Abstract
Background:
Studies investigating the clinical outcome of intravascular ultrasound (IVUS)‐guided primary percutaneous coronary intervention (PPCI) in patients with ST‐segment elevation myocardial infarction (STEMI) show conflicting results. The aim of our study was to evaluate whether IVUS‐guidedPPCI with drug‐eluting stents (DESs) in STEMI patients improves clinical outcome.
Hypothesis:
IVUS‐guided PPCI is superior to angio‐guided PPCI.
Methods:
Three hundred forty‐one patients who underwent PPCI for STEMI and survived the hospitalization were enrolled in this study. Two hundred sixteen (63.3%) patients were treated with angio‐guided PPCI and 125 (36.7%) patients were treated with IVUS‐guided PPCI. The primary endpoint was defined as the composite of death, myocardial infarction, target vessel revascularization, and target lesion revascularization at the 3‐year follow‐up visit.
Results:
Male gender, dyslipidemia, and smoking were frequent in the IVUS‐guided PPCI group. These patients had a higher rate of radial approach, adjunctive ballooning, thrombectomy, and the use of a glycoprotein IIb/IIIa inhibitor. The number and length of implanted stents were higher in the IVUS‐guided PPCI group. The primary end point (18.1% vs 12.8%, P = 0.22) and stent thrombosis (2.8% vs 2.4%, P = 1.00) was not different between the groups.
Conclusions:
In our observational study, IVUS‐guided PPCI with DESs in patients with STEMI did not improve clinical outcome or stent thrombosis. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Although the benefit of drug‐eluting stents (DESs) compared with bare‐metal stents in the treatment of coronary artery stenosis has been demonstrated in many studies, DESs are not free of restenosis and are limited by late stent thrombosis.1, 2, 3, 4, 5
Several intravascular ultrasound (IVUS) studies have shown that suboptimal deployment of DESs, including stent malapposition, incomplete stent expansion, and smaller minimal stent area correlated with restenosis and stent thrombosis.6, 7, 8, 9, 10 However, studies investigating whether IVUS‐guided percutaneous coronary intervention (PCI) affected the clinical outcomeof patients with ST‐segment elevation myocardial infarction (STEMI) showed conflicting results. Recently, Roy et al reported that IVUS‐guided PCI reduced both DES thrombosis and the need for repeat revascularization in all‐comer patients undergoing DES implantation. However, this study included only 17% of patientswith STEMI.11 On the contrary, Maluenda et al reported that IVUS‐guided PCI did not improve clinical outcome in STEMI patients.12
Because the role of IVUS‐guided PPCI with DESs in patients with STEMI remains unclear, we evaluated whether this modality could improve the clinical outcome and stent thrombosis in patients treated at our center.
Methods
Study Population and Design
Three hundred sixty‐sevenpatients were enrolled from an ongoing registry of consecutive patients who underwent DES implantation for STEMI from May 2003 to December 2008 at Wonju Christian Hospital. Of these, the 341 patients who survived hospitalization were assessed for clinical outcome to avoid potential selection bias imposed by the use of IVUS in the acute PCI scenario. Two hundred sixteen (63.3%) patients were treated with angiographic‐guided PPCI, and 125 (36.7%) patients were treated with IVUS‐guided PPCI. This data set is presented in Figure 1.
Figure 1.
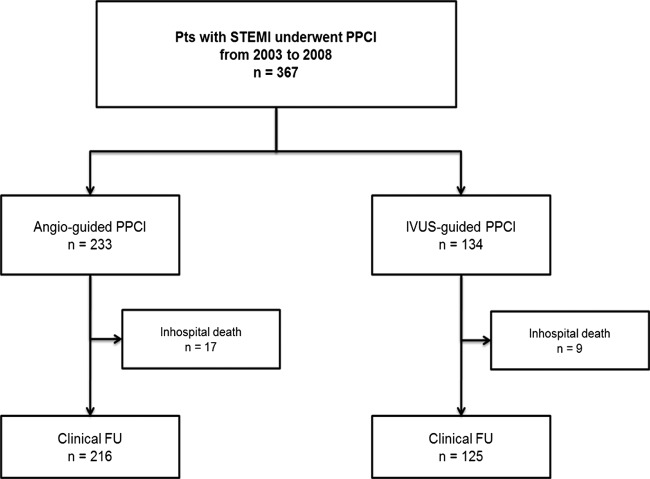
Data set for study population. Abbreviations: FU, follow‐up; IVUS, intravascular ultrasound; PPCI, primary percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Study patients were followed up for 3 years in this observational study. All patients provided written informed consent for the PCI procedure. This study was approved by our institutional review board.
