Abstract
Background:
Randomized trials have established the benefit of medical therapy and revascularization in the treatment of acute myocardial infarction (MI). Cancer and cardiovascular disease are the 2 most common diseases worldwide. In clinical practice, cancer patients are frequently afflicted with MI. The benefit of medical and/or revascularization therapy in the cancer population with MI is less well known.
Hypothesis:
Medical and revascularization therapy reduces mortality in cancer patients with MI.
Methods:
After approval by the institutional review board, we retrospectively reviewed all patients with a discharge diagnosis of acute MI who were admitted to the University of Texas MD Anderson Cancer Center between December 2000 and October 2006 and evaluated the association between cardiac treatments with survival outcomes.
Results:
A total of 456 patients with a discharge diagnosis of acute MI were identified and included in the study, of which 386 had non–ST‐segment elevation MI (NSTEMI) and 70 had ST‐segment elevation MI (STEMI). Compared with patients with NSTEMI, patients who had STEMI were more often prescribed aspirin (66% vs 43%; P = 0.004), β‐blockers (61% vs 46%; P = 0.018), and thrombolytic therapy (9% vs 0.3%; P = 0.0001). In the multivariable analysis, aspirin use was associated with a 23% decreased risk of death (hazard ratio [HR]: 0.77, 95% confidence interval [CI]: 0.60‐0.98, P = 0.033) and β‐blocker use was associated with a 36% decreased risk of death (HR: 0.64, 95% CI: 0.51–0.81, P = 0.0002). Statins (HR: 0.82, P = 0.18) and catheter‐based revascularization (HR: 0.57, P = 0.09) did not have an impact on the risk of death. Compared with patients with limited cancer, advanced cancer patients were twice as likely to die (HR: 2.12, 95 CI: 1.47–3.04, P < 0.0001). Previous chemotherapy (P = 0.005) and chest radiotherapy (P = 0.017) were associated with increased 1‐year mortality, whereas hyperlipidemia (P = 0.018) was protective.
Conclusions:
In this study of cancer patients with MI, medical therapy with aspirin and β‐blockers was associated with improved survival.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Cancer and cardiovascular disease are the 2 leading causes of mortality and morbidity worldwide.1 Coronary artery disease (CAD) is frequently encountered in younger patients with Hodgkin disease treated with radiotherapy.2 The majority of elderly patients with cancer also suffer from other serious comorbidities, of which heart and vascular disease are most prevalent.3 Among newly diagnosed cancer patients age >75 years, the prevalence of heart disease is 20%.3 An increased risk of cardiac events is seen in long‐term survivors of testicular cancer.4 One or more cardiovascular risk factors (hypertension, hypercholesterolemia, smoking, or a positive family history) are seen in up to 97% of testicular cancer patients treated with chemotherapy.4 There appears to be a significant increase in coronary events in the 2 years before the cancer diagnosis.5 Another study has shown that the risk of CAD was increased during first 6 months after cancer diagnosis, and metastasis was associated with an increased risk of CAD.6 Coronary artery disease and cancer share common risk factors, such as smoking, and there is a moderately increased risk of tobacco‐related cancers among survivors of myocardial infarction (MI).7
Coronary artery disease may predate the development of cancer or may result from treatment of cancer itself. Hence in clinical practice, CAD is frequently present in patients with cancer. The treatment options available for these patients are based on studies done in general population. Patients with cancer have been largely excluded from all trials of acute coronary syndrome; hence, the evidence‐based treatment regimen for MI in this group of patients is unknown. In this study, we have looked at the clinical characteristics, treatment, and outcomes of acute MI in cancer patients.
Methods
The protocol was approved by the institutional review board. No extramural funding was used to support this study. We retrospectively reviewed all patients with a discharge diagnosis of acute MI who were admitted to the University of Texas MD Anderson Cancer Center between December 2000 and October 2006 and evaluated the association between cardiac treatments with survival outcomes.
All charts were reviewed and the following data were collected: demographic characteristics, clinical symptoms, electrocardiogram (ECG) findings, treatment, complications, and mortality. The medical records were also reviewed for bleeding complications and contraindications to aspirin therapy, defined as any major internal‐organ bleeding within 6 months of presentation with index MI, allergy to aspirin, and thrombocytopenia (platelet count <100 K/µL).
ST‐elevation ECG was defined as ST elevation in 2 contiguous leads of ≥1 mm in the limb leads or 2 mm in the precordial leads. All other ECGs were considered non–ST elevation.
