Abstract
Background:
Elevated admission glucose level is a strong predictor of short‐term adverse outcome in patients with acute coronary syndrome (ACS). However, the prognostic value of diabetic control (ie, hemoglobin A1c levels) in patients with ACS is still undefined.
Hypothesis:
Hemoglobin A1c level may predict short‐term outcome in patients with ACS.
Methods:
We conducted a retrospective study with prospective follow‐up in 317 diabetic patients with ACS. Patients were stratified into 2 groups based on HbA1c level, checked within 8 weeks of the index admission (optimal control group, HbA1c ≤7%; suboptimal control group, HbA1c >7%). All patients were followed up prospectively for major adverse cardiovascular events (MACE) and mortality for 6 months. Short‐term clinical outcomes were also compared between the 2 study groups.
Results:
In our cohort, 27.4%, 46.4%, and 26.2% patients had unstable angina, non–ST‐segment elevation myocardial infarction, and ST‐segment elevation myocardial infarction, respectively. In‐hospital mortality was similar in both HbA1c groups (3.37% vs 2.88%, P = 0.803). Six‐month MACE was also similar (26.40% vs 26.47%, P = 0.919). All‐cause mortality, cardiovascular mortality, symptom‐driven revascularization, rehospitalization for angina, and hospitalization for heart failure were also similar in both groups. The hazard ratios for 6‐month MACE and individual endpoints were also similar in both groups.
Conclusions:
This study suggests that HbA1c levels before admission are not associated with short‐term cardiovascular outcome in diabetic patients subsequently admitted with ACS. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Diabetes mellitus (DM) is associated with high risk of coronary heart disease and a higher risk of death after acute myocardial infarction (MI).1, 2, 3, 4 Numerous trials have shown that blood glucose concentration on admission is a prognostic factor of short‐term and long‐term mortality in acute coronary syndromes (ACS) in both nondiabetic and diabetic patients.5, 6, 7, 8, 9, 10, 11 Although admission glucose has good prognostic value on outcome in ACS, it may be affected by meals, the circadian cycle, and also the stress response. Glycated hemoglobin A1c (HbA1c) is a better marker of diabetic control, as it provides a good reflection of plasma glucose concentrations over 8 to 12 weeks with no effect from meals or the circadian cycle.
The value of admission HbA1c level in patients with ACS has been studied in a limited number of small‐scale trials.12, 13, 14 In addition, the role of optimal DM control as reflected by HbA1c levels on short‐term outcomes in diabetic patients with ACS has not yet been defined. Therefore, the primary objective of this study is to define the relationship between HbA1c levels (ie, DM control) and the short‐term outcome in diabetic patients with ACS in a larger population size.
Methods
Patient Characteristics
Consecutive patients admitted to our hospital for suspected ACS between January 1, 2008, and December 31, 2009, were eligible in this prospective follow‐up study. All hospitalized patients are screened for suspected ACS on the basis of admission diagnoses. The whole spectrum of ACS, including unstable angina, non–ST‐segment elevation myocardial infarction (NSTEMI), and ST‐segment elevation MI (STEMI), was studied.
All diabetic patients, including undiagnosed and known DM with the diagnosis of ACS, were included. The diagnosis of ACS was based on American College of Cardiology (ACC)/American Heart Association (AHA) guidelines. Analysis of HbA1c on admission was done in every patient with ACS and a known history of DM. However, HbA1c level within 8 weeks of the index admission was an acceptable figure for analysis, although this was not recommended. The HbA1c level on admission was also done in every patient with random glucose >11.1 mmol/L or fasting glucose >7.0 mmol/L to include patients with undiagnosed DM. The measurement of HbA1c was done by the National Glycohemoglobin Standardization Program–certified PDQ Plus Ultra 2 System (Primus Diagnostics, Kansas City, MO) by boronate affinity chromatography.
Patients were classified as having known DM before enrollment in the study if their medical records contained documentation of past history of DM or past laboratory results compatible with the diagnosis of DM, according to the American Diabetes Association (ADA) 2010 Revised Clinical Practice Guidelines for diabetes diagnosis.15 The diagnosis was also established if the patient had been informed of the diagnosis by a physician before the admission or was on oral antihyperglycemic agents, insulin, or diet therapy. The definition of known DM was regardless of duration of disease or the need for antidiabetic agents. However, admission glucose was not used as the sole diagnostic criterion for DM, as it may be affected by stress response. The diagnosis of “undiagnosed diabetes mellitus” was made if patients with fasting glucose >7.0 mmol/L or random glucose >11.1 mmol/L together with an admission HbA1c >6.5% according to the latest ADA recommendations.15
A total of 926 patients with ACS were screened. All diabetic patients without HbA1c checked within 8 weeks before admission or during the index admission period were excluded, resulting in a sample size of 317 patients for analysis.