Procedures and Adjunctive Medical Therapy
PCI was performed using the standard technique and via the radial approach, except in those with cardiogenic shock, those requiring large bore catheters, and those with contraindications to the radial approach (absence of radial artery pulse, negative Allen's test, ipsilateral renal dialysis fistula, known atherosclerosis, known severe tortuosity of upper extremity arteries, or Raynaud's disease). All patients received DESs, including sirolimus‐eluting stents (SESs)(Cypher, Cordis; Johnson & Johnson, New Brunswick, NJ), paclitaxel‐eluting stents (Taxus; Boston Scientific, Natick, MA or Pico Elite; amg International GmbH, Raesfeld‐Erle, Germany), zotarolimus‐eluting stents (Endeavor Sprint or Endeavor Resolute; Medtronic, Minneapolis, MN), and everolimus‐eluting stents (Xience; Abbott Laboratories, Abbott Park, IL or Promus; Boston Scientific). We defined the Cypher stent and Taxus stent as a 1st generation DES and the others as 2nd generation DESs. All patients received aspirin (325 mg) and clopidogrel (600 mg) before PCI. Dual antiplatelet therapy was recommended to all patients for a minimum of 1 year postprocedure. During PCI, a fixed dose of unfractionated heparin (10000 U) was administered. Additional heparin (unfractionated heparin or enoxaparin), a glycoprotein IIb/IIIa inhibitor, and thrombus aspiration were given at the operator's discretion. IVUS was performed using the standard technique, before stent implantation, after stent implantation, or both at the operator's discretion. Prestent IVUS use was defined as any IVUS use before stent implantation. This could be performed before or after preballooning. Prestent IVUS was used to evaluate the characterization of plaque and to estimate the reference diameter or the length of the lesion. Poststent IVUS use was defined as any IVUS use after stent implantation. Poststent IVUS was used to detect suboptimal stent deployment, such as stent malapposition, stent underexpansion, or edge dissection. According to the results of the poststent IVUS, additional ballooning or stenting was performed. One of the 2 commercially available systems: iLab (Boston Scientific) or Eagle Eye (Volcano Therapeutics, San Diego, CA) was used. IVUS images were interpreted by attending physicians or experienced IVUS technicians. Response to IVUS, including adjunctive ballooning and deployment of additional stents, was also performed at the operator's discretion.
Clinical End Point and Definitions
The primary endpoint was defined as the composite of death, myocardial infarction, target vessel revascularization (TVR), and target lesion revascularization (TLR) at the 3‐year follow‐up appointment (major adverse cardiac event [MACE]). Death was defined as mortality from all causes. Myocardial infarction was defined as an elevation in creatine kinase‐MB greater than or equal to twice the upper limit of normal (5 ng/mL) or new ST‐segment elevation on electrocardiography in 2 or more contiguous leads after discharge. TVR was defined as a percutaneous or surgical revascularization of the stented lesion, including 5‐mm margin segments, and more proximal or distal newly developed lesions. TLR was defined as a percutaneous or surgical revascularization of the stented lesion and 5‐mm segments immediately proximal and distal to the stent. Stent thrombosis (ST) was classified by the Academic Research Consortium definition of definite, probable, or possible. The definition of definite ST required the presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion. Probable ST included unexplained death 30 days after the procedure or acute myocardial infarction involving the target vessel territory without angiographic confirmation. Possible ST included unexplained death after 30 days. Angiographic success was defined as a residual stenosis of <30% with thrombolysis in myocardial infarction (TIMI) 3 flow.
Data Collection and Follow‐Up
Demographic, clinical, and procedural data and in‐hospital outcomes were collected and entered into a prospective database. These data were obtained from hospital chart review and angiography review by independent research personnel blinded to the study objectives. Clinical follow‐up was collected by chart review, telephone contact, or revisit.