Patient characteristics including age, sex, race, smoking status, cancer type, cancer status, comorbidities, previous chest radiotherapy, chemotherapy, laboratory values and the use of aspirin, β‐blockers, statins, thrombolytics, cardiac catheterization, and angiotensin‐converting enzyme (ACE) inhibitors were tabulated between the non–ST elevation and ST‐elevation groups. Advanced cancer was defined as stage >T2 and/or N1 and/or M1; any malignancy considered refractory, relapsing, or recurrent; and cancer treated with transplantation.8 The relapsing and recurrent cancers treated with transplantation were mainly applicable to liquid tumors.
Groups were compared with the χ 2 test or the Wilcoxon 2‐sample tests as appropriate. Overall survival was measured from the date of index MI to the date of death or last follow‐up. One‐year overall survival was calculated using the Kaplan‐Meier product limit method with 95% confidence intervals (CIs) by groups; groups were compared with the log‐rank statistic. Cox proportional hazard models were fitted to determine the association of the use of cardiac treatment with survival outcomes after adjustment for other patient and disease characteristics. Factors that had significant univariate associations with overall survival were included in the models. Results are expressed in hazard ratios (HR) and 95% CIs. P values <0.05 were considered statistically significant; all tests were 2‐sided. Statistical analyses were carried out using SAS 9.1 (SAS Institute Inc., Cary, NC) and S‐Plus 7.0 (Insightful Corporation, Seattle, WA).
Results
A total of 456 patients diagnosed with acute MI were identified and included in the study, of which 386 had non–ST‐elevation MI (NSTEMI) and 70 had ST‐elevation MI (STEMI).
Baseline demographics and clinic characteristics are summarized in Table 1. Compared with NSTEMI, there was a higher prevalence of cerebrovascular accident (15% vs 6%; P = 0.01), chest pain (44.3% vs 27.7%; P = 0.006), and higher peak troponin level (mean 27 vs 7.8; median 4.1 vs 2.4; P = 0.0002) in patients with STEMI. Hypotension was more prevalent in the NSTEMI group (25.3% vs 8.5%; P = 0.01). A higher number of patients in NSTEMI group had left ventricular ejection fraction >50% (61.1% vs 41.9%; P = 0.03). Other risk factors and comorbidities were not significantly different between the 2 groups.
Table 1.
Patient and Clinical Characteristics by ST‐Elevation on Electrocardiogram
| All Patients [n = 456] | NSTEMI [n = 386] | STEMI [n = 70] | P Value | |
|---|---|---|---|---|
| Age, y, median | 67 | 67 | 66.5 | 0.48 |
| Male sex, n (%) | 295 (64.7) | 252 (65.3) | 43 (61.4) | 0.53 |
| BMI, mean (SD) | 26.6 (6.0) | 26.5 (6.1) | 26.8 (5.4) | 0.43 |
| Smoker yes, n (%) | 177 (38.9) | 152 (39.5) | 25 (35.7) | 0.55 |
| Cancer type, n (%) | ||||
| All solid tumors | 279 (61.2) | 234 (60.6) | 45 (64.3) | |
| Lymphoma + leukemia | 150 (32.9) | 130 (33.7) | 20 (28.6) | |
| Multiple cancer | 27 (5.9) | 22 (5.7) | 5 (7.1) | 0.67 |
| Cancer status, n (%) | ||||
| Advanced | 403 (88.4) | 340 (88.1) | 63 (90.0) | |
| Limited | 53 (11.6) | 46 (11.9) | 7 (10.0) | 0.65 |
| Previous history of, n (%) | ||||
| CAD | 145 (31.9) | 124 (32.3) | 21 (30.0) | 0.71 |
| CVA | 33 (7.4) | 23 (6.1) | 10 (14.9) | 0.01 |
| Hyperlidemia | 92 (20.9) | 76 (20.3) | 16 (24.2) | 0.47 |
| DM | 108 (24) | 93 (24.3) | 15 (22.1) | 0.68 |
| HTN | 229 (50.2) | 192 (49.7) | 37 (52.9) | 0.63 |
| Chest radiotherapy | 84 (18.6) | 72 (18.8) | 12 (17.6) | 0.82 |