Data Collection
Clinical information from each patient was gathered through review of clinical notes and charts. Demographic data and past medical history, including cardiovascular (CV) risk factors and comorbidities, were collected. The investigation results including blood tests and electrocardiographic findings were also recorded. The type of reperfusion therapy (thrombolytic therapy, percutaneous coronary intervention (PCI), and coronary artery bypass grafting) was documented. For patients managed with an invasive strategy as recommended by the attending cardiologist, diagnostic coronary angiography was performed. In case of significant coronary artery stenosis, PCI or coronary artery bypass grafting was performed according to the ACC Foundation/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons/American Association for Thoracic Surgery/AHA/American Society of Nuclear Cardiology Appropriateness Criteria for Coronary Revascularization.16 Drug treatments before admission, during the hospital stay, and also at discharge were recorded.
All patients were followed up for ≥6 months by telephone or clinic assessment. Scheduled follow‐up was conducted not later than 6 months post‐discharge. For all deceased patients, cause of death was analyzed individually to define CV vs non‐CV death.
Endpoints
The composite primary endpoints of this study were the correlation of HbA1c level with major adverse cardiac events (MACE) at 6 months and in‐hospital death. Six‐month MACE included CV mortality, MI, malignant arrhythmia, cardiac arrest, cardiogenic shock, congestive heart failure, rehospitalization for angina, and rehospitalization for heart failure. Cardiogenic shock was defined as systolic blood pressure <90 mm Hg or a drop of mean arterial pressure >30 mm Hg with a pulse >60 beats per minute to exclude shock secondary to bradycardia and/or low urine output (<0.5 mL/kg/h) with or without evidence of organ congestion.17 Malignant arrhythmia was defined as symptomatic sustained ventricular tachycardia and also ventricular fibrillation, irrespective of symptoms or hemodynamic stability. Secondary endpoints included 6‐month CV mortality rate, 6‐month all‐cause mortality rate, 6‐month rehospitalization for angina, 6‐month rehospitalization for heart failure, and 6‐month symptom driven coronary artery revascularization.
Statistical Analysis
We used SPSS version 17.0 (SPSS Inc., Chicago, IL) for statistical analysis. For the purpose of present analysis, patients were divided into 2 groups based on admission HbA1c: group 1, HbA1c >7% (the suboptimal DM control group) and group 2, HbA1c ≤7% (the optimal DM control group). In addition, the analysis was applied without separating into groups, but according to the exact HbA1c level as a continuous variable.
Continuous variables were presented as mean ± SD and categorical variables were presented as number of patients and percentage. Baseline characteristics of the 2 groups were compared using the χ 2 test or the Fisher exact test for categorical variables and the Student unpaired t test for continuous variables, as appropriate. The date of all‐cause mortality, CV‐related death, and MACE were recorded. Univariate Cox regression modeling followed by Cox multivariate analyses were used to determine predictors of 6‐month MACE, 6‐month all‐cause mortality, and 6‐month CV mortality. Significant variables were entered in a forward stepwise manner if the univariate analysis of the defined study group was statistically significant. The significance level was set at P<0.05. The hazard ratio (HR) of the study group related to 6‐month MACE, 6‐month all‐cause mortality, and 6‐month CV mortality, and other secondary endpoints were calculated. The estimate of the predictive effect of HbA1c in continuous variable manner was also tested by similar stepwise model for the primary endpoint. Six‐month event‐free survival was estimated by the Kaplan‐Meier method and was compared with the log‐rank test.
Results
Demographic Data
The baseline patient characteristics are shown in Table 1. No significant differences were found between the 2 groups, except that more patients in the optimal DM control group had hypertension, previous stroke/transient ischemic attack, and renal impairment. About 6% of patients in both groups had undiagnosed DM. The mean HbA1c in undiagnosed DM patients was 6.67% ± 0.155% in the optimal control group and 9.09% ± 1.7% in the suboptimal control group. Other potential clinical outcome predictors (eg, type of ACS, vital signs, and blood tests on admission) were also well‐balanced, except there was a higher admission glucose level in the suboptimal DM control group and a higher creatinine level in the optimal control group.