Statistics
Statistical analysis was performed using SPSS version 15 (SPSS, Inc., Chicago, IL). Continuous variables were expressed as the mean ± standard deviation or as the median with interquartile range. Categorical variables were expressed as absolute numbers and percentages. To compare the groups, the Student unpaired t test was used for continuous variables, and the χ 2 test or Fisher exact test was used for categorical variables. The Kaplan‐Meier log‐rank test was used to compare the incidence of MACE and stent thrombosis between 2 groups. Multivariate logistic regression analysis was used to determine the predictors for MACE. Variables known to be risk factors for MACE were entered in a forward step‐wise manner into this model. Age; sex; door‐to‐balloon time; ejection fraction; Killip classification; use of cilostazol, β‐blocker, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, and statin at discharge; history of myocardial infarction and smoking; culprit lesion (left anterior descending vs non‐left anterior descending); approach site (radial vs femoral approach); disease extent; American College of Cardiology/American Heart Association (ACC/AHA) lesion type (type A vs type B1‐C); number of implanted stents; total stent length; stent diameter; use of thrombectomy; use of adjuvant balloon; initial TIMI flow grade (TIMI flow grade 0–1 vs TIMI flow grade 2–3); final TIMI flow grade (TIMI flow grade 3 vs TIMI flow grade 0–2); implanted stent class (1st generation DES vs 2nd generation DES); and use of IVUS were included in the model for MACE at 3 years. The same variables except medications at discharge were included in the model for in‐hospital mortality. A P value of <0.05 was considered to be statistically significant.
Results
Patient Characteristics
Clinical characteristics are presented in Table 1. More males underwent IVUS‐guided PPCI (63.0% vs 74.4%, P = 0.032), but age was similar between the 2 groups. Dyslipidemia (11.1% vs 22.4%, P = 0.007) and smoking (57.9% vs 75.2%, P = 0.002) were frequent in the IVUS‐guided PPCI group, but other medical history was similar. Laboratory examination and blood pressure at admission was similar between the groups, but heart rate was higher in the IVUS‐guided PPCI group (74 ± 23 bpm vs 80 ± 22 bpm, P = 0.038). Left ventricular ejection pressure measured by echocardiography was higher in the angio‐guided PPCI group (48.0 ± 11.5% vs 45.1 ± 9.1%, P = 0.009). Killip classification and symptom‐to‐door time was similar between the groups. Discharge medications were similar between the 2 groups, but the use of cilostazol was frequent in the angio‐guided PPCI group (17.6% vs 7.2%, P = 0.009).
Table 1.
Patient Demographics and Clinical Characteristics
| Angio‐Guided PPCI n = 216 | IVUS‐Guided PPCI n = 125 | P Value | |
|---|---|---|---|
| Male sex, n (%) | 136 (63.0) | 93 (74.4) | 0.032 |
| Age, y | 61.4 ± 11.6 | 60.0 ± 12.9 | 0.259 |
| Past history, n (%) | |||
| Diabetes | 71 (32.9) | 34 (27.2) | 0.330 |
| Hypertension | 111 (51.4) | 64 (50.4) | 0.911 |
| Dyslipidemia | 24 (11.1) | 28 (22.4) | 0.007 |
| Myocardial infarction | 13 (6.0) | 12 (9.6) | 0.281 |
| Previous PCI | 12 (5.6) | 13 (10.4) | 0.130 |
| Previous CABG | 2 (0.9) | 0 (0) | 0.534 |
| Smoking | 125 (57.9) | 94 (75.2) | 0.002 |