| Chemotherapy | 291 (66.1) | 241 (64.6) | 50 (74.6) | 0.11 |
| Clinical features and laboratory values | ||||
| Chest pain, n (%) | 138 (30.3) | 107 (27.7) | 31 (44.3) | 0.006 |
| Dyspnea, n (%) | 202 (44.3) | 164 (42.5) | 38 (54.3) | 0.07 |
| Hypotension, n (%) | 68 (22.7) | 64 (25.3) | 4 (8.5) | 0.01 |
| LVEF >50%, n (%) | 169 (58.3) | 151 (61.1) | 18 (41.9) | 0.03a |
| Creatinine (mg/dL), mean (SD) | 1.4 (1.1) | 1.5 (1.1) | 1.3 (1.2) | 0.38b |
| Platelets (K/µL), mean (SD) | 157 (136) | 151 (134) | 189 (147) | 0.043b |
| WBC (K/µL) | 12 (15) | 11.9 (16) | 12.5 (8) | 0.035b |
| Hb (g/dL) | 10 (1.9) | 10.1 (1.9) | 10.4 (1.7) | 0.18b |
| Peak troponin (ng/mL) | ||||
| Mean (SD) | 10.9 (43) | 7.8 (25) | 27 (91) | 0.0002b |
| Median | 2.7 | 2.4 | 4.1 | |
| Albumin, mean (SD) | 3.4 (15.6) | 3.5 (16.9) | 2.8 (0.7) | 0.19 |
| Bilirubin, mean (SD) | 1.5 (2.1) | 1.6 (2.2) | 1.3 (1.5) | 0.64 |
| AST, mean (SD) | 361 (1493) | 404 (1615) | 116 (151) | 0.18 |
| ALT, mean (SD) | 219 (1107) | 233 (1179) | 140 (565) | 0.92 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CAD, coronary artery disease; CVA, cerebrovascular accident; DM, diabetes mellitus; Hb, hemoglobin; HTN, hypertension; LVEF, left ventricular ejection fraction; NSTEMI, non–ST‐elevation myocardial infarction; SD, standard deviation; STEMI, ST‐elevation myocardial infarction; WBC, white blood cell count.
Fisher exact test.
Wilcoxon 2‐sample test.
We added a comparison of the variables including history of hyperlipidemia (no vs yes), chest radiation (no vs yes), and chemotherapy (no vs yes) by cancer status (limited vs advanced) and found no association between hyperlipidemia, chemotherapy, or chest radiation with cancer status.
Cardiac treatment including the use of aspirin, β‐blockers, statins, ACE inhibitors, thrombolytics, and catheter‐based revascularization is summarized in Table 2. Compared with patients with NSTEMI, patients who had STEMI were more often prescribed aspirin (66% vs 43%; P = 0.004), β‐blockers (61% vs 46%; P = 0.018), and thrombolytics (9.0% vs 0.3%; P = 0.0001). There was no difference in the use of aspirin and β‐blockers between patients with limited and advanced cancer. Aspirin was prescribed to 2 of 53 (4%) patients with limited cancer vs 43 of 403 (10.7%) patients with advanced cancer (P = 0.11), and β‐blockers were prescribed to 8 of 43 (15%) patients with limited cancer vs 47 of 403 (11.7%) patients with advanced cancer (P = 0.47). Both aspirin and β‐blockers were prescribed to 23 of 53 (43.4%) patients with limited cancer vs 143 of 403 (35.5%) patients with advanced cancer (P = 0.26).
Table 2.
Cardiac Treatment According to Myocardial Infarction Category
| All Patients [n = 456] | NSTEMI [n = 386] | STEMI [n = 70] | P Value | |
|---|---|---|---|---|
| Aspirin | 211 (46.3) | 165 (42.7) | 46 (65.7) | 0.0004 |
| β‐Blocker | 221 (48.5) | 178 (46.1) | 43 (61.4) | 0.018 |
| Statin | 94 (20.6) | 74 (19.2) | 20 (28.6) | 0.07 |
| ACE inhibitor | 47 (10.3) | 38 (9.8) | 9 (12.9) | 0.44 |
| Thrombolytic | 7 (1.5) | 1 (0.3) | 6 (8.6) | 0.0001 |
| Catheter‐based revascularization | 15 (3.3) | 11 (2.8) | 4 (5.7) | 0.21 |
Abbreviations: ACE, angiotensin‐converting enzyme; NSTEMI, non–ST‐elevation myocardial infarction; STEMI, ST‐elevation myocardial infarction.
Data are given as n (%).
Among patients with thrombocytopenia (platelet count <150 K/µL), 34% received aspirin, compared with 63% in patients with normal platelets (platelet count >150 K/µL; P < 0.001).