Table 1.
Patient Baseline Characteristics.
| Variable | HbA1c ≤ 7.0% n = 178 | HbA1c > 7.0% n = 139 | P value |
|---|---|---|---|
| Demographics | |||
| Age, Mean (SD) Years | 70.83 ± 10.754 | 68.98 ± 11.793 | NS |
| Male, n (%) | 95 (53.73%) | 75 (53.96%) | NS |
| Cardiovascular risk factors | |||
| Active Smoker, n (%) | 20 (11.24%) | 24 (17.27%) | NS |
| Hypertension, n (%) | 147 (82.58%) | 96 (69.06%) | 0.005 |
| Hyperlipidemia, n (%) | 58 (32.58%) | 55 (39.57%) | NS |
| Known Diabetes, n (%) | 167 (93.82%) | 130 (93.52%) | NS |
| Undiagnosed Diabetes, n (%) | 11 (6.18%) | 9 (6.47%) | NS |
| Previous IHD, n (%) | 70 (39.32%) | 60 (43.17%) | NS |
| Previous MI, n (%) | 22 (12.36%) | 21 (15.11%) | NS |
| Previous CHF, n (%) | 22 (12.36%) | 15 (10.79%) | NS |
| Previous Coronary revascularization therapy, n (%) | 30 (16.85%) | 27 (19.42%) | NS |
| Previous Stroke/TIA, n(%) | 36 (20.22%) | 14 (10.07%) | 0.014 |
| Documented History of PVD, n (%) | 9 (5.06%) | 3 (2.16%) | NS |
| History of Renal impairment, n (%) | 34 (19.1%) | 15 (10.79%) | 0.042 |
| Type of Acute coronary syndrome | |||
| Unstable Angina, n (%) | 53 (29.78%) | 34 (24.46%) | NS |
| NSTEMI, n (%) | 85 (47.75%) | 62 (44.6%) | NS |
| STEMI, n (%) | 40 (22.47%) | 43 (30.94%) | NS |
| Vital sign on admission | |||
| Admission Systolic Blood Pressure, (mmHg) | 150.07 ± 34.353 | 147.35 ± 30.132 | NS |
| Admission Diastolic Blood Pressure, (mmHg) | 77.05 ± 19.462 | 77.25 ± 18.749 | NS |
| Admission Heart Rate, (BPM) | 86.85 ± 23.205 | 82.68 ± 19.091 | NS |
| Lab Result | |||
| HbA1c (%) | 6.226 ± 0.5468 | 8.486 ± 1.3733 | <0.001 |
| Hemoglobin, (g/dl) | 12.181 ± 2.3112 | 13.178 ± 2.0944 | NS |
| Albumin, (g/l) | 38.563 ± 7.1672 | 39.419 ± 5.6524 | NS |
| Total Cholesterol, (mmolŁ) | 4.4231 ± 1.1736 | 4.6574 ± 1.0998 | NS |
| Triglycerides, (mmol/L) | 1.7676 ± 1.0626 | 1.9247 ± 1.0524 | NS |
| HDL Cholestrol, (mmol/L) | 1.2104 ± 0.4735 | 1.1209 ± 0.3034 | NS |
| LDL Cholestrol, (mmol/L) | 2.4901 ± 1.0377 | 2.6969 ± 0.9761 | NS |
| Fasting glucose, (mmol/L) | 7.347 ± 2.3547 | 10.409 ± 3.5757 | 0.001 |
| Admission Glucose (mmol/L) | 9.809 ± 4.6496 | 13.720 ± 3.5757 | 0.012 |
| TnT Peak, | 1.4206 ± 3.3952 | 1.4596 ± 3.3287 | NS |
| Creatinine, (umol/l) | 177 ± 185.5663 | 126.633 ± 113.1787 | 0.008 |
Abbreviations: CHF, congestive heart failure; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein cholesterol; IHD, ischemic heart disease; LDL, low‐density lipoprotein cholesterol; MI, myocardial infarction; NS, not significant; NSTEMI, non–ST‐segment elevation myocardial infarction; PVD, peripheral vascular disease; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack; TnT, troponin T.