| Congestive heart failure | 4 (1.9) | 3 (2.4) | 0.710 |
| Cerebral infarction | 16 (7.4) | 6 (4.8) | 0.493 |
| Chronic renal insufficiency | 6 (2.8) | 3 (2.4) | 1.000 |
| Laboratory tests at admission | |||
| TC, mg/dL | 178.0 ± 42.0 | 172.6 ± 40.0 | 0.407 |
| TG, mg/dL | 134.8 ± 105.3 | 128.4 ± 90.1 | 0.690 |
| HDLC, mg/dL | 44.3 ± 10.9 | 43.9 ± 10.9 | 0.797 |
| LDLC, mg/dL | 103.1 ± 38.2 | 103.3 ± 36.3 | 0.980 |
| CK‐MB initial, ng/mL | 37.3 ± 68.3 | 49.2 ± 79.5 | 0.149 |
| Creatine, mg/dL | 1.02 ± 0.94 | 1.01 ± 0.78 | 0.874 |
| Hb, g/dL | 14.2 ± 3.3 | 15.3 ± 13.6 | 0.255 |
| hs‐CRP, mg/dL | 1.95 ± 3.82 | 1.77 ± 3.72 | 0.757 |
| BNP, pg/dL | 128.9 ± 336.1 | 138.6 ± 284.2 | 0.824 |
| Initial vital sign | |||
| SBP, mmHg | 122 ± 37 | 125 ± 35 | 0.569 |
| DBP, mmHg | 74 ± 22 | 76 ± 24 | 0.521 |
| HR, beat/min | 74 ± 23 | 80 ± 22 | 0.038 |
| LVEF by echocardiography, % | 48.0 ± 11.5 | 45.1 ± 9.1 | 0.009 |
| Killip classification II or more, n (%) | 51 (23.6) | 25 (20.0) | 0.500 |
| Symptom‐to‐door time, min, median (IQR) | 180 (109, 305) | 154 (85, 305) | 0.994 |
| Discharge medication, n (%) | |||
| Aspirin | 216 (100.0) | 124 (99.2) | 0.367 |
| Clopidogrel | 215 (99.5) | 124 (99.2) | 1.000 |
| Cilostazol | 38 (17.6) | 9 (7.2) | 0.009 |
| Statin | 119 (55.1) | 75 (60.0) | 0.427 |
| β‐blocker | 139 (64.4) | 86 (68.8) | 0.477 |
| ACEI or ARB | 148 (68.5) | 81 (64.8) | 0.550 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass graft; CK‐MB; creatine kinase‐MB; DBP, diastolic blood pressure; Hb, hemoglobin; HDLC, high‐density lipoprotein cholesterol; HR, heart rate; hs‐CRP, high sensitive CRP; IQR, interquartile range; IVUS, intravascular ultrasound; LDLC, low‐density lipoprotein cholesterol; LVEF, left‐ventricular ejection fraction; PCI, percutaneous coronaryintervention; PPCI, primary percutaneous coronary intervention; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Angiographic and Procedural Characteristics
Angiographic and procedural details are presented in Table 2. Distribution of the culprit lesion, disease extent, and ACC/AHA lesion type were not different between the 2 groups. Compared to angio‐guided PPCI, patients in the IVUS‐guided PPCI group had a higher rate of radial approach (74.1% vs 87.7%, P = 0.004) and adjunctive balloon (33.7% vs 54.9%, P<0.001). More numbers (1.2 ± 0.5 vs 1.4 ± 0.6, P = 0.010), longer length (29.5 ± 10.2 mm vs 34.8 ± 10.1 mm, P<0.001), and a larger‐diameter (3.03 ± 0.41 mm vs 3.18 ± 0.48 mm, P = 0.004) DES was used in the IVUS‐guided PPCI group despite the similarity of quantitative coronary angiography (QCA) at preintervention. These resulted in a larger minimal luminal diameter (2.75 ± 0.43 mm vs 2.87 ± 0.47 mm, P = 0.021) and smaller residual stenosis (14.3 ± 8.4% vs 10.7 ± 6.9%, P<0.001) at postintervention by QCA. SES was more frequently used in the angio‐guided PPCI group (62.5% vs 21.6%, P<0.001). The use of a glycoprotein IIb/IIIa inhibitor (2.8% vs 16.0%, P<0.001) and thrombectomy (21.8% vs 43.2%, P<0.001) was more frequently used in the IVUS‐guided PPCI group, but there was no difference in incidence of no or slow reflow phenomena (11.1% vs 13.1%, P = 0.597). Final TIMI flow grade (88.9% vs 86.9%, P = 0.597) and angiographic success rate (82.9%vs 86.9%, P = 0.428) were similar between groups.
Table 2.