The median follow‐up among all patients was 1.25 months (range, 0–99 months). The overall survival estimates with 95% CIs are listed in Table 3.
Table 3.
One‐Year Overall Survival Estimates and Patient/Clinical Characteristics
| No. of Patients | 1‐Year Estimates (95% CI) | Log‐Rank P Value | |
|---|---|---|---|
| All | 456 | 0.26 (0.22–0.3) | |
| ST elevation | |||
| No | 386 | 0.26 (0.22–0.31) | |
| Yes | 70 | 0.22 (0.13–0.33) | 0.28 |
| Sex | |||
| F | 161 | 0.26 (0.2–0.33) | |
| M | 295 | 0.25 (0.2–0.3) | 0.77 |
| Smoker | |||
| No | 366 | 0.26 (0.22–0.31) | |
| Yes | 80 | 0.24 (0.15–0.34) | 0.99 |
| Cancer type | |||
| All solid tumors | 279 | 0.29 (0.24–0.35) | |
| Lymphoma/ leukemia | 150 | 0.20 (0.14–0.26) | |
| Multiple cancer | 27 | 0.22 (0.09–0.39) | 0.07 |
| Cancer status | |||
| Advanced | 403 | 0.22 (0.18–0.26) | |
| Limited | 53 | 0.53 (0.39–0.65) | <0.001 |
| CAD | |||
| No | 309 | 0.23 (0.18–0.28) | |
| Yes | 145 | 0.31 (0.24–0.39) | 0.09 |
| CVA | |||
| No | 414 | 0.27 (0.23–0.31) | |
| Yes | 33 | 0.14 (0.05–0.28) | 0.49 |
| Hyperlipidemia | |||
| No | 348 | 0.22 (0.18–0.27) | |
| Yes | 92 | 0.40 (0.30–0.50) | 0.001 |
| DM | |||
| No | 342 | 0.26 (0.22–0.31) | |
| Yes | 108 | 0.25 (0.17–0.33) | 0.50 |
| HTN | |||
| No | 227 | 0.22 (0.17–0.28) | |
| Yes | 229 | 0.29 (0.24–0.35) | 0.13 |
| Chest radiotherapy | |||
| No | 367 | 0.28 (0.24–0.33) | |
| Yes | 84 | 0.16 (0.09–0.24) | 0.0065 |
| Chemotherapy | |||
| No | 149 | 0.37 (0.29–0.44) | |
| Yes | 291 | 0.20 (0.15–0.24) | <0.001 |
| Aspirin | |||
| No | 245 | 0.18 (0.14–0.24) | |
| Yes | 211 | 0.34 (0.28–0.41) | <0.001 |
| β‐Blocker | |||
| No | 235 | 0.16 (0.12–0.21) | |
| Yes | 221 | 0.36 (0.3–0.42) | <0.001 |
| Statin | |||
| No | 362 | 0.22 (0.18–0.27) | |
| Yes | 94 | 0.39 (0.29–0.49) | <0.001 |
| ACE inhibitor | |||
| No | 409 | 0.25 (0.21–0.3) | |
| Yes | 47 | 0.28 (0.16–0.41) | 0.28 |
| Thrombolytic | |||
| No | 449 | 0.26 (0.22–0.3) | |
| Yes | 7 | 0.14 (0.01–0.46) | 0.73 |
| Catheter‐based revascularization | |||
| No | 441 | 0.24 (0.2–0.28) | |
| Yes | 15 | 0.67 (0.38–0.85) | <0.001 |
Abbreviations: ACE, angiotensin‐converting enzyme; CAD, coronary artery disease; CI, confidence interval; CVA, cerebrovascular accident; DM, diabetes mellitus; F, female; HTN, hypertension; M, male.