The treatment modality, coronary anatomy, left ventricular systolic function, and medication usage before admission were also well‐balanced in both groups, as shown in Table 2.
Table 2.
Medication Before Admission, Treatment Modality, Coronary Anatomy, Left Ventricular Systolic Function
| Variable | HbA1c ≤ 7.0% n = 178 | HbA1c > 7.0% n = 139 | P value |
|---|---|---|---|
| Medication before admission | |||
| Aspirin before admission, n (%) | 99 (55.62%) | 60 (43.17%) | 0.028 |
| Clopidigrel before admission, (n%) | 4 (2.25%) | 6 (4.31%) | NS |
| Statin, n(%) | 73 (41.01) | 46 (33.09) | NS |
| Anti‐diabetic therapy, n (%) | 112 (62.92%) | 95 (68.35%) | NS |
| Beta‐blocker, n (%) | 82 (46.07%) | 51 (36.69%) | NS |
| Angiotensin converting enzyme inhibitor, n(%) | 77 (43.26%) | 54 (38.85%) | NS |
| Angiotensin II receptor antagonist, n (%) | 9 (5.06%) | 12 (8.63%) | NS |
| Treatment Modality | |||
| Convervative, n (%) | 71 (39.89%) | 54 (38.85%) | NS |
| PCI, n (%) | 103 (57.87%) | 81 (58.27%) | NS |
| CABG, n (%) | 4 (2.24%) 4 (2.88%) | NS | |
| Coronary Anatomy | |||
| LMS Disease, n (%) | 11 (6.18%) | 8 (5.76%) | NS |
| One vessel disease, n (%) | 40 (22.47%) | 24 (17.27%) | NS |
| Two vessel disease, n (%) | 32 (17.98%) | 25 (17.99%) | NS |
| Three vessel disease, n (%) | 24 (13.48%) | 28 (20.14%) | NS |
| Left Ventricular Systolic Function | |||
| Ejection Fraction, (%) mean ± SD | 50.32% ± 14.48 | 50.32% ± 16.47 | NS |
Abbreviations: CABG, coronary artery bypass grafting; HbA1c, glycated hemoglobin; LMS, left main stem; NS, not significant; PCI, percutaneous coronary intervention; SD, standard deviation.
Outcomes
As shown in Figure 1, there was no significant difference between the 2 groups in the primary endpoint, 6‐month MACE rate. In addition, there were no differences in the rate of individual endpoints between the 2 groups. Apart from that, there were also no significant differences in the HR between the 2 groups for 6‐month MACE and individual endpoints, as shown in Table 3. The MACE‐free Kaplan‐Meier survival curve also did not demonstrate significant difference between 2 study groups (Figure 2).
Figure 1.
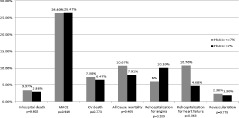
Rates of in‐hospital mortality, 6‐month MACE, 6‐month CV death, rehospitalization for angina, rehospitalization for heart failure, and symptom‐driven revascularization in both groups. Abbreviations: CV, cardiovascular; HbA1c, glycated hemoglobin; MACE, major adverse cardiovascular events.
Table 3.
Hazard Ratio for Primary and Secondary Endpoints in Optimal DM Control Group Compared With Suboptimal Control Group
| Hazard Ratio (95% C.I.) | |
|---|---|
| 6‐month MACE | 1.041 (0.674–16.07) p = 0.856 |
| In‐hosptial all cause mortality | 1.177 (0.326–4.256) p = 0.803 |
| 6‐month cardiovasular mortality | 1.041 (0.674–16.07) p = 0.856 |
| 6‐month all cause mortality | 1.36 (0.647–2.857) p = 0.417 |
| Rehospitalisation for angina | 0.576 (0.243–1.367) p = 0.211 |
| Rehospitalisation for heart failure | 2.263 (0.892–5.739) p = 0.086 |
| Symptom driven revascularisation | 0.795 (0.16–3.938) p = 0.779 |
Abbreviations: CI, confidence interval; MACE, major adverse cardiovascular events.
Figure 2.
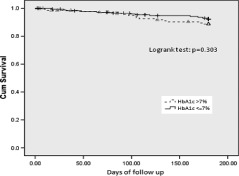
MACE‐free survival curve. Abbreviations: Cum, cumulative; HbA1c, glycated hemoglobin; MACE, major adverse cardiovascular events.