Details of Lesions on Coronary Angiography and Intervention
| Angio‐Guided PPCI n = 216 | IVUS‐Guided PPCI n = 125 | P Value | |
|---|---|---|---|
| Lesion characteristics | |||
| Culprit lesion, n (%) | 0.113 | ||
| LAD | 101 (46.8) | 75 (60.0) | |
| LCX | 21 (9.7) | 7 (5.6) | |
| RCA | 92 (42.6) | 42 (33.6) | |
| LM | 2 (0.9) | 1 (0.8) | |
| Disease extent, n (%) | 0.471 | ||
| 1VD | 94 (43.5) | 57 (45.6) | |
| 2VD | 72 (33.3) | 46 (36.8) | |
| 3VD | 50 (23.1) | 22 (17.6) | |
| ACC/AHA type C lesion, n (%) | 163 (75.5) | 102 (81.6) | 0.225 |
| Procedural factors | |||
| Radial approach for PCI, n (%) | 149 (74.1) | 107 (87.7) | 0.004 |
| GP IIb/IIIa inhibitor n (%) | 6 (2.8) | 20 (16.0) | <0.001 |
| Thrombectomy, n (%) | 47 (21.8) | 54 (43.2) | <0.001 |
| IVUS use, n (%) | |||
| Prestent IVUS | 90 (73.8) | ||
| Poststent IVUS | 86 (70.5) | ||
| Adjuvant ballooning n (%) | 67 (33.7) | 67 (54.9) | <0.001 |
| Adjuvant ballooning as result of IVUS n (%) | 41 (33.6) | ||
| Adjuvant ballooning without guide of IVUS n (%) | 67 (33.7) | 26 (21.3) | 0.022 |
| Multivessel PCI, n (%) | 39 (18.1) | 22 (17.6) | 1.000 |
| Stent data | |||
| Implanted DES, n (%) | <0.001 | ||
| SES | 135 (62.5) | 27 (21.6) | |
| PES | 40 (18.5) | 32 (25.6) | |
| ZES | 36 (16.7) | 36 (28.8) | |
| EES | 5 (2.3) | 30 (24.0) | |
| Implanted stent per lesion | |||
| Numbers, n | 1.2 ± 0.5 | 1.4 ± 0.6 | 0.010 |
| Length, mm | 29.5 ± 10.2 | 34.8 ± 10.1 | <0.001 |
| Diameter, mm | 3.03 ± 0.41 | 3.18 ± 0.48 | 0.004 |
| Angiographic data | |||
| Initial TIMI flow grade 0 or 1 n (%) | 149 (71.0) | 102 (82.9) | 0.017 |
| Final TIMI flow grade 3 n (%) | 117 (88.9) | 106 (86.9) | 0.597 |
| Slow or no reflow n (%) | 22 (11.1) | 16 (13.1) | 0.597 |
| Angiographic success, n (%) | 165 (82.9) | 106 (86.9) | 0.428 |
| QCA data | |||
| Preintervention RD, mm | 3.03 ± 0.43 | 3.02 ± 0.52 | 0.837 |
| Preintervention MLD, mm | 0.09 ± 0.24 | 0.11 ± 0.34 | 0.588 |
| Preintervention DS, % | 97.0 ± 8.1 | 96.5 ± 10.2 | 0.654 |
| Postintervention RD, mm | 3.21 ± 0.44 | 3.22 ± 0.51 | 0.841 |
| Postintervention MLD, mm | 2.75 ± 0.43 | 2.87 ± 0.47 | 0.021 |
| Postintervention DS, % | 14.1 ± 8.4 | 10.7 ± 6.9 | <0.001 |
| Postintervention acute gain, mm | 2.66 ± 0.49 | 2.76 ± 0.54 | 0.085 |
| DTB time, median min (IQR) | 92 (62, 135) | 67 (53, 83) | <0.001 |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; DS, diameter stenosis; DTB, door to balloon; EES, everolimus‐eluting stent; GP, glycoprotein; IQR, interquartile range; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main coronary artery; MLD, minimal luminal diameter; PCI, percutaneous coronary intervention; PES, paclitaxel‐eluting stent; PPCI, primary percutaneous coronary intervention; QCA, quantitative coronary analysis; RCA, right coronary artery; RD, reference diameter; SES, sirolimus‐eluting stent; TIMI, thrombolysis in myocardail infarction; VD, vessel disease; ZES, zotarolimus‐eluting stent.
Clinical Outcome
Clinical outcomes are presented in Table 3. Median follow‐up duration was similar between the groups (654 days vs 644 days). MACE rates were similar between the angio‐guided PPCI and IVUS‐guided PPCI groups at 30 days (0.5% vs 1.6%, P = 0.557), 1 year (13.9% vs 9.6%, P = 0.306), and 3‐year follow‐up (18.1% vs 12.8%, P = 0.224). Figure 2 shows the Kaplan‐Meier survival curve for free from MACE over 3 years for both groups. Mortality rate, myocardial infarction, revascularization, and stent thrombosis also showed no difference between the groups.