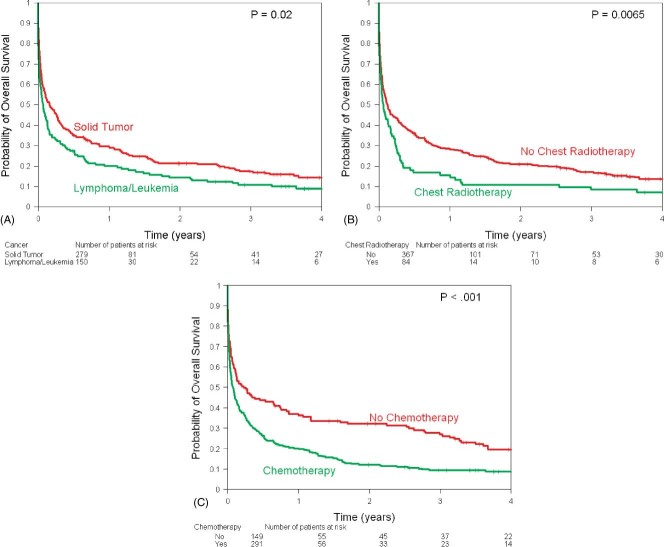
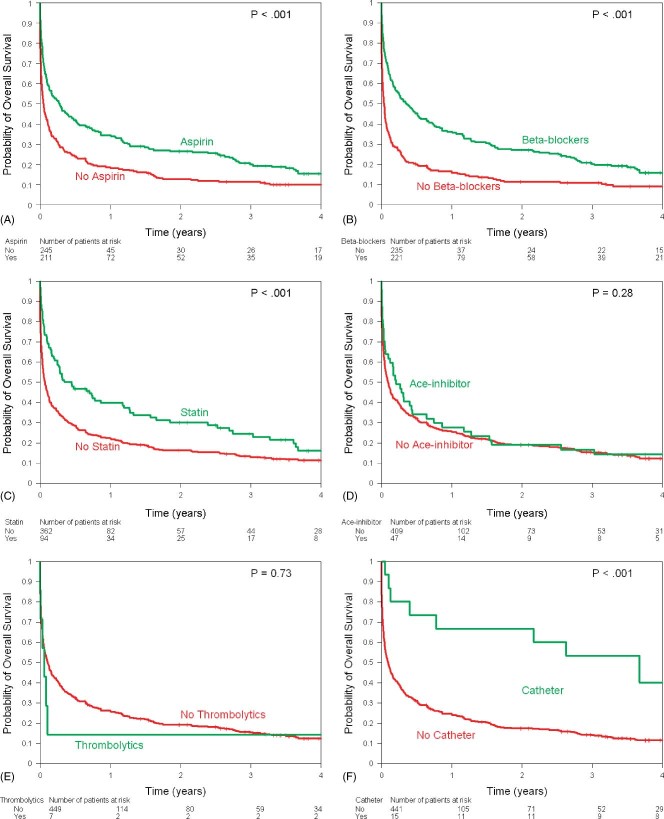
There were 407 deaths, 342 (88%) in the NSTEMI group and 65 (94%) in the STEMI group. The 1‐year survival rate was 26% (95% CI: 22%‐30%) among all patients and was 26% in the NSTEMI group vs 22% in the STEMI group (P = 0.28). Use of aspirin (1‐year survival, 34% vs 18%; P < 0.001), β‐blockers (1‐year survival 36% vs 16%; P < 0.001), statins (1‐year survival, 39% vs 22%; P < 0.001), and catheter‐based revascularization (1‐year survival, 67% vs 24%; P < 0.001) was associated with better survival. Univariate log‐rank tests show that limited cancer status (P < 0.001) and high lipids (P = 0.001) were associated with higher survival rates, whereas chemotherapy use (P < 0.001) and chest radiotherapy (P = 0.0065) were associated with lower survival rates (Table 3).
The Kaplan‐Meier estimates of overall survival stratified by ECG changes showed similar survival in the 2 groups (Figure 1). Overall survival was worse in patients with history of lymphoma/leukemia (Figure 2A), chest radiotherapy (Figure 2B), and chemotherapy (Figure 2C). Use of aspirin, β‐blockers, statins, and catheter‐based revascularization was associated with improved survival (Figures 3A, B, C, F). Use of ACE inhibitors and thrombolytics did not show any benefit (Figures 3D, E).
Figure 1.
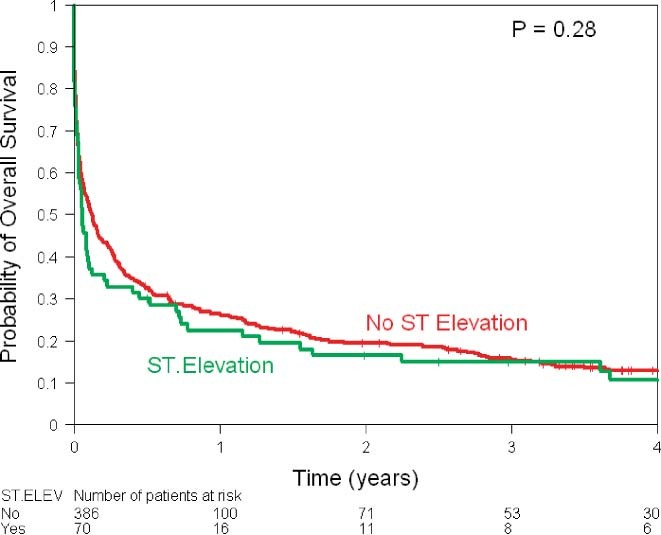
Kaplan‐Meier estimates of overall survival according to ECG findings. Abbreviations: ECG, electrocardiographic; ELEV, elevation.