In addition to the categorical assessment, HbA1c was analyzed as a continuous variable. However, it also failed to predict the primary endpoint (ie, 6‐month MACE). The calculated HR was 1.013 (95% CI: 0.876–1.170, P = 0.866). It also did not predict 6‐month CV death and all‐cause mortality. The HR for 6‐month CV death was 0.814 (95% CI: 0.582–1.137, P = 0.227), and the HR for 6‐month all‐cause mortality was 0.717 (95% CI: 0.448–1.147, P = 0.166).
The admission glucose level was studied in our cohort. Patients were stratified into 2 groups, with admission glucose <11.1 and ≥11.1. Multivariate Cox regression analysis showed that admission hyperglycemia was an independent predictor for 30‐day MACE (HR: 3.541, 95% CI: 1.32–9.501, P = 0.012).
Discussion
The main finding of the current study is that HbA1c within 8 weeks of admission is not the predictor of short‐term outcome in our cohort. However, admission hyperglycemia is a significant independent predictor of 6‐month MACE. Our study detected similar rates of in‐hospital death and MACE between the 2 groups in the 6‐month follow‐up period. And there was no significant correlation between HbA1c levels and short‐term outcome (ie, in‐hospital death, composite MACE, and CV death in 6 months), despite the fact that the analysis was performed as a continuous variable. Although there was a slight difference in the baseline characteristics between the 2 groups (eg, renal impairment), this observation might be a random event. In order to address this issue, these factors had been included in the analysis of the primary endpoint. The result suggests that optimal diabetic control 8 weeks before admission is not a major predictor for short‐term clinical outcome.
Numerous previous reports5, 6, 7, 8, 9, 10, 11 have found that elevated admission glucose levels were associated with adverse short‐term outcome in patients presenting with ACS. The predictive effect of admission glucose level is valid across the whole spectrum of patients presenting with ACS, including elderly patients,9 and also irrespective of the treatment modality, whether primary PCI17 or lytic9 or conservative mangement.13 This observation was further confirmed by a large multinational observational registry, the Global Registry of Acute Coronary Events (GRACE).18 In GRACE, admission glucose and fasting glucose levels were proved to predict in‐hospital mortality. The relationship was extended to patients presenting with STEMI, NSTEMI, and unstable angina. However, a high glucose level may only be the marker of stress hyperglycemia, and not represent the general glucometabolic state.
The measurement of glycated forms of hemoglobin provides a reliable reflection of the degree of general glucometabolic state in the previous 8–12 weeks. It serves as a marker for diabetic control. There has been conflicting evidence about the prognostic value of HbA1c levels on short‐term outcomes in ACS. The prognostic relationship between HbA1c and mortality after STEMI in patients with or without DM has also been demonstrated in a small‐scale trial.12 However, other trials, which included diabetic and nondiabetic patients, did not show such a relationship.13, 14 In our study, we focused on diabetic patients only. After the modification of the diagnostic criteria of DM in the latest 2010 ADA recommendation, our study was able to include undiagnosed diabetic patients, which was not possible in the past.
This study set out to determine whether or not optimal diabetic control (ie, HbA1c ≤7%) will improve short‐term outcome in ACS. This cutoff value is the same as the current definition of optimal diabetic control recommended by major clinical guidelines. However, there was no significant correlation between optimal diabetic control and short‐term outcomes of ACS. This observation echoes the conclusion of the Diabetes Control and Complications Trial (DCCT)19, 20 and the United Kingdom Prospective Diabetes Study (UKPDS)21, 22 that longer time is need to obtain the macrovascular benefit from tight diabetic control. Our study also assessed the potential correlation of clinical outcome and HbA1c level as a continuous variable. However, it also failed to predict clinical outcomes.
Although there a large body of data to demonstrate that admission and fasting glucose levels are related to prognosis in ACS, this appears not to be the case for HbA1c levels, as shown in our study. Our study added further information to the understanding of the prognostic value of glucose level in ACS. The HbA1c level is a reflection of the general glucometabolic state and is not affected by acute stress and also acute glucose management. Because our study suggested that HbA1c level is not a marker for short‐term clinical outcome, poor short‐term clinical outcome associated with a high glucose level should be explained by mechanisms other than abnormal general glucometabolism. Thus, high glucose levels in patients with poor outcome may only be a marker of a stress response, instead of a precipitating factor of the poor outcome.