Table 3.
Clinical Outcome at 3 Years
| Angio‐Guided PPCI, n = 216 | IVUS‐Guided PPCI, n = 125 | P Value | |
|---|---|---|---|
| Follow‐up duration, d, median (IQR) | 654 (253, 1100) | 644 (307, 931) | |
| MACE n (%) | |||
| 30 days | 1 (0.5) | 2 (1.6) | 0.557 |
| 1 year | 30 (13.9) | 12 (9.6) | 0.306 |
| 3 years | 39 (18.1) | 16 (12.8) | 0.224 |
| Death n (%) | |||
| 30 days | 0 | 0 | |
| 1 year | 5 (2.3) | 1 (0.8) | 0.421 |
| 3 years | 8 (3.7) | 1 (0.8) | 0.163 |
| Myocardial infarction n (%) | |||
| 30 days | 0 | 0 | |
| 1 year | 6 (2.8) | 2 (1.6) | 0.715 |
| 3 years | 8 (3.7) | 3 (2.4) | 0.752 |
| Target vessel revascularization n (%) | |||
| 30 days | 1 (0.5) | 2 (1.6) | 0.557 |
| 1 year | 23 (10.6) | 12 (9.6) | 0.854 |
| 3 years | 29 (13.4) | 15 (12.0) | 0.741 |
| Target lesion revascularization n (%) | |||
| 30 days | 0 | 2 (1.6) | 0.134 |
| 1 year | 13 (6.0) | 8 (6.4) | 1.000 |
| 3 years | 17 (7.9) | 10 (8.0) | 1.000 |
| Definite ST n (%) | |||
| 30 days | 0 | 1 (0.8) | 0.367 |
| 1 year | 2 (0.9) | 2 (1.6) | 0.626 |
| 3 years | 3 (1.4) | 3 (2.4) | 0.673 |
| Definite and probable ST n (%) | |||
| 30 days | 0 | 1 (0.8) | 0.367 |
| 1 year | 3 (1.4) | 2 (1.6) | 1.000 |
| 3 years | 4 (1.9) | 3 (2.4) | 0.710 |
| Definite, probable, and possible ST n (%) | |||
| 30 days | 0 | 1 (0.3) | 0.367 |
| 1 year | 4 (1.9) | 2 (1.6) | 1.000 |
| 3 years | 6 (2.8) | 3 (2.4) | 1.000 |
Abbreviations: IQR, interquartile range; IVUS, intravascular ultrasound; MACE, major adverse cardiac event; ST, stent thrombosis.
Figure 2.
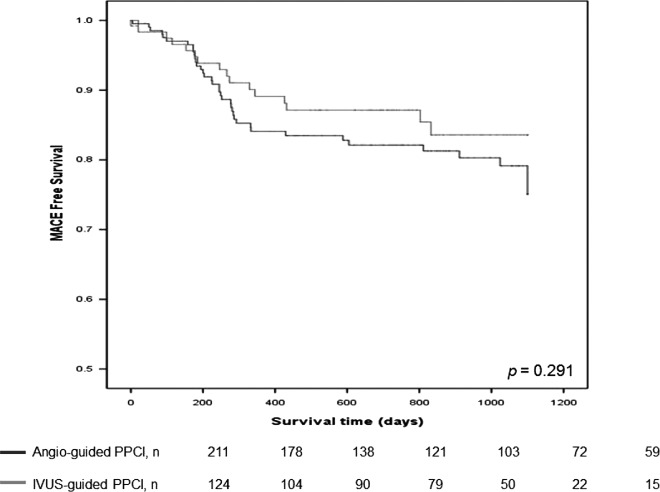
Kaplan‐Meier curves illustrating freedom from major adverse cardiac event (MACE) in angio‐guided primary percutaneous coronary intervention (PCI) group and intravascular ultrasound (IVUS)‐guided primary PCI group in patients (P = 0.291). Abbreviations: PPCI, primary percutaneous coronary intervention.