Figure 2.
Kaplan‐Meier estimates of overall survival by (A) cancer type (solid tumor, lymphoma/leukemia), (B) chest radiotherapy, and (C) chemotherapy.
Figure 3.
Kaplan‐Meier estimates of overall survival by use of (A) aspirin, (B) β‐blockers, (C) statins, (D) ACE inhibitors, (E) thrombolytics, and (F) catheter‐based revascularization. Abbreviations: ACE, angiotensin‐converting enzyme.
Table 4 shows the multivariable models for the association of aspirin, β‐blockers, statins, and catheter‐based revascularization with overall survival adjusting for patients and clinical characteristics. After adjustment, aspirin use was associated with a 23% decreased risk of death (HR: 0.77, 95% CI: 0.60–0.98, P = 0.033) and β‐blocker use was associated with a 36% decreased risk of death (HR: 0.64, 95% CI: 0.51–0.81, P = 0.0002). Statins (HR: 0.82, P = 0.18) and catheter‐based revascularization (HR: 0.57, P = 0.09) did not have an impact on the risk of death.
Table 4.
Multivariable Cox Proportional Hazards Model on Overall Survival Outcomes
| HR | 95% CI | P Value | |
|---|---|---|---|
| Aspirin: yes vs no | 0.77 | 0.6–0.98 | 0.033 |
| β‐Blocker: yes vs no | 0.64 | 0.51–0.81 | 0.0002 |
| Statin: yes vs no | 0.82 | 0.62–1.10 | 0.18 |
| Catheter‐based revascularization: yes vs no | 0.57 | 0.29–1.10 | 0.09 |
| Age, y | 1.01 | 1.00–1.02 | 0.07 |
| Cancer: lymphoma/leukemia vs solid tumors | 1.19 | 0.94–1.51 | 0.15 |
| Cancer status: advanced vs limited | 2.12 | 1.47–3.04 | <0.0001 |
| Hyperlipidemiaa | 0.73 | 0.56–0.95 | 0.018 |
| Previous chemotherapya | 1.42 | 1.11–1.81 | 0.005 |
| Chest radiotherapya | 1.40 | 1.06–1.84 | 0.017 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Compared with no history.
Compared with patients with limited cancer, advanced cancer patients were twice as likely to die (HR: 2.12, 95% CI: 1.47–3.04, P < 0.0001). Hyperlipidemia (P = 0.018) was protective, whereas chemotherapy use (P = 0.005) and chest radiotherapy (P = 0.017) were associated with increased risk of death.
There was no difference in albumin, bilirubin, aspartate transaminase (AST), and alanine transaminase (ALT) between patients with no history of hyperlipidemia vs hyperlipidemia. The mean body mass index was 26.2 for patients with no hyperlipidemia vs 27.7 for patients with hyperlipidemia (P = 0.03). When the albumin, bilirubin, body mass index, AST, and ALT were added to the multivariate model, none of the factors had a significant association with survival.
Review of charts to identify possible reasons for not prescribing aspirin showed that among patients who did not get aspirin, a major bleed within 6 months of the index MI was present in 69 (28%) patients, allergy to aspirin in 10 (4%), and thrombocytopenia (platelet count <100 K/µL) in 178 (73%) of the patients. In 66 (27%) patients there were no obvious contraindications to aspirin therapy. A bleeding complication (internal organ) following aspirin therapy was present in 8 (3.8%) patients, compared with 14 (5.7%) among patients who did not get aspirin (P = 0.34).
Discussion
In general population, use of aspirin,9 β‐blockers,10 statins,11 and coronary revascularization12 improves outcome in patients with acute MI. A similar result was obtained in a heterogeneous group of the cancer population.
We found that the result of treatment of MI in the general population is also applicable to the cancer population. The majority of our patients had NSTEMI, which is in keeping with studies in the general population.13 Dyspnea was the most common symptom, followed by chest pain and findings of hypotension. This is in contrast to the general and even the elderly population, where chest pain is the most frequent presenting symptom of MI.14 In our patient population, the symptoms of chest pain may have been masked by analgesics and narcotics, which are often prescribed to treat cancer pain.