Study Limitations
The retrospective design constitutes the major limitation of this study. This study was an observational and nonrandomized study. Only patients with HbA1c levels checked 8 weeks before or during the index admission were included. This might result in selection bias. However, it was the standard practice to check HbA1c in every diabetic patient to optimize patient management. Therefore, most of the diabetic patients were included, except for those undiagnosed diabetic patients. Apart from that, the study population was relatively small. This might result in inadequate power to detect a slight difference in clinical outcomes between the 2 groups. Therefore, it will be more informative if a larger sample size is studied.
Conclusion
In conclusion, this study suggests that HbA1c levels before admission are not associated with short‐term CV outcome in diabetic patients subsequently admitted with ACS.
References
- 1. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA. 1979;241:2035–2038. [DOI] [PubMed] [Google Scholar]
- 2. Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. [DOI] [PubMed] [Google Scholar]
- 3. Behar S, Boyko V, Reicher‐Reiss H, et al. Ten‐year survival after acute myocardial infarction: comparison of patients with and without diabetes: Secondary Prevention Reinfarction Israeli Nifedipine Trial. Am Heart J. 1997;133:290–296. [DOI] [PubMed] [Google Scholar]
- 4. Rydén L, Strandl E, Bartnik M, et al. Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases: executive summary. The Task Force of Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28:88–136. [DOI] [PubMed] [Google Scholar]
- 5. Wahab NN, Cowden EA, Pearce NJ, et al. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748–1754. [DOI] [PubMed] [Google Scholar]
- 6. Schiele F, Descotes‐Genon V, Seronde MF, et al. Predictive value of admission hyperglycaemia on mortality in patients with acute myocardial infarction. Diabet Med. 2006;23:1370–1376. [DOI] [PubMed] [Google Scholar]
- 7. Aronson D, Hammerman H, Kapeliovich MR, et al. Fasting glucose in acute myocardial infarction—incremental value for long‐term mortality and relationship with left ventricular systolic function. Diabetes Care. 2007;30:960–966. [DOI] [PubMed] [Google Scholar]
- 8. Ainla T, Barburin A, Teesalu R, et al. The association between hyperglycemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med. 2005;22:1321–1325. [DOI] [PubMed] [Google Scholar]
- 9. Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–3086. [DOI] [PubMed] [Google Scholar]
- 10. Dziewierz A, Giszterowicz D, Siudak Z, et al. Impact of admission glucose level and presence of diabetes mellitus on mortality in patients with non–ST‐segment elevation acute coronary syndrome treated conservatively.Am J Cardiol. 2009;103:954–958. [DOI] [PubMed] [Google Scholar]
- 11. Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. [DOI] [PubMed] [Google Scholar]
- 12. Cakmak M, Cakmak N, Cetemen S, et al. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol. 2008;24:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hadjadj S, Coisne D, Mauco G, et al. Prognostic value of admission plasma glucose and HbA in acute myocardial infarction. Diabet Med. 2005;22:509–510. [DOI] [PubMed] [Google Scholar]
- 14. Timmer JR, Ottervanger JP, Bilo HJ, et al. Prognostic value of admission glucose and glycosylated haemoglobin levels in acute coronary syndromes. QJM. 2006;99:237–243. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association .Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel MR, Dehmer GJ, Hirshfeld JW, et al. 2009. ACCF/SCAI/ STS/AATS/AHA/ASNC Appropriateness Criteria for Coronary Revascularization. J Am Coll Cardiol. 2009;53:530–553. [DOI] [PubMed] [Google Scholar]
- 17. Lavi S, Kapeliovich M, Gruberg L, et al. Hyperglycemia during acute myocardial infarction in patients who are treated by primary percutaneous coronary intervention: impact on long‐term prognosis. Int J Cardiol. 2008;123:117–122. [DOI] [PubMed] [Google Scholar]
- 18. Sinnaeve PR, Steg PG, Fox KA, et al; GRACE Investigators . Association of elevated fasting glucose with increased short‐term and 6‐month mortality in ST‐segment elevation and non–ST‐segment elevation acute coronary syndromes. Arch Intern Med. 2009;169:402–409. [DOI] [PubMed] [Google Scholar]
- 19. Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 20. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352: 837–853. [PubMed] [Google Scholar]
- 22. Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359: 1577–1589. [DOI] [PubMed] [Google Scholar]