After adjustment by the multivariate Cox regression model using previously described risk factors (including IVUS‐guided PPCI), IVUS guidance was not an independent predictor for 3‐year MACE. Although multiple factors, including left anterior descending myocardial infarction (odds ratio [OR]: 49.36, 95% confidence interval (CI): 3.15–773.23, P = 0.005), disease extent (OR: 15.05, 95% CI: 1.98–114.32, P = 0.009), final TIMI flow grade 3 (OR: 0.06, 95% CI: 0.01–0.50, P = 0.010), and number of implanted stents (OR: 3.97, 95% CI: 1.00–15.74, P = 0.049) were independently associated with in‐hospital mortality. Only an implanted stent diameter (OR: 0.41, 95% CI: 0.18–0.93, P = 0.033) was an independent risk factor for 3‐year MACE.
Discussion
This study evaluates the impact of IVUS‐guided PPCI in patients with STEMI on clinical outcome and stent thrombosis. Although IVUS‐guided PPCI was associated with a more favorable immediate procedural result with increasing luminal diameter after DES implantation, this did not improve clinical outcome or prevent stent thrombosis.
We found that IVUS guidance during PPCI did not improve clinical outcome and stent thrombosis, despite the fact that IVUS has the potential to reduce events by detecting the suboptimal DES deployment, which is reported to reduce the risk of restenosis and stent thrombosis.6, 7, 8, 9 Our results are similar to Maluenda's,12 who reported that the routine use of IVUS guidance for stent deployment in patients presenting with acute myocardial infarction and undergoing PPCI could not reduce the MACE and stent thrombosis compared with the use of angiography guidance. They provided the following possible explanations. First, because ruptured plaques are typically characterized by a thin fibrous cap with less calcification that does not usually result in severe fibrotic narrowing,13, 14, 15 these plaques usually do not require postdilatation for proper stent implantation with high pressure. Second, the rate of additional balloon inflations, which may increase the risk of subsequent distal embolization associated with substantial morbidity and mortality after PCI, was significantly higher in the IVUS‐guided PCI group.16
In our series, the rate of adjunctive ballooning was significantly higher in the IVUS‐guided PPCI group. However, there was no difference in the final TIMI flow grade and the slow or no‐reflow phenomena between groups. We presumed that the higher rate of glycoprotein IIb/IIIa inhibitor use and thrombectomy, which are known to reduce the slow or no‐reflow phenomena, would result in a similar rate of final TIMI flow grade and slow or no‐reflow phenomena in the groups.17, 18
We can offer 1 explanation for why the MACE rate and stent thrombosis was similar in our study, despite the higher rate of using glycoprotein IIb/IIIa inhibitor and thrombectomy. In our study, a longer length or a greater number of stents was used in the IVUS‐guided PPCI group. It is well known that the length of the stented segment was independently associated with restenosis, myocardial infarction, death, and stent thrombosis after DES implantation.19, 20 Although the use of IVUS was associated with the use of a larger diameter DES, and larger minimal luminal diameters postintervention, the use of IVUS‐guided PPCI resulted in implantation of longer or more DESs, minimizing the potential benefits of IVUS utility in patients with STEMI.
Finally, we found that multiple factors, including left anterior descending territory myocardial infarction, disease extent, final TIMI flow grade, and number of implanted stents, were associated with in‐hospital mortality in multivariate analysis, but only stent diameter was an independent predictor for long‐term clinical outcome in patients with survival discharge. This result was similar to that of Parodi et al, in which anonoptimal PPCI result was strongly predictive of early mortality. However, in patients surviving the early phase, the incidence of clinical events at long‐term follow‐up appears to be similar to successfully reperfused acute myocardial infarction patients.21 We attempted to evaluate the risk factors for long‐term clinical outcome, not including patients who did not survive hospitalization. Our study showed that only larger stent diameter was independently associated with fewer clinical events. This indicates that whether the operator uses IVUS during DES implantation or not, every effort must be made to achieve a larger cross‐sectional area that would favor the long‐term clinical outcome.
Limitations
There were several limitations to our study. First, this was a non‐randomized, single‐center, observational study. Although we used multivariate analysis, unaccounted for variables might potentially influence outcomes. Second, the number of patients was relatively small. Although we could not find statistical differences between the 2 groups, IVUS‐guided PPCI showed 29% of relative risk reduction for MACE at 3 years in our study. This difference could be statistically significant in a study with a larger number of patients. Third, we reported a similar rate of discharge medications except cilostazol. But we did not report the patients' compliance to medical therapy during follow‐up, which could be associated with clinical outcome. Finally, the use of IVUS was at the operator's discretion, but the use of IVUS was a routine practice in our institution since 2008 (72.8% since 2008 vs 27.2% before 2008, P<0.001). It means that most of the patients in the angio‐guided PPCI were enrolled before 2008. Because of the time difference of enrollment, unmeasured differences of trends in treatment of patient with STEMI could affect the results. In addition, we already excluded the nonsurvivors to avoid potential selection bias imposed by the use of IVUS in the acute PCI scenario. But the patients with unstable hemodynamic status or complex coronary lesions, such as severe tortuosity or heavy calcification that are difficult to use IVUS, could be included to the angio‐guided PPCI group.