Compared with the general population, the mortality in cancer patients was high, with a 1‐year survival rate of only 26%. Both lack of appropriate medical therapy for MI and cancer with its complications may have contributed to the poor survival in this population. Similar to the elderly population, in view of the high mortality cancer patients are more likely to benefit from aggressive medical therapy of MI, and the number needed to treat to save 1 life may be considerably lower than in the general population.
Only a minority of patients received reperfusion/ revascularization therapy; hence, the data on thrombolytic and coronary revascularization should be taken with caution. They compose a very small group of patients and may have been selectively chosen to receive this form of treatment; selection bias was likely present. Proportionally more patients with STEMI were prescribed aspirin, β‐blockers, and thrombolytics, a finding similar to other studies in the general population.13 The reasons for the difference in prescription of aspirin and β‐blockers are unclear, as the indications for the use of these medications are generally similar for both types of MI. Perhaps this is a reflection of more aggressive therapy for patients with STEMI.
Patients with liquid tumors (lymphoma/leukemia) have a worse outcome than patients with solid tumors, likely as they may have concomitant infection and thrombocytopenia and hence were less likely to be given aspirin. Aspirin should not be withheld from this group of patients, as previous study has shown that in the absence of aspirin therapy, patients with MI and thrombocytopenia have a worse outcome.15
In the present study, patients with history of hyperlipidemia had a better survival, whereas ACE inhibitors conferred no survival benefit. We can only speculate that a history of hyperlipidemia was protective because these patients may already be taking lipid‐lowering agents and hence be protected against adverse cardiovascular events.
Although ACE inhibitors generally provide benefit in patients with MI, the effects of ACE inhibitors in the treatment of acute MI are mixed, and negative results have been observed in some studies. In fact, when compared with placebo, early intravenous use (within 24 hours) of ACE inhibitors is not associated with improved survival during the 6 months after MI.16 Due to the retrospective nature of the study, we were unable to assess whether or not hypotension related to ACE inhibitors was a contributing factor.
In the present study, only 46% of patients received aspirin, only 48% were given β‐blockers, and only 21% were prescribed statin therapy. A minority of patients received heparin and clopidogrel; only 17.6% of patients received heparin, and clopidogrel was prescribed to only 11% of patients. Of the total number of patients undergoing reperfusion/revascularization therapy, about 32% of patients were given thrombolytics. One of the reasons for the comparatively higher use of thrombolytics is that our institution does not have primary percutaneous coronary intervention facilities, and perhaps in these cases a transfer to an outside facility would have considerably prolonged the time to treatment.
Although evidence‐based treatment for MI is underused in the general population and in particular in the elderly population,17 the utilization rate of medical therapy in our patient population is considerably lower than in general population. Our data do not allow evaluation of exactly why only a minority of patients received appropriate medical therapy. Perhaps a physician's desire to avoid side effects (particularly in the case of thrombolytics) may have contributed to the low administration of some drugs. In some cases, contraindications, concomitant comorbidities, cancer staging, and complications of cancer therapy may have directly affected the physician's decision. Irrespective of the reason for withholding appropriate therapy, the results of our study indicate our ineffectiveness to translate results from important clinical trials into everyday practice.
The results of our study show that all cancer patients with MI should receive optimal medical therapy. Due to the small number of patients receiving thrombolytics and percutaneous coronary intervention, any conclusive recommendations regarding this form of therapy cannot be made; but all cancer patients who have a reasonable prognosis should be considered for this form of therapy. However, prior to any aggressive therapy, other conditions that can cause ST elevation and mimic MI in cancer patients—intracranial bleeding,18 tumor invasion,19 5FU‐based chemotherapy,20 MI due to endocarditis,21 or pulmonary embolism22 —should always be considered in the differential diagnosis.
The present study has limitations inherent to all retrospective studies, including the unavailability of specific‐cause mortality on a consistent basis. In addition, due to the different types of cancer and staging, we were unable to find a comparable group with no MI and hence were unable to determine whether survival was solely dependent on MI or other related conditions. But to date this is one of the largest series in the cancer population and shows that simple therapy, such as aspirin and β‐blockers, saves lives in cancer patients with MI. Due to the proven benefits of medical and revascularization therapy in MI, a prospective trial of such therapy in cancer patients would be unethical. Hence, we advocate the development of a prospective registry for cancer patients with MI among the leading oncological centers of the world.
