and diastolic heart failure and renal impairment. Considering that 20‐mm Hg or 10‐mm Hg reductions in systolic and diastolic blood pressure, respectively, are associated with a halving of cardiovascular mortality,3 the opportunity to influence clinical outcomes through hypertension management is paramount.
The design and methods of this trial satisfy the regulatory requirements to test whether renal denervation therapy is a safe and effective treatment for patients who remain hypertensive despite adherence to maximally titrated polypharmacy. The study will provide insights into the critical role of renal sympathetic and somatic nerves in mediating resistant hypertension, and should it meet regulatory approval, it will introduce a significant addition to our treatment strategy for this unique cohort of patients, who currently have not been able to reduce their hypertensive cardiovascular risk. The trial will form the basis of further studies that will address the potential value of selective renal sympathetic and somatic denervation in patients with various hypertensive syndromes, for patients who may not adhere to lifelong polypharmacy, and for additional disease states that are linked to sympathetic hyperactivity.
The novelty of this intervention, selectively interfering with renal sympathetic efferent and afferent signals, underlies much of the excitement about this strategy. Thus, in part, the importance of this therapy is linked to the insights into hypertension pathogenesis. Current pharmacologic strategies attempting to control blood pressure in patients with resistant hypertension are limited by the lack of effectiveness of existing antihypertensive drugs, even when administered in combination, as well as by their adverse effect profiles. The opportunity to use a low‐risk interventional procedure to safely and durably reduce blood pressure offers the hope of successful treatment for the populations of hypertensive patients unwilling or unable to take maximal polypharmacy. The potential for treating additional diseases with this intervention is also a topic for further research.
Attenuation of sympathetic activity may have a multitude of effects beyond those directly related to hypertension. Increased sympathetic nervous system activity, for example, is associated with heightened risk of death among heart failure patients.15 Further, salt and water retention in some forms of heart failure may be mediated in large part by renal sympathetic activity, and selective renal denervation may play a role in treatment or prevention of heart failure and the cardiorenal syndrome.34 Recent reports in patients with insulin resistance or type II diabetes mellitus, polycystic ovary syndrome, and hypertension have also suggested improved insulin resistance and glycemic control with denervation therapy.35., 36. Whether interruption of renal sympathetic nerve activity permanently alters these and other disease states attributed to hyperadrenergic conditions will expectedly be a focus for further investigation.
Conclusion
The results of the SYMPLICITY HTN‐3 pivotal trial will be submitted to the US Food and Drug Administration for approval of catheter‐based renal denervation as a treatment option for resistant hypertension. This trial will define the efficacy and safety of renal denervation in a rigorous manner and is certain to be the basis for the design of future interventional trials.
Acknowledgements
The authors gratefully acknowledge Dr. Sidney A. Cohen for expert review of the manuscript and Dr. Minglei Liu for statistical review. Colleen Gilbert provided technical assistance in the preparation of the manuscript. All are employees of Medtronic, Inc.
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365: 217–223. [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics 2012 update; a report from the American Heart Association. Circulation. 2012;125:e12–e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd‐Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. [DOI] [PubMed] [Google Scholar]
- 5. Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control—continued disparities in adults: United States, 2005–2006. NCHS Data Brief No. 3. Hyattsville, MD: National Center for Health Statistics; 2008.. [PubMed]
- 6. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 7. Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52: 1749–1757. [DOI] [PubMed] [Google Scholar]
- 8. Conlin PR, Gerth WC, Fox J, et al. Four‐year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Ther. 2001;23:1999–2010. [DOI] [PubMed] [Google Scholar]
- 9. Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: A scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. [DOI] [PubMed] [Google Scholar]
- 10. Mancia G, De Backer G, Dominiczak A, et al. 2007. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 11. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 12. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 13. Esler M, Jennings G, Korner P, et al. Total and organ‐specific noradrenaline plasma kinetics in essential hypertension. Clin Exp Hypertens A. 1984:6:507–521. [DOI] [PubMed] [Google Scholar]
- 14. Esler M, Jennings G, Korner P, et al. The assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. [DOI] [PubMed] [Google Scholar]
- 15. Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. [DOI] [PubMed] [Google Scholar]
- 16. Narkiewicz K, Pesek CA, Kato M, et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. [DOI] [PubMed] [Google Scholar]
- 17. Vollenweider P, Tappy L, Randin D, et al. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest. 1993;92:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huggett RJ, Scott EM, Gilbey SG, et al. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. [DOI] [PubMed] [Google Scholar]
- 19. Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–920. [DOI] [PubMed] [Google Scholar]
- 20. Hausberg M, Kosch M, Harmelink P, et al. Sympathetic nerve activity in end‐stage renal disease. Circulation. 2002;106: 1974–1979. [DOI] [PubMed] [Google Scholar]
- 21. DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. [DOI] [PubMed] [Google Scholar]
- 22. Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension. Results in 1,266 cases. JAMA. 1953;152:1501–1504. [DOI] [PubMed] [Google Scholar]
- 23. Longland CJ, Gibb WE. Sympathectomy in the treatment of benign and malignant hypertension. A review of 74 patients. Br J Surg. 1954;41:382–392. [DOI] [PubMed] [Google Scholar]
- 24. Smithwick RH. Surgical treatment of hypertension. Am J Med. 1948:4:744–759. [DOI] [PubMed] [Google Scholar]
- 25. Smith PA, Graham LN, Mackintosh AF, et al. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. [DOI] [PubMed] [Google Scholar]
- 26. Converse RL, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. [DOI] [PubMed] [Google Scholar]
- 27. Schlaich MP, Sobotka PA, Krum H, et al. Renal sympathetic‐nerve ablation for uncontrolled hypertension. N Engl J Med. 2009; 361:932–934. [DOI] [PubMed] [Google Scholar]
- 28. Krum H, Sobotka P, Mahfoud F, et al. Device‐Based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209–215. [DOI] [PubMed] [Google Scholar]
- 29. Krum H, Schlaich M, Whitbourn R, et al. Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 30. Symplicity HTN‐1 Investigators. Catheter‐based renal sympathetic denervation for resistant hypertension: Durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. [DOI] [PubMed] [Google Scholar]
- 31. Symplicity HTN‐2 Investigators. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 32. Schlaich MP, Krum H, Sobotka PA, et al. Renal denervation and hypertension. Am J Hypertension. 2011;24:635–642. [DOI] [PubMed] [Google Scholar]
- 33. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
- 34. Sobotka PA, Mahfoud F, Schlaich MP, et al. Sympatho‐renal axis in chronic disease. Clin Res Cardiol. 2011;100:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123: 1940–1946. [DOI] [PubMed] [Google Scholar]
- 36. Schlaich MP, Straznicky N, Grima M, et al Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens. 2011;29:991–996. [DOI] [PubMed] [Google Scholar]