Conclusion
IVUS‐guided PPCI during DES implantation in patients with STEMI did not improve clinical outcome and stent thrombosis. To confirm these findings, a large‐scale, randomized, controlled trial should be performed.
References
- 1. Moses JW, Leon MB, Popma JJ, et al. Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. [DOI] [PubMed] [Google Scholar]
- 2. Stone GW, Ellis SG, Cox DA, et al. A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. [DOI] [PubMed] [Google Scholar]
- 3. Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus‐or paclitaxel‐eluting stents. Circulation. 2006;113:2293–2300. [DOI] [PubMed] [Google Scholar]
- 4. Pfisterer M, Brunner‐LaRocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug‐eluting stents: an observational study of drug‐eluting versus bare‐metal stents. J Am CollCardiol. 2006;48:2584–2591. [DOI] [PubMed] [Google Scholar]
- 5. Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study. Lancet. 2007;369:667–678. [DOI] [PubMed] [Google Scholar]
- 6. Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus‐eluting stent implantation. Eur Heart J. 2006;27:1305–1310. [DOI] [PubMed] [Google Scholar]
- 7. Fujii K, Mintz GS, Kobayashi Y, et al. Contribution of stent underexpansion to recurrence after sirolimus‐eluting stent implantation for in‐stent restenosis. Circulation. 2004;109:1085–1088. [DOI] [PubMed] [Google Scholar]
- 8. Cheneau E, Leborgne L, Mintz GS, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108:43–47. [DOI] [PubMed] [Google Scholar]
- 9. Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus‐eluting stent implantation: an intravascular ultrasound study. J Am CollCardiol. 2005;45:995–998. [DOI] [PubMed] [Google Scholar]
- 10. Okabe T, Mintz GS, Buch AN, et al. Intravascular ultrasound parameters associated with stent thrombosis after drug‐eluting stent deployment. Am J Cardiol. 2007;100:615–620. [DOI] [PubMed] [Google Scholar]
- 11. Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug‐eluting stents. Eur Heart J. 2008;29:1851–1857. [DOI] [PubMed] [Google Scholar]
- 12. Maluenda G, Lemesle G, Ben‐Dor I, et al. Impact of intravascular ultrasound guidance in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Catheter CardiovascInterv. 2010;75:86–92. [DOI] [PubMed] [Google Scholar]
- 13. Fuster V, Badimon J, Chesebro JH, et al. Plaque rupture, thrombosis, and therapeutic implications. Haemostasis. 1996;26(suppl 4):269–284. [DOI] [PubMed] [Google Scholar]
- 14. Virmani R, Burke AP, Farb A. Plaque morphology in sudden coronary death. Cardiologia. 1998;43:267–271. [PubMed] [Google Scholar]
- 15. Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 suppl):C13–C18. [DOI] [PubMed] [Google Scholar]
- 16. Ndrepepa G, Tiroch K, Fusaro M, et al. 5‐year prognostic value of no‐reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383–2389. [DOI] [PubMed] [Google Scholar]
- 17. Movahed MR, Butman SM. The pathogenesis and treatment of no‐reflow occurring during percutaneous coronary intervention. Cardiovasc Revasc Med. 2008;9:56–61. [DOI] [PubMed] [Google Scholar]
- 18. Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1‐year follow‐up study. Lancet. 2008;371:1915–1920. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi Y, De Gregorio J, Kobayashi N, et al. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol. 1999;34:651–659. [DOI] [PubMed] [Google Scholar]
- 20. Suh J, Park DW, Lee JY, et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug‐eluting stent implantation. JACC Cardiovasc Interv. 2010;3:383–389. [DOI] [PubMed] [Google Scholar]
- 21. Parodi G, Valenti R, Carrabba N, et al. Long‐term prognostic implications of nonoptimal primary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2006;68:50–55. [DOI] [PubMed] [Google Scholar]