Conclusion
Myocardial infarction in cancer patients often presents without chest pain and is associated with a high mortality. Medical therapy improves survival.
Acknowledgements
The authors would like to thank Dr Yasmine Elgharably and Dr Faisal Zaeem for their help in collecting the data.
References
- 1. Fuster V, Voute J. MDGs: chronic diseases are not on the agenda. Lancet. 2005;366:1512–1514. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Schinttger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25:43–49. [DOI] [PubMed] [Google Scholar]
- 3. Coebergh JW, Janssen‐Heijnen ML, Post PN, et al. Serious co‐morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993‐1996. J Clin Epidemiol. 1999;52:1131–1136. [DOI] [PubMed] [Google Scholar]
- 4. Meinardi MT, Gietma JA, van der Graaf WT, et al. Cardiovascular mortality in long‐term survivors of metastatic testicular cancer. J Clin Oncol. 2000;18:1725–1732. [DOI] [PubMed] [Google Scholar]
- 5. Naschitz JE, Yeshurun D, Abrahamson J, et al. Ischemic heart disease precipitated by occult cancer. Cancer. 1992;69:2712–2720. [DOI] [PubMed] [Google Scholar]
- 6. Zöller B, Ji J, Sundquist J, et al. Risk of coronary heart disease in patients with cancer: a nationwide follow‐up study from Sweden. Eur J Cancer. 2012;48:121–128. [DOI] [PubMed] [Google Scholar]
- 7. Dreyer L, Olsen JH. Cancer risk of patients discharged with acute myocardial infarction. Epidemiology. 1998;9:178–183. [PubMed] [Google Scholar]
- 8. Yusuf SW, Gladish G, Lenihan DJ, et al. Computerized tomographic finding of saddle pulmonary embolism is associated with high mortality in cancer patients. Intern Med J. 2010;40: 293–299. [DOI] [PubMed] [Google Scholar]
- 9. ISIS‐2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS‐2. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 10. ISIS‐1 (First International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS‐1. Lancet. 1986;2:57–66. [PubMed] [Google Scholar]
- 11. Stenestrand U, Wallntin L; for the Swedish Register of Cardiac Intensive Care (RIKS‐HIA). Early statin treatment following acute myocardial infarction and 1‐year survival. JAMA. 2001;285:430–436. [DOI] [PubMed] [Google Scholar]
- 12. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomized trials. Lancet. 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 13. McManus DD, Gore J, Yarzebski J, et al. Recent trends in the incidence, treatment and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yusuf SW, McLean KA, Mishra RM. Thrombolytic therapy for eligible elderly patients with acute myocardial infarction. Am Heart J. 2005;149:e25–e26. [DOI] [PubMed] [Google Scholar]
- 15. Sarkiss MG, Yusuf SW, Warneke CL. Impact of aspirin therapy in patients with thrombocytopenia and acute coronary syndrome. Cancer. 2007;109:621–627. [DOI] [PubMed] [Google Scholar]
- 16. Swedberg K, Held P, Kjekshus J, et al. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction: results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). N Engl J Med. 1992;327:678–684. [DOI] [PubMed] [Google Scholar]
- 17. Tran CT, Laupacis A, Mamdani MM, et al. Effect of age on the use of evidence‐based therapies for acute myocardial infarction. Am Heart J. 2004;148:834–841. [DOI] [PubMed] [Google Scholar]
- 18. Yusuf SW, Bhalla KS, Champion JC. Intracranial bleeding mimicking acute myocardial infarction. Intern Med J. 2007;37: 339–340. [DOI] [PubMed] [Google Scholar]
- 19. Yusuf SW, Durand JB, Lenihan DJ. Wrap beats. Am J Med. 2007;20:417–419. [DOI] [PubMed] [Google Scholar]
- 20. Bathina JD, Yusuf SW. 5‐Fluorouracil–induced coronary vasospasm. J Cardiovasc Med (Hagerstown). 2010;11:281–284. [DOI] [PubMed] [Google Scholar]
- 21. Bathina JD, Daher IN, Plana JC, et al. Acute myocardial infarction associated with nonbacterial thrombotic endocarditis. Tex Heart Inst J. 2010;37:208–212. [PMC free article] [PubMed] [Google Scholar]
- 22. Goslar T, Podbregar M. Acute ECG ST‐segment elevation mimicking myocardial infarction in a patient with pulmonary embolism. Cardiovasc Ultrasound. 2010;24;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]